Abstract
Objective
Uncertainties remain regarding the relationship between sarcopenic obesity and frailty. This study aimed to explore the association of these two common geriatric syndromes among community-dwelling older adults.
Methods
Baseline data from the West China Health and Aging Trend (WCHAT) study was used. Sarcopenia was assessed based on the criteria established by the Asian working group for sarcopenia. Body fat percentages above the 60th percentile specified by sex were classified as obesity. Sarcopenic obesity was defined as the concurrence of obesity and sarcopenia. Frailty was assessed by Fried criteria. Multinomial logistic regression was adopted to explore associations of sarcopenic obesity with frailty.
Results
Overall, 2372 older adults (mean age 67.6 ± 5.9) were involved in this study. The prevalence of frailty and sarcopenic obesity was 6.2 and 6.28%, respectively. After adjusting for covariates, sarcopenic obesity was significantly associated with prefrailty (OR = 1.74, 95% CI = 1.15–2.64, P = 0.009) and frailty (OR = 4.42, 95% CI = 2.19–8.93, P < 0.001) compared to nonsarcopenia and nonobesity.
Conclusions
Sarcopenic obesity was significantly correlated with prefrailty and frailty among older adults. Intervention for sarcopenic obesity may contribute to the prevention of incident frailty.
Similar content being viewed by others
Introduction
Frailty, characterized by increased susceptibility to stressors and decreased physiological reserves [1], is a multidimensional geriatric condition incorporating physical, psychological and social domains [2]. Frailty is a highly prevalent and health-threatening issue among older adults. Presently, several operational definitions of frailty have been proposed, among which the Fried phenotype [3] and the Frailty Index (FI) [4] are most frequently used. The prevalence of frailty differs significantly, ranging from 4 to 59% due to the lack of a unique definition [5]. The adverse outcomes of frailty are wide-ranging. Disability [6], falls [7], fractures, mortality [8], loneliness, depression [9], cognitive impairment, dementia [10] and hospitalization [11] are all reported to be correlated with frailty.
As a dynamic condition, prefrailty and frailty are believed to be reversible to some extent. Among numerous studies conducted on the management of frailty, the European SPRINTT project (sarcopenia and physical frailty in older people: multicomponent treatment strategies), a multicomponent strategy composed of nutritional and technological intervention, physical activity and educational counseling, has drawn our attention [12, 13]. It has been demonstrated that this multicomponent intervention could reduce the incidence of mobility disability [14] in physically frail or sarcopenic older adults. Despite the inspiring results of the project, identifying modifiable risk factors for frailty is still a priority for healthy aging.
Body composition changes with aging, and muscle mass usually decreases in conjunction with fat mass gain. The concurrence of excessive adiposity and low muscle mass is emerging as a major health problem termed ‘sarcopenic obesity’ [15]. Sarcopenic obesity consists of two components, namely, sarcopenia and obesity. Sarcopenia per se is closely related to frailty and has been regarded as a biological substrate of physical frailty [16]. Obesity has also been linked to frailty. A meta-analysis conducted by Yuan et al. revealed that both abdominal obesity (relative risk (RR) = 1.57, 95% confidence interval (CI) = 1.29–1.91) defined by waist circumference and general obesity defined by body mass (RR = 1.40, 95% CI = 1.17–1.67) could increase the risk of frailty [17]. In addition, an increased body fat percentage has also been reported to be associated with frailty (β = 0.97 ± 0.43, p = 0.03) [18]. Although no consensus has been reached regarding the diagnostic criteria of sarcopenic obesity, the hazardous effect of sarcopenic obesity should never be neglected.
Presently, associations of frailty with decreased muscle mass or increased body fat have been explored separately. However, little is known regarding the association between sarcopenic obesity and frailty. Whether sarcopenic obesity augments the deleterious effect of each condition remains unclear.
To bridge this gap, we conducted this study, which aimed to shed light on the prevalence of sarcopenic obesity, as well as the association between sarcopenic obesity and frailty in older adults.
Methods
Study design and sample selection
This was a retrospective, cross-sectional analysis of baseline data from the West China Health and Aging Trend (WCHAT) study. Details of the WCHAT study have been described elsewhere [19]. The WCHAT study was approved by the Ethics Committee of West China Hospital, Sichuan University (reference: 2017–445) and was carried out under the guidance of the Helsinki Declaration. This study was also registered at the Chinese Clinical Trial Registry (number ChiCTR1800018895; date of first registration 16/10/2018). Before enrollment, informed consent was obtained from each participant.
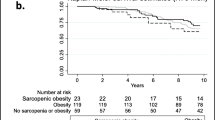
A total of 7536 participants were enrolled in the WCHAT study. Eventually, we included 2372 participants after excluding 3022 participants under 60 years old, 1578 with missing data for bioimpedance analysis, and 528 missing data for grip strength, gait speed, body fat percentage and frailty phenotype (Fig. 1).
Assessment of frailty
Frailty was assessed based on the modified Fried phenotype [3]. Five components were used to define frailty, including shrinking, weakness, exhaustion and slowness. Participants were divided into 3 groups according to the number of components involved (0 component for robust, 1 or 2 components for prefrailty and 3 or more components for frailty). The details of each component are described below.
-
(1)
Shrinking: shrinking was defined as an unintentional weight loss of more than 4.5 kg during the past year or a body mass index (BMI) < 18.5 kg/m2.
-
(2)
Weakness: Weakness was defined as grip strength of the dominant hand in the lowest quintile of the population distribution, adjusted for sex and body mass index (BMI).
-
(3)
Exhaustion: meeting any one of the criteria below was considered exhaustion. (1) I felt extremely fatigued for the majority of the time; (2) I felt extremely weak for the majority of the time; (3) A self-reported energy score of three or less was reported when a score of ten represents the condition with the greatest power.
-
(4)
Slowness: 4-m walking time in the lowest quintile of the population distribution, adjusted for sex and height.
-
(5)
Low physical activity: Sex-adjusted kilocalories in the lowest quintile based on a validated China Leisure Time Physical Activity Questionnaire (CLTPAQ) [20]
Assessment of sarcopenia, obesity and sarcopenic obesity
Sarcopenia was assessed based on the criteria established by the Asian Working Group for Sarcopenia (AWGS) in 2019 [21]. The appendicular skeletal muscle index (SMI) was used as an indicator for muscle mass. SMI and body fat percentage were calculated with a bioimpedance analyzer (InBody 770, Biospace, Korea). The cutoffs for low muscle mass were 7.0 kg/m2 and 5.7 kg/m2 in men and women, respectively. Dynamometers (EH101; Camry, Zhongshan, China) were used to measure grip strength. The cutoffs for low grip strength were 28 kg for males and 18 kg for females. A cutoff of 1.0 m/s for gait speed was used to estimate physical function. Body fat percentages exceeding the 60th percentile specified by sex were classified as obesity [22]. Concurrence of obesity and sarcopenia was defined as sarcopenic obesity [23].
Covariates
Information including age, sex, education level (illiteracy/primary school/secondary school or above), ethnicities (Han/Yi/Tibetan/Qiang/other ethnic minorities), smoking history, alcohol history, marital status (married/single), and number of chronic diseases (0/1/≥ 2) were collected via face-to-face interviews. Nutrition status was categorized using the Mini Nutrition Assessment-Short Form (MNA-SF) scale (0 ~ 11 scores as malnutrition risk; 12 ~ 14 scores as well nourished) [24].
Statistical analysis
We conducted the analyses with Stata software, version 14.0 (Stata Corp, College Station, TX, USA). Continuous data are presented as the means ± standard deviations (SD) or medians and interquartile range (IQR), while categorical variables are presented as counts (percentages). Group differences were tested by ANOVA or Kruskal-Wallis for normally distributed or skewed continuous variables and the chi square test for categorical variables, respectively. Multinomial logistic regression was adopted to explore the associations of frailty with sarcopenic obesity. Variables such as age, sex, ethnicity, education level, marital status, smoking history, drinking history, number of chronic diseases, and risk of malnutrition were included in the adjusted model. Each statistical test was two-sided, and P < 0.05 was set as the significance level.
Results
In total, 2372 participants (mean age 67.6 ± 5.9 years; 60.24% female) were included in this analysis. The prevalence rates of obesity alone, sarcopenia alone and sarcopenic obesity were 33.05, 23.31 and 6.28%, respectively. The percentages of prefrailty and frailty were 46.96 and 6.2%, respectively.
Table 1 presents the characteristics of the participants according to sarcopenia and obesity status. Significant differences regarding age, sex, ethnicities, education level, smoking history, marital status, number of chronic diseases, nutritional status and frailty status, were observed among the 4 groups. Participants with sarcopenia alone or sarcopenic obesity were older than those in the obesity alone group or the nonobese and nonsarcopenia group.
Table 2 shows the results of logistic regression about the association of frailty with sarcopenic obesity. We found that in the unadjusted model, sarcopenic obesity and sarcopenia alone were significantly related to prefrailty and frailty compared with the nonobesity and nonsarcopenia groups, whereas obesity alone was not. The odds ratios for prefrailty were 1.77 (95% CI = 1.42–2.22, P < 0.001) in the sarcopenia alone group and 1.97 (95% CI = 1.34–2.89, P < 0.001) in the sarcopenic obesity group. In addition, the odds ratios for frailty were 4.14 (95% CI = 2.60–6.59, P < 0.001) in the sarcopenia alone group and 7.00 (95% CI = 3.79–12.93, P < 0.001) in the sarcopenic obesity group. However, after adjustment for confounders, only sarcopenic obesity was independently associated with prefrailty and frailty. The respective odds ratios for prefrailty and frailty were 1.74 (95% CI = 1.15–2.64, P = 0.009) and 4.42 (95% CI = 2.19–8.93, P < 0.001), respectively.
We further explored sex and age differences regarding the association of frailty with sarcopenic obesity in a fully adjusted model. After stratification by sex, the association of frailty with sarcopenic obesity remained significant. The respective odds ratios were 7.14 (95% CI = 2.19–23.97, P = 0.001) and 4.18 (95% CI = 1.63–10.72, P = 0.003) for males and females, respectively. However, an association of sarcopenic obesity with prefrailty was observed only in males (OR = 2.00, 95% CI = 1.12–3.57, P = 0.018) and not in females (OR = 1.40, 95% CI = 0.76–2.61, P = 0.276) (Table 3). Regarding different age groups, sarcopenic obesity was demonstrated to be significantly associated with prefrailty (OR = 2.84, 95% CI = 1.32–6.13, P = 0.007) and frailty (OR = 6.86, 95% CI = 2.52–18.64, P < 0.001) in participants aged 70 years and over. However, in participants aged 60–69 years, sarcopenic obesity was only related to frailty (OR = 3.79, 95% CI = 1.16–12.42, P = 0.027) rather than prefrailty (OR = 1.45, 95% CI = 0.86–2.44, P = 0.153) (Table 4).
Discussion
This study first examined the association of sarcopenic obesity with frailty among community-dwelling older adults in western China. Our results revealed that individuals with sarcopenic obesity had 1.74 times and 4.42 times increased risks for prefrailty and frailty, respectively.
In our study, the prevalence of frailty was 6.2%, which was in accordance with previously reported data in the Chinese community [25]. The prevalence of frailty was 15.4, 10.3 and 4.5% in the sarcopenic obesity group, sarcopenia alone group and obesity alone group, respectively. Our study indicated that sarcopenic obesity significantly increased the risks of prefrailty and frailty. These findings were supported by Hirani and colleagues, who reported that men with sarcopenic obesity were more prone to frailty, with an odds ratio of 2.0 (95% CI = 1.42–2.82) [26]. In addition, Saitoh et al. also reported that sarcopenic obesity could increase the risk of frailty with an odds ratio of 4.518 (95% CI = 1.218–16.752, P = 0.024) in patients undergoing hemodialysis [27].
The results of our study demonstrated that neither sarcopenia alone nor obesity alone fully captured the vital link to frailty, which was partially different from previous studies. This discrepancy possibly resulted from ethnic differences as well as variance in diagnostic criteria for obesity, sarcopenia and frailty. Meanwhile, decreased muscle mass along with increased body fat may not have been taken into consideration simultaneously in these studies, which may obscure the role of obesity in sarcopenia and vice versa. After age stratification, a significant association between sarcopenic obesity and prefrailty was found only in individuals aged 70 and over. This is possibly because of the dynamic nature and complex diagnostic components of prefrailty as well as the dominant role of aging in the development of prefrailty. Presently, most studies have focused on associations of frailty with obesity or sarcopenia separately. For example, Falsarella et al. reported that a significant difference in body fat percentage was observed between nonfrail and frail individuals [28]. In addition, sarcopenia components, including decreased muscle mass [29], decreased grip strength [30] and decreased gait speed [31], have also been reported to be related to frailty. However, a recent study disclosed that sarcopenia predicted frailty with a high specificity (> 97%) but a low sensitivity (< 10%) [32]. Considering that an aging-related increase in fat mass is always coupled with a decrease in muscle mass, the combined effect of sarcopenia and obesity could be more predictive.
The mechanism linking sarcopenic obesity with frailty remains unclear. Here, we provide some insights into the similarities between the two diseases. Biological factors contributing to the development of frailty overlap significantly with those described for sarcopenic obesity. Weakness is reported to present as an initial sign of frailty [33]. Fat infiltration including intermuscular adipose tissue (IMAT) and intramyocellular lipids (IMCLs) in skeletal muscle is reported to be associated with muscle weakness [34]. On the one hand, IMCLs could impair mitochondrial function, reduce lipid β-oxidation and enhance reactive oxygen species (ROS) production. Increased ROS could activate stress pathways such as c-jun N-terminal kinase(JNK), IκB kinase (IKK), and p38-mitogen-activated protein kinase (p38-MAPK), which compromise the function of muscle protein [35]. On the other hand, inflammation is regarded as the common pathogenesis of sarcopenia, sarcopenic obesity and frailty [36, 37]. IMAT is reported to enhance whole-body inflammation as well as induce local inflammation and insulin resistance by releasing a number of proinflammatory cytokines, which could impair muscle function [38, 39]. A meta-analysis showed that inflammatory markers such as C reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) are associated with lower grip strength (CRP; r = − 0.10, p < 0.001, IL-6; r = − 0.13, p < 0.001, TNFα; r = − 0.08, p < 0.00) [40]. It has been reported that CRP can inhibit Akt phosphorylation, downregulate the mammalian target of rapamycin (mTORC1) pathway, and inhibit the synthesis of muscle fibrin [41]. In addition, IL-6 may exert detrimental effects by upregulating ubiquitin, E3 ligase, and proteasome activity as well as by activating the nuclear factor kappa-B (NF-κB) pathway. Moreover, TNF-α can not only downregulate the expression of myogenic genes but also upregulate atrophy-associated genes such as muscle atrophy F-box (MuRF1) and muscle ring-finger protein 1 (MAFbx) by activating ubiquitin proteasome signaling and the NF-κB pathway [42].
The strength of our study was that we first investigated the association of sarcopenic obesity with frailty based on a relatively large sample size in western China. Nevertheless, there inevitably existed some limitations. First, the causal association of sarcopenic obesity with frailty cannot be confirmed by a cross-sectional study. In the future, longitudinal studies are needed to verify their relationship. Second, studies on sarcopenic obesity are hindered by the absence of a unified definition to a great extent. Third, the retrospective nature of the study may have introduced bias. Finally, there were other potential confounders we failed to address, such as dietary preference and medical conditions (chronic diseases, drug use and hospitalization et.al).
Conclusion
Our findings indicated that sarcopenic obesity was significantly associated with frailty among older adults. As sarcopenic obesity is a hazardous but potentially modifiable condition, intervention for sarcopenic obesity may contribute to the prevention of incident frailty.
Availability of data and materials
The data that support the findings of this study are available from the National Clinical Research Center for Geriatrics, West China Hospital, but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available from the corresponding author upon reasonable request and with permission of the National Clinical Research Center for Geriatrics, West China Hospital.
Abbreviations
- SPRINTT:
-
Sarcopenia and physical frailty in older people: multicomponent treatment strategies
- RR:
-
Relative risk
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- WCHAT:
-
West China Health and Aging Trend study
- BMI:
-
Body mass index
- CLTPAQ:
-
China leisure time physical activity questionnaire
- AWGS:
-
Asian Working Group for Sarcopenia
- SMI:
-
Appendicular skeletal muscle index
- MNA-SF:
-
Mini Nutrition Assessment-Short Form
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- IMAT:
-
Intermuscular adipose tissue
- IMCLs:
-
Intramyocellular lipids
- ROS:
-
Reactive oxygen species
- JNK:
-
c-jun N-terminal kinase
- IKK:
-
IκB kinase
- p38-MAPK:
-
p38-mitogen-activated protein kinase
- CRP:
-
C reactive protein
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor α
- mTORC1:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor kappa-B
- MuRF1:
-
Muscle atrophy F-box
- MAFbx:
-
Muscle ring-finger protein 1
- NA:
-
Nonapplicable
References
Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15.
Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook. 2010;58(2):76–86.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59(6):M627–32.
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92.
Kojima G. Frailty as a predictor of disabilities among community-dwelling older people: a systematic review and meta-analysis. Disabil Rehabil. 2017;39(19):1897–908.
Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and Meta-analysis. J Am Med Dir Assoc. 2015;16(12):1027–33.
Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62(7):744–51.
Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87.
Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: a systematic review and Meta-analysis. J Am Med Dir Assoc. 2016;17(10):881–8.
Kojima G. Frailty as a predictor of hospitalisation among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(7):722–9.
Longobucco Y, Benedetti C, Tagliaferri S, Angileri VV, Adorni E, Pessina M, et al. Proactive interception and care of frailty and multimorbidity in older persons: the experience of the European innovation partnership on active and healthy ageing and the response of Parma local health trust and lab through European projects. Acta Biomed. 2019;90(2):364–74.
Jyvakorpi SK, Ramel A, Strandberg TE, Piotrowicz K, Blaszczyk-Bebenek E, Urtamo A, et al. The sarcopenia and physical frailty in older people: multi-component treatment strategies (SPRINTT) project: description and feasibility of a nutrition intervention in community-dwelling older Europeans. Eur Geriatr Med. 2021;12(2):303–12.
Bernabei R, Landi F, Calvani R, Cesari M, Del Signore S, Anker SD, et al. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ. 2022;377:e068788.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–46.
Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(4):1118–28.
Crow RS, Lohman MC, Titus AJ, Cook SB, Bruce ML, Mackenzie TA, et al. Association of Obesity and Frailty in older adults: NHANES 1999-2004. J Nutr Health Aging. 2019;23(2):138–44.
Hou L, Liu X, Zhang Y, Zhao W, Xia X, Chen X, et al. Cohort profile: West China health and aging trend (WCHAT). J Nutr Health Aging. 2021;25(3):302–10.
Wang Y, Deng C, Ding D, Song Y, Taiping L, Jirong YJPG. Development and validation of the China Leisure Time Physical Activity Questionnaire in the elderly. Prac Geriatr. 2019;33(3):229–33.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307 e302.
Rolland Y, Lauwers-Cances V, Cristini C. Abellan van Kan G, Janssen I, Morley JE, Vellas B: difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) study. Am J Clin Nutr. 2009;89(6):1895–900.
Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clinical nutrition (Edinburgh, Scotland). 2020;39(8):2368–88.
Zhang L, Wang C, Sha SY, Kwauk S, Miller AR, Xie MS, et al. Mini-nutrition assessment, malnutrition, and postoperative complications in elderly Chinese patients with lung cancer. J BUON. 2012;17(2):323–6.
Wu C, Smit E, Xue QL, Odden MC. Prevalence and correlates of frailty among community-dwelling Chinese older adults: the China health and retirement longitudinal study. J Gerontol A Biol Sci Med Sci. 2017;73(1):102–8.
Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: the Concord health and ageing in men project. Age Ageing. 2017;46(3):413–20.
Saitoh M, Ogawa M, Kondo H, Suga K, Takahashi T, Itoh H, Tabata YJRRT. Sarcopenic obesity and its association with frailty and protein-energy wasting in hemodialysis patients: preliminary data from a single center in Japan. Ren Replace Ther. 2019;5(1):1–9.
Falsarella GR, Gasparotto LP, Barcelos CC, Coimbra IB, Moretto MC, Pascoa MA, et al. Body composition as a frailty marker for the elderly community. Clin Interv Aging. 2015;10:1661–6.
Chan KS, Chan YM, Chin YS, Mohd Shariff Z. Dietary quality, sleep quality and muscle mass predicted frailty among Chinese postmenopausal women in Malaysia. Int J Environ Res Public Health. 2022;19(5):2565.
Dudzinska-Griszek J, Szuster K, Szewieczek J. Grip strength as a frailty diagnostic component in geriatric inpatients. Clin Interv Aging. 2017;12:1151–7.
Jung HW, Jang IY, Lee CK, Yu SS, Hwang JK, Jeon C, et al. Usual gait speed is associated with frailty status, institutionalization, and mortality in community-dwelling rural older adults: a longitudinal analysis of the aging study of Pyeongchang rural area. Clin Interv Aging. 2018;13:1079–89.
Davies B, Garcia F, Ara I, Artalejo FR, Rodriguez-Manas L, Walter S. Relationship between sarcopenia and frailty in the Toledo study of healthy aging: a population based cross-sectional study. J Am Med Dir Assoc. 2018;19(4):282–6.
Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women's health and aging study II. J Gerontol A Biol Sci Med Sci. 2008;63(9):984–90.
Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–85.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nat. 2000;408(6809):239–47.
Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–21.
Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–22.
Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring, Md). 2009;17(5):1062–9.
Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, et al. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014;18(5):532–8.
Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev. 2020;64:101185.
Yang H, Jiang X, Li B, Yang HJ, Miller M, Yang A, et al. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature. 2017;552(7685):368–73.
Wang T. Searching for the link between inflammaging and sarcopenia. Ageing Res Rev. 2022;77:101611.
Acknowledgements
We thank all the participants for their contribution in the WCHAT study.
Funding
This work was funded by the National Key R&D Program of China (2020YFC2005600, 2020YFC2005602 and 2017YFC0840101); “Chengdu Science and Technology Bureau Major Science and Technology Application Demonstration Project” (2019YF0900083SN); National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University Z2021JC003.
Author information
Authors and Affiliations
Contributions
MY formulated the research question, designed the study, analyzed the data, and drafted the paper. MH, YZ, WZ, MG, SJ, and XS designed the study, analyzed the data, and revised the paper. BD assisted with formulating the research question, interpreting the data, and supervising the quality of the paper. All authors reviewed, provided feedback to, and confirmed the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of West China Hospital, Sichuan University (reference: 2017–445). All participants gave written informed consent before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, M., Hu, M., Zhang, Y. et al. Sarcopenic obesity is associated with frailty among community-dwelling older adults: findings from the WCHAT study. BMC Geriatr 22, 863 (2022). https://doi.org/10.1186/s12877-022-03617-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03617-z