Abstract
Introduction
The study of polymorphisms and their relationship with diseases is very important for risk assessment. The aim of this study was to determine the relationship between early risk of coronary artery disease(CAD) with renin-angiotensin(RAS) genes and endothelial nitric oxide synthase(eNOS) in a sample of the Iranian population.
Methods & materials
In this cross-sectional study, 63 patients with premature CAD and 72 healthy samples were enrolled. Polymorphism of the promotor region of eNOS- and ACE-I/D (Angiotensin Converting Enzyme-I/D) polymorphism was evaluated. Polymerase chain reaction (PCR) test and PCR-RFLP (Restriction Fragment Length Polymorphism) was performed for ACE and eNOS-786 gene, respectively.
Results
The frequency of deletion(D) for the ACE gene was significantly higher in patients(96% versus 61%; P < 0.001). Conversely, the number of defective C alleles for the eNOS gene was similar in both groups (p > 0.9).
Conclusion
ACE polymorphism seems to be an independent risk factor for premature CAD.
Similar content being viewed by others
Introduction
Despite of decrease in mortality by improvement of treatment options, one of the main etiology of death all over the world is still coronary artery disease (CAD) [18]. Many potential risk factors like environmental, biochemical, and inherited cause CAD due to progress in atherosclerotic lesions [7, 30]. Although there are some known genetic loci associated with CAD, less heritability of CAD is explained [10]. It seems that genetic risk factors have marked effect on etiology of CAD in young patients, because they have not enough time to expose to environmental factors [27]. Premature CAD is defined as: CAD in women younger than 55 years and men younger than 45 years, but this age range varies from 45 to 65 years in different studies [22].
Nowadays, the study on polymorphisms and their relationship with diseases is very important for risk assessment. Therefore, the analyzing of the association of any polymorphism in different population is specific to that groups and can be considered as a marker to determine the risk of disorder [28]. Many recognized polymorphisms have been shown to be associated with the risk of premature CAD. Although the list of genetic variants correlated with cardiovascular disease is still expanding, the genes of the renin angiotensin system (RAS) polymorphisms have been extensively studied to date. This is based on the fact that RAS has a great impact on the cardiovascular system and plays a key role in stimulating vascular smooth muscle cell proliferation, intimal fibrosis, inflammatory reactions, pro-thrombotic processes, and plaque calcification [11]. Numerous studies have shown polymorphisms of various components of the angiotensin-converting enzyme (ACE) system with the progression of CAD in some individuals and the efficacy of ACE inhibitors and angiotensin II receptor blockers [2]. Three common polymorphisms of different RAS genes have been commonly attributed to the risk of premature CAD or MI in previous investigations. These polymorphisms include insertion/deletion polymorphism in the angiotensin converting enzyme (ACE) gene, A1166C polymorphism in the ATR1 receptor gene (cell surface receptor for angiotensin II), and M235T polymorphism in the angiotensinogen gene [21]. One of the most important endothelial cell products is nitric oxide (NO), an important endothelium-dependent vasodilator mediator produced by endothelial cells from l-arginine. Nitric oxide also controls the function of angiotensin II in cardiovascular tissues, which itself regulates nitric oxide synthesis (10). Another notable gene would be the endothelial NOS gene. Nitric oxide (NO) is a molecule synthesized enzymatically from l-arginine (l-Arg) by three isoforms of NO synthase (NOS), i.e. neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) [19]. NO synthesized by eNOS is important for maintaining endothelial homeostasis. It mediates vasodilatation and suppresses smooth muscle cells proliferation. It also has an antioxidant effect. It promotes aggregation and proliferation of thrombocytes or inhibition of apoptosis of myocytes. Thus, polymorphisms within eNOS gene are suggested to be associated with the CAD development [1].
In this study, we evaluated the association of polymorphisms of renin angiotensin system and endothelial nitric oxide synthase genes with premature cardiovascular disease in an Iranian population.
Materials and methods
This was a case-control study in which we enrolled patients with premature CAD. Patients who referred to the cardiology department of Ghaem and Imam Reza hospitals, Mashhad, during 2020–2021 were selected. ACE I/D and eNOS polymorphism was tested on 63 patients and 72 controls.
Sample size
Eligibility
Inclusion criteria were the age of onset of CAD less than 50 years and at least 50% of obstruction in large arteries or one of the branches diagnosed by angiography.
Definitions
Arterial hypertension was defined as systolic blood pressure equal to or greater than 140 mmHg and diastolic blood pressure equal to or greater than 90 mmHg as well as patients treated with antihypertensive drugs.
Patients with a history of diabetes and fasting blood sugar above 126 mg/dl and also patients treated with insulin or oral hypoglycemic drugs were defined as diabetes mellitus.
Study design
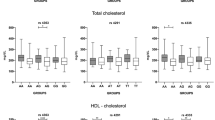
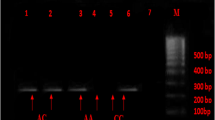
For each patient, a checklist including demographic data, clinical risk factors (diabetes mellitus, hypertension, hypercholesterolemia, and smoking) and laboratory data (triglyceride, total cholesterol, LDL cholesterol, HDL cholesterol) were recorded. From the blood samples of the subjects, DNA extraction was performed by Kiagen kit. To evaluate the polymorphism of the studied genes, first the primer sequence of each gene was extracted from the relevant articles and their accuracy was confirmed by the NCBI site of the PRIMER BLAST section. PCR test was performed for ACE gene alone. For eNOS-786 gene, the PCR product was exposed to MboI enzymes at 37 ° C for 8 hours and then electrophoresed on 3% agarose gel and the bands related to each allele were examined. Genotyping of eNOS-786 region of endothelial nitric oxide synthetize enzyme by PCR amplification method with direct and inverse primers (sense: 5’-CACCCAGGCCCACCCCAACT-3 ‘; antisense: 5’-GCCGCAGGTCGACAGAGACT-3’) was performed according to the protocol of Esposti et al [12] (Fig. 1). PCR of ACE gene was done based on the methods described previously and using ACE primers (5’-GCCCTGCAGGTGTCTGCAGCATGT-3 ‘and 5’-GGATGGCTCTCCCCCGCCTTGTCTC-3’) based on the protocol of Lindpaintner et al [17] (Fig. 2).
Statistics
SPSS version 16 was used for statistical analysis in this study. Descriptive data are displayed as mean ± SD (mean ± SD). Genotype distribution and frequency of alleles were compared between the two groups using chi-square test. Freeman-Halton extensions associated with the Fisher exact test were used for alleles with a frequency of less than five. Logistic regression was used to determine the role of alleles in predicting disease. Significance level less than 0.05 was considered.
Ethics
This study was approved by ethical committee of Mashhad University of Medical Sciences (Ethical ID: IR.MUMS.MEDICAL.REC.1398.723). All steps of the study was in accordance with the Declaration of Helsinki.
Results
A total of 135 patients (83, 61.5% male and 52, 38.5% female) entered the study. The mean ± SD age was 43.5 ± 4.3 years. Fifteen patients had diabetes (23.8%), 20 patients had hypertension (31.7%), 21 patients had hyperlipidemia (33.3%) and 11 patients were smokers (17.5%). Of them, 32% had one vessel involvement, 59% had two-vessel involvement and 9% had three coronary vessels involvement. Table 1 demonstrates the number and percentage of ACE, Dallel, Dhomozygote and eNOS-786 by study groups and statistical test results.
It is demonstrated that 95.2% (n = 60) of participants in the case group and 13.9% (n = 10) in control group had DD allele. Three patients (4.8%) and 38.9% (n = 28) of the control group had allele II. In the case group, no person had D/I but 47.2% of the control group had D/I. One person in each group had eNOS-786 of TC type in which the difference was not statistically significant (P > 0.99). Table 2 shows the number and percentage of variables of diabetes, hypertension, hyperlipidemia and smoking and the results of statistical tests.
Table 3 shows the number and percentage of ACE and eNOS-786 B parameters. In both groups of the ACE gene DD and II alleles, most individuals had two vessels disease. Subjects with one vessel disease were almost identical in both groups, but those with three-vessel disease were only in the D allele group. There was no significant difference between the two groups in terms of the number of involved vessels (P > 0.99). In the eNOS-786 TC group, there was only one subject who had three vessels’ involvement. There was also no significant difference between the two groups in terms of the number of stenotic arteries (P = 0.095).
Discussion
RAS plays an important role in the pathophysiology of heart diseases such as coronary artery stenosis. Coronary artery stenosis is a polygenic disease and its occurrence and severity depends on environmental and genetic factors. The association of RAS polymorphism with classical risk factors for CAD such as hypertension, obesity, diabetes and hyperlipidemia has been reported. ACE I / D gene polymorphism is a strong risk factor for CAD. In this study, patients with premature CAD and healthy control group were evaluated to determine the relationship between CAD and RAS gene and eNOS-786 polymorphism in a sample of the Iranian population. Although available data show that assessment of a single gene role in the development of CAD is insufficient, most case–control studies focus to analyze only one genetic polymorphism in regarding to the disease. To draw meaningful conclusions in the field, several polymorphisms with specific gene–gene as well as gene-environment interactions should be studied. This has been confirmed by earlier researches [27]. We found that there is a strong association between DD polymorphism and CAD. According to genotype studies, DD is associated with MI, hypertrophic cardiomyopathy and coronary artery occlusion, as well as other heart diseases. DD genotype is more closely related to CAD than the other two genotypes (II and ID). The DD genotype of the ACE gene has been identified as a risk factor for heart disease in other communities, such as the Caucasus, Australia, and China [2]. In another study in Saudi Arabia examining of diabetic patients showed that there is a clear correlation between ACE DD and CAD [3].
Some other previous investigations worldwide [2, 4, 9, 11, 14], demonstrated that there is a significant correlation between ACE polymorphism and CAD. RAS gene polymorphism is not only an independent risk factor for CAD progression, but also can affect other CAD risk factors such as hypertension, atherosclerosis and thrombosis. The result is an increased risk of CAD in people who carry these polymorphisms [24]. Conversely, another study in Brazil, in patients with obstructive lesions, there was no association between ACE gene polymorphism and CAD in both Caucasian and Brazilian African breeds [5]. Rallidis et al. found no association between RAS gene polymorphism and premature CAD in a small sample size of patients with early MI [23]. On the other hand, in an investigation in United Kingdom on 684 patients with acute MI and 537 control people, there was no association between ACE I/D polymorphism and MI risk [26]. In another study, the relative risk of the D allele was estimated 1.07 for ischemic heart disease and 1.05 for MI, so the ACE genotype does not significantly increase the risk of ischemic heart disease and MI [17]. Studies support the idea that in the larger statistical population there is an association between RAS gene polymorphisms and CAD and early MI [23].
Our results showed that there was no significant relationship between eNOS-786 polymorphism and CAD. There was no association between the type of polymorphism in eNOS-786 gene and CAD in younger patients in previous studies [1, 15, 20, 29]. In the study by Zigra et al. [31] no association was found in analyzing the whole CAD group. However, the authors demonstrated that prevalence of TT homozygotes were significantly more common only in patients with ‘normal’ or ‘near normal’ coronary arteries compared to controls [31]. In turn, a strong significant association between one of eNOS-786 genotypes and the disease was found by Cam et al. [6]. They showed that NOS3 TT genotype was an independent risk factor for premature CAD with OR = 15.35. Saini et al. [25] observed that mean NO level was significantly lower in subject with GT genotype than in those bearing GG genotype. However, the mean NO levels in the CAD patients with GT genotype were lower comparing to the control group. According to authors, the T allele of 894GT polymorphism in NOS3 gene may be a marker for endothelial dysfunction which is characteristic for CAD. Kim et al. found no association between eNOS G894T and CAD risk [16]. Similarly, Granath et al., in a Caucasian Australian population with premature CAD and younger than 50 years, showed that there was no significant relationship between any of the eNOS-786 gene polymorphisms and early CAD in this population [13]. However, some studies have shown an association between this polymorphism and the risk of CAD. Colombo et al. studied 451 people and showed that the T786C-eNOS gene polymorphism could significantly increase the chance of developing CAD in Italian populations [8]. The discrepancy between the results obtained in the present study and other researches can be attributed to differences in the ethnic and racial backgrounds of the participants in our study with other populations. In addition, environmental factors and the interaction between genes and the environment can also contribute to these differences.
Conclusion
The carrier-state of DD allele of RAS polymorphism was associated with premature CAD but eNOS-786 was not. Recognition of this polymorphism in different populations could help healthcare policy makers to manage the burden of cardiovascular disorders, logically. Performing large-scale study in different regions of Iran could be helpful to mapping the distribution of polymorphism and programming for their future treatments.
Data Availability
Data are available from the authors upon reasonable request and with permission of the corresponding author.
Abbreviations
- RAS:
-
Renin angiotensin
- eNOS:
-
Endothelial nitric oxide synthase
- ACE:
-
Angiotensin Converting Enzyme
- I/D:
-
Insertion/ Deletion
- PCR:
-
Polymerase chain reaction
- RFLP:
-
Restriction Fragment Length Polymorphism
- CAD:
-
Coronary artery disease
- MI:
-
Myocardial infarction
- NO:
-
Nitric oxide
- l-Arg:
-
l-arginine
- nNOS:
-
Neuronal NOS
- iNOS:
-
Inducible NOS
References
Abdel-Aziz T, Mohamed R. Association of endothelial nitric oxide synthase gene polymorphisms with classical risk factors in development of premature coronary artery disease. Mol Biol Rep. 2013;40:3065–71. https://doi.org/10.1007/s11033-012-2380-7.
Al-Hazzani A, Daoud MS, Ataya FS, Fouad D, Al-Jafari AA. Renin–angiotensin system gene polymorphisms among saudi patients with coronary artery disease. J Biol Res (Thessalon). 2014;21:1–9. https://doi.org/10.1186/2241-5793-21-8.
Al-Jafari AA, Daoud MS, Ataya FS. Renin-angiotensin system gene polymorphisms and coronary artery disease in saudi patients with diabetes mellitus. Int J Clin Exp Pathol. 2017;10:10505.
Berdeli A, Sekuri C, Cam FS, Ercan E, Sagcan A, Tengiz I, Eser E, Akın M. Association between the eNOS (Glu298Asp) and the RAS genes polymorphisms and premature coronary artery disease in a turkish population. Clin Chim Acta. 2005;351:87–94. https://doi.org/10.1016/j.cccn.2004.08.015.
Bonfim-Silva R, Guimaraes LO, Santos JS, Pereira JF, Barbosa AAL, Rios DLS. Case–control association study of polymorphisms in the angiotensinogen and angiotensin-converting enzyme genes and coronary artery disease and systemic artery hypertension in african-brazilians and caucasian-brazilians. J Genet. 2016;95:63–9. https://doi.org/10.1007/s12041-015-0599-5.
Cam SF, Sekuri C, Tengiz I, Ercan E, Sagcan A, Akin M, Berdeli A. The G894T polymorphism on endothelial nitric oxide synthase gene is associated with premature coronary artery disease in a turkish population. Thromb Res. 2005;116:287–92. https://doi.org/10.1016/j.thromres.2004.12.002.
Chen H, Ding S, Liu X, Wu Y, Wu X. Association of interleukin-6 genetic polymorphisms and environment factors interactions with coronary artery disease in a chinese Han population. Clin Exp Hypertens. 2018;40:514–7. https://doi.org/10.1080/10641963.2017.1403618.
Colombo MG, Paradossi U, Andreassi MG, Botto N, Manfredi S, Masetti S, Biagini A, ClERICO A. Endothelial nitric oxide synthase gene polymorphisms and risk of coronary artery disease. Clin Chem. 2003;49:389–95. https://doi.org/10.1373/49.3.389.
Dalal AB, Tewari D, Tewari S, Sharma MK, Pradhan M, Gupta UR, Sinha N, Agarwal S. Association of coronary artery disease with polymorphisms of angiotensin-converting enzyme and methylenetetrahydrofolate reductase gene. Indian Heart J. 2006;58:330.
Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. https://doi.org/10.1038/ng.2480.
Dzielińska Z, Małek ŁA, Roszczynko M, Szperl M, Demkow M, Kądziela J, Prejbisz A, Zieliński T, Januszewicz A, Rużyłło W. Combined renin–angiotensin system gene polymorphisms and outcomes in coronary artery disease—a preliminary report. Kardiol Pol. 2011;69:688–95.
Esposti R, Sponton C, Malagrino P, Carvalho F, Peres E, Puga G, Novais I, Albuquerque D, Rodovalho C, Bacci M. Influence of eNOS gene polymorphism on cardiometabolic parameters in response to physical training in postmenopausal women. Braz J Med Biol Res. 2011;44:855–63. https://doi.org/10.1590/S0100-879X2011007500106.
Granath B, Taylor RR, van Bockxmeer FM, Mamotte CD. Lack of evidence for association between endothelial nitric oxide synthase gene polymorphisms and coronary artery disease in the australian caucasian population. Eur J Cardiovasc Prev Rehabil. 2001;8:235–41. https://doi.org/10.1177/174182670100800408.
Guney A, Ergec D, Kirac D, Ozturhan H, Caner M, Koc G, Kaspar C, Ulucan K, Agirbasli M. Effects of ACE polymorphisms and other risk factors on the severity of coronary artery disease. Genet Mol Res. 2013;12:6895–906. https://doi.org/10.4238/2013.December.19.8.
Joshaghani HR, Salehi A, Samadian E, Gharaei R, Ahmadi AR. Association between NOS3 G894T, T-786 C and 4a/4b variants and coronary artery diseases in iranian population. Iran J Public Health. 2018;47:1891.
Kim HS, Bae S-C, Kim T-H, Kim S-Y. Endothelial nitric oxide synthase gene polymorphisms and the risk of osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop. 2013;37:2289–96. https://doi.org/10.1007/s00264-013-1966-6.
Lindpaintner K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, Buring J, Hennekens CH. A prospective evaluation of an angiotensin-converting–enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332:706–12. https://doi.org/10.1056/NEJM199503163321103.
Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–407. https://doi.org/10.1056/NEJMoa1915922.
Marsden PA, Heng H, Scherer S, Stewart R, Hall A, Shi X, Tsui L, Schappert K. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268:17478–88.
Mathew J, Narayanan P, Sundaram R, Jayaraman B, Dutta TK, Raman SK, Chandrasekaran A. Lack of association between glu (298) asp polymorphism of endothelial nitric oxide synthase (eNOS) gene and coronary artery disease in Tamilian population. Indian Heart J. 2008;60:223–7.
Mocan O, Radulescu D, Buzdugan E, Cozma A, Leucuta DC, Procopciuc LM. Association between M235T-AGT and I/D-ACE polymorphisms and carotid atheromatosis in hypertensive patients: a cross-sectional study. in vivo. 2020;34:2811–9. https://doi.org/10.21873/invivo.12107.
Panwar RB, Gupta R, Gupta BK, Raja S, Vaishnav J, Khatri M, Agrawal A. Atherothrombotic risk factors & premature coronary heart disease in India: a case-control study. Indian J Med Res. 2011;134:26.
Rallidis LS, Gialeraki A, Varounis C, Dagres N, Kotakos C, Travlou A, Lekakis J, Kremastinos DT. Lack of association of angiotensin-converting enzyme insertion/deletion polymorphism and myocardial infarction at very young ages. Biomarkers. 2009;14:401–5. https://doi.org/10.1080/13547500903039966.
Riad M, Adhikari P, Bhattarai S, Gupta A, Ali E, Ali M, Mostafa JA. Risk Assessment using the Association between renin-angiotensin genes polymorphisms and coronary artery disease. Cureus. 2021;13. https://doi.org/10.7759/cureus.14083.
Saini V, Bhatnagar M, Bhattacharjee J. Association of endothelial dysfunction with endothelin, nitric oxide and eNOS Glu298Asp gene polymorphism in coronary artery disease. Dis Markers. 2011;31:215–22. https://doi.org/10.3233/DMA-2011-0819.
Samani NJ, O’Toole L, Martin D, Rai H, Fletcher S, Lodwick D, Thompson JR, Morice AH, Channer K, Woods KL. Insertion/deletion polymorphism in the angiotensin-converting enzyme gene and risk of and prognosis after myocardial infarction. J Am Coll Cardiol. 1996;28:338–44. https://doi.org/10.1016/0735-1097(96)00139-8.
Sarecka-Hujar B. Association between 894G > T polymorphism within endothelial nitric oxide synthase (eNOS) gene and coronary artery disease in young adults. Eur J Med Technol. 2020;1:26.
Shankarishan P, Borah PK, Mahanta J. Renin-angiotensin system gene polymorphisms and hypertension. J Assoc Physicians India. 2018;66:79–84.
Vasilakou M, Votteas V, Kasparian C, Pantazopoulos N, Dedoussis G, Deltas C, Nastos P, Nikolakis D, Lamnissou K. Lack of association between endothelial nitric oxide synthase gene polymorphisms and risk of premature coronary artery disease in the greek population. Acta Cardiol. 2008;63:609–14. https://doi.org/10.2143/AC.63.5.2033229.
Ying Y, Luo Y, Peng H. EBF1 gene polymorphism and its interaction with smoking and drinking on the risk of coronary artery disease for chinese patients. Biosci Rep. 2018;38:BSR20180324. https://doi.org/10.1042/BSR20180324.
Zigra A-M, Rallidis LS, Anastasiou G, Merkouri E, Gialeraki A. eNOS gene variants and the risk of premature myocardial infarction. Dis Markers. 2013;34:431–6. https://doi.org/10.3233/DMA-130987.
Acknowledgements
Mashhad University of Medical Sciences supported this study.
None.
Funding
This study is funded by Mashhad University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Dr. H.P. designed the study, prepared the first draft of the manuscript, analyzed the echocardiographic views, and read and approved the final version of the manuscript. Dr. B.F. helped in doing the molecular analysis and reported them. Dr. O.Kh. collected the data, filled the checklists wrote the first manuscript and read and approved the final version of the manuscript. Dr. A.Gh. helped in data collection and management of the patients. Dr. N.M. helped in data analysis and read and approved the final version of the manuscript. Dr. F.K participated in analysis of the results, and read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical committee of the medical faculty of Mashhad University of Medical Sciences. The ethical code of the study was IR.MUMS.MEDICAL.REC.1398.723. All patients filled a written informed consent for participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Poorzand, H., Fazeli, B., Khajavi, O. et al. Association of polymorphisms of renin angiotensin system and endothelial nitric oxide synthase genes with premature cardiovascular disease in an Iranian population. BMC Cardiovasc Disord 23, 254 (2023). https://doi.org/10.1186/s12872-023-03276-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03276-x