Abstract
Background
In dry and semi-arid areas, salinity is the most serious hazard to agriculture, which can affect plant growth and development adversely. Over-accumulation of Na+ in plant organs can cause an osmotic effect and an imbalance in nutrient uptake. However, its harmful impact can vary depending on genotype, period of exposure to stress, plant development stage, and concentration and content of salt. To overcome the unfavorable effect of salinity, plants have developed two kinds of tolerance strategies based on either minimizing the entrance of salts by the roots or administering their concentration and diffusion.
Results
Having sufficient knowledge of Na+ accumulation mechanisms and an understanding of the function of genes involved in transport activity will present a new option to enhance the salinity tolerance of vegetables related to food security in arid regions. Considerable improvements in tolerance mechanisms can be employed for breeding vegetables with boosted yield performance under salt stress. A conventional breeding method demands exhaustive research work in crops, while new techniques of molecular breeding, such as cutting-edge molecular tools and CRISPR technology are now available in economically important vegetables and give a fair chance for the development of genetically modified organisms.
Conclusions
Therefore, this review highlights the molecular mechanisms of salinity tolerance, various molecular methods of breeding, and many sources of genetic variation for inducing tolerance to salinity stress.
Similar content being viewed by others
Background
Salt stress is one of the main environmental factors reducing yield in arid and semi-arid areas where the irrigation water is saline and the rainfall is low. In other words, the accumulation of chlorides of sodium, calcium, magnesium, sulfates, and carbonates in soil solution can be defined as a climatic threat. Salt-impacted soils are varied in their physical and chemical characteristics. Hence, they vary in salt content, salt type, pH of the soil, temperature, distribution of salts in the soil profile, and the content of clay. Under such inconsistent situations, only a few plant species that are not susceptible occur naturally [1,2,3].
Regarding the hazardous effect of salinity, high levels of salinity can cause modifications in the plants’ performance, including the morphological, physiological, biochemical, and molecular functions characters. It changes photosynthetic activities, such as stomatal conductance, carboxylation, the substomatal concentration of CO2 (Ci), and the maximum quantum yield of photosystem II (PSII). Ultimately, it destabilizes the structure and permeability of the cell membrane and, the structure and function of the proteins, causing cell death [4, 5].
Vegetable crops are one of the main sources of natural antioxidants, such as various pigments, phenolic acids, and flavonoids [2, 6]. These are not just crucial sources of natural antioxidants but may also donate to human health due to the existence of various minerals, dietary fibers, and vitamins. Nonetheless, vegetables show various reactions to salt stress. Some vegetables, such as Asparagus officinalis, are tolerant to salinity stress, but some crops, such as bean (Phaseolus vulgaris), carrot (Daucus carota), and onion (Allium cepa) are susceptible to salinity stress. Accordingly, knowledge of the genetic basis of salinity tolerance in vegetables is a prerequisite for plant breeders to develop superior genotypes by adopting the traditional breeding procedure. In view of the truth that there is no single mechanism by which tension can be ameliorated, this review shall focus on salt tension, especially in terms of genomics. An attempt has been made to discuss the vision of salinity tolerance, the adaptive strategy, characteristics conferring salinity tolerance, and their use in conventional/traditional breeding activities for vegetable improvement.
Impact of salinity stress on the plants
The response of plants to salinity is complicated, and salinity can damage plants via osmotic stress and ion toxicity, nutrient uptake balance, hormonal imbalance, and activities of antioxidants [6, 7]. The first response of physiological traits against salinity stress is the stomatal closing and a decline in leaf extension. In the lower leaves of plants, we can find the over-accumulation of Na+ and Cl− and its toxic impact on ions concentration. Consequently, the accumulation of salts and imbalance in nutrient uptake leads to a decrease in the leaf area index [8, 9].
Additionally, in sodic soils, hyperosmotic pressure causes the cells to lose more water than they take in. Water deficiencies cause a chain reaction of physical, signaling, gene expression, metabolic, and physiological pathways and activities, leading to a decrease in leaf area index, photosynthesis, and biomass accumulation and, eventually, reducing yield [2, 10,11,12]. In sodic soils, NaCl includes 50–80% of soluble salts, and this is the main cause of increasing the concentration of Na+ and Cl− ions and toxicity in plants [13, 14]. These ions impact the biochemical process of enzyme activities or the signaling pathway of photosynthetic activities significantly [3, 15]. Also, due to physical, chemical, and transport system interactions between Na+ and K+, the Na+ in the solution of saline soils competes with K+ for absorption and can lead to the deficiency of K+ in plant tissues [7, 16, 17]. The conducted deficiency in K+ concentration in tissues especially in leaves minimizes growth since it performs an essential role in photosynthetic parameters, such as stomatal conductance and function, and regulation of cell turgor [18, 19].
Photosynthesis in vegetables is a complex plant-physiological and biochemical mechanism that is influenced by salt tension extremely [3, 20]. Stomata closure can be conducted in a decrease in carboxylation and photosynthesis efficiency in salt-sensitive vegetables. Salinity stress also impacts photosynthesis by chlorophyll degradation through overexpression of pheophorbide a oxygenase (CaPAO) [3]. Furthermore, salinity can cause disorders in PSII function and oxygen-evolving complex, and cyclic electron transport [4, 5]. In parallel, it was documented that salinity stress reduced transpiration rates and stomatal conductance, and caused to degradation of pigments and light-harvesting complexes, and as result, it led to a reduction in quantum yield of photosynthetic, and dissipation of energy by non-photochemical strategies in lettuce, onion, tomato, and sweet potato [20,21,22,23].
Regardless of the primary impacts of salt stress, the secondary effect of salinity on plant cells is accumulation of reactive oxygen species (ROS), which are toxic and cause DNA methylation and affect gene expression [24, 25]. Moreover, ROS can cause lipid peroxidation, which improves the membrane’s fluidity and permeability [26]. However, plants have developed extensive internal tolerance systems, such as enzymatic and non-enzymatic antioxidants to overcome the results of ROS accumulation [3, 27]. Salt tolerance of some vegetables is shown in Table 1.
Mechanism of salt tolerance
Some vegetable crops, such as Beta vulgaris have the natural capability of tolerating the adverse impacts of high NaCl in the root zone or on the leaves without adversely affecting their yield by having the highest leaf relative water content (LRWC), leaf area index, biomass accumulation, and photosynthesis. Some crops are mitigated the adverse impact of NaCl via minimizing the absorption of Na+ and Cl− ions to the shoots, and osmotic adjustment. In the root, salts can penetrate root membranes through active or passive transport mechanisms. Ion path has been revealed to be selectively adjusted through plasmalemma, tonoplast, or specialized cells along with xylem parenchyma. Individual ion-specific ATP’s that adjust this ion activity can be impacted by the concentration of other ions. Consequently, a higher variation can be found among the genotypes in their capability in adjusting the concentration of ions and the production and accumulation of the osmotic substances in reaction to sodic conditions. Nonetheless, some organic acids and amino acids, such as malate, oxalate, glycine betaine, and proline have been identified as potential osmotic mechanisms in higher plants. Carbohydrates can also be selectively aggregated in response to drought and salinity stress [6, 7, and 28].
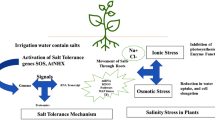
Melatonin, dopamine, and eATP are Neurotransmitters, which can adjust K+ and Ca2+ permeable ion channels and also plays a main function in ionic homeostasis under NaCl stress in plants. Among neurotransmitters, melatonin is isolated from tryptophan and can induce tolerance to salinity in plants by activating antioxidant systems and adjustment of the expression of K+ uptake (AKT), Na+/H+ exchanger (NHX), and salt overly sensitive (SOS) (Fig. 1), which are known as ion channels genes [29, 30]. In Brassica, the treatment of melatonin by activating NHX1, and SOS2 improved vacuolar Na+ sequestration and Na+ exclusion for reducing Na+ toxicity under salt stress [29].
The suggested model depicts melatonin’s signaling function in conferring salt stress resistance in plants. By being exposed to salinity, Na+ produces ROS, which activates antioxidant and non-antioxidant enzymes as well as melatonin. Melatonin activates antioxidant and non-antioxidant enzymes, as well as, increases SOS and NHX activities. SOS transports excess Na+ to the extracellular space using the plasma membrane proton gradient. NHX on the vacuole tonoplast contributes to Na+ homeostasis (created using Inkscape software ver.1.2.2). Na+/H+ exchanger1 (NHX1); Salt overly sensitive1 (SOS1); Salt overly sensitive2 (SOS2); Ascorbate peroxidase (APX); Superoxide dismutase (SOD); Catalase (CAT); Peroxidase (POD); Glutathione peroxidase (GPX); Glutathione reductase (GR); Glutathione transferase (GST)
Dopamine, 3, 4-dihydroxyphenethylamineis, is delivered from tyrosine amino acid in plants and is a non-protein amino acid that belongs to the families of catecholamine and phenethylamine. Under salinity stress, dopamine can also enable vegetables, such as Brassica species to overcome osmotic stress through sensing osmotic pressure alterations by adjusting or triggering mechano-sensitive cation channels. Notably, the mentioned channels may be practicable candidates for discerning toxicity or deficiency of ions in plant organs, as they could alter mechanical modifications influenced by turgor pressure into electrical signals [9, 29, and 31].
Besides the above-mentioned mechanism, under adverse conditions, plants evolved antioxidant defense systems that used ROS-scavenging processes. Antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) are employed to relieve ROS to maintain redox homeostasis under salinity stress [9]. Furthermore, salinity-induced osmotic stress stimulates the synthesis of the phytohormone abscisic acid (ABA), which controls plant physiological activities in response to diverse abiotic stress. A higher level of salinity can stimulate ABA synthesis in plants by triggering the expression of biosynthesis pathway genes. ABA not only transmits salinity signals to cells, but it also protects them from salt stress by altering the expression of stress-responsive genes [29].
Plants, nonetheless, respond to and adjust to different environmental factors via morphological, anatomical, and biological acclimation at the cellular and plant levels. These morphological, anatomical, and physiological adaptations assist the plant in coping with environmental stress. As is widely known, the anatomical flexibility of leaf and root in plants is vital in conferring tolerance under numerous abiotic stresses, such as salinity and drought stresses [30]. An enhancement in both leaf cuticle and leaf epidermal thickness is one of the anatomical traits in salt-tolerant genotypes in general. Also, physiological traits, such as low photosynthesis, closed stomata, high leaf temperature, and a decrease in leaf turgor and chlorophyll content are well-known physiological mechanisms for tolerating salinity stress in vegetables. Furthermore, intact bulliform cells, bulliform cell area, chloroplast content, and chloroplast ultrastructure, particularly the length, width, and width/length of chloroplasts, can be efficient indicators for salt-unsusceptible genotypes in monocotyledon vegetables, such as sweet corn, asparagus, yams, leeks, and onions [7].
Genetic mechanism of salinity tolerance
The next generation of sequencing, the technology of high-throughput sequencing technology, offered the way for the whole genome sequencing of vegetables. Because of the high capability of sequencing and decrease in sequencing costs, it has led to the discovery of multiple molecular strategies in abiotic tension and therewith contributed to the elaboration of functional genomics of tolerance to abiotic stress, such as salinity in vegetables [32]. Nonetheless, because of the heterozygous nature of most vegetables, and also polyploidy, diversity, and complexity, sequencing of whole genome has been completed in a little number of crops. Also, most of the quantitative potential characters in the vegetables are polygenic traits controlled by numerous alleles/genes located across the entire genome [33]. Due to the forenamed limitations, we can find a shortage of genome information and varied molecular strategies for tolerance to abiotic stress in vegetables. Hence, next-generation sequencing must be done through the whole genome of important vegetables. Recently, advanced bioinformatics methods and genotyping-by-sequencing (GBS) data have offered novel genetic resources of tolerance to environmental stress in vegetables [34]. Because of this, we can find the development of various molecular markers, such as SNP (single nucleotide polymorphism), SSR (simple sequence repeats), and RNA-based molecular markers. Through them, the election cycle has been shortened, resulting in the development of genetic gain, mapping for quantitative trait locus (QTLs) related to tolerance to abiotic stress, and identification of abiotic characters. Among vegetables, reference genomes for various Solanaceae and Cucurbitaceae genotypes have been sequenced as well as assembled, such as tomatoes, peppers, cucumbers, watermelons, and melons. In tomatoes, various association mapping studies, such as genome-wide association studies (GWAS), have been performed for fruit traits, volatiles, and abiotic/biotic tolerance [35, 36]. Genome sequencing also revealed more than 22,000 candidate regions across the whole genome that could act to develop different SSR and SNP markers in Dioscorea rotundata (white guinea yam). Under salinity stress conditions, silico gene expression analysis of the transcriptomic profile identified 6,233 SSR markers in Ipomoea imperati [37]. These markers are valuable for comparative genomics research and indirect selection in the development of salt tolerance in sweet potatoes [38,39,40]. Nonetheless, Liu et al. [41] assessed the mechanisms of miRNA-mediated gene adjusting in grafted watermelon under normal conditions. They also identified the expression of 47 miRNAs in watermelon grafted on Shintozwa’ (Cucurbita maxima×Cucurbita moschata) and 20 miRNAs in the ones grafted on Lagenaria sicerariawere significantly, relative to self-grafted watermelon. Nonetheless, Ram et al. [42] identified 31 putative TLP gene families in watermelon and named them ClaTLP1 to ClaTLP31, and this gene family is distributed randomly on some chromosomes rather than covering of whole genome of watermelon under salinity and drought stresses. Chromosome 10 represented the maximum number of ClaTLP genes. Out of 31 gene families, five ClaTLP genes (16%) showed tandem duplication whereas only 4 genes (12.90%) resided in segmentally duplicated blocks on watermelon chromosomes.
However, there are few reports on vegetables that use genomic resources to develop lines with salt-tolerant traits. Accordingly, future studies must focus on QTL mapping to discover characters and genes for salt tolerance. GWAS, and sequencing can also accelerate breeding by shortening the breeding cycle, as well as discover salt-tolerant functional genotypes by re-sequencing. As a result, manipulating these sensitive genes and cross-transfer between genotypes will be critical in instilling tolerance to salinity and comprehending the underlying molecular strategies.
Nonetheless, polyploidy is an effective strategy to improve crop abiotic stress tolerance by affecting morphological and anatomic traits, as well as physiology, biochemistry, and gene expression. Many plants that are currently grown and exploited for world food are also polyploidy. In the case of polyploidy induction, it is a procedure that gives the plant breeder the opportunity to modify a plant by altering the number of chromosomes and, consequently, the proportion of allelic genes that contribute to the appearance of characters. Also, polyploidy plants can tolerate abiotic stress better than diploid genotypes. For instance, polyploidization of Lettuce sativa var. crespa promoted chromosomal duplication in originally diploid lettuce seedlings (2n = 18), and it was discovered that polyploidy-induced seedlings had a higher dry weight and leaf area per seedling under saline stress [43].
Transcriptomics
Advancements in the capability of next-generation sequencing techniques and a decrease in sequencing costs have precipitated transcriptomic studies on abiotic and biotic stress in crops. The primary function of this technique is that it decodes various transcripts or genes which have a significant function in imparting tolerance to salinity in crops [44]. Exposing plants to adverse conditions, such as salinity is associated with the upregulation or downregulation of various genes with many functions, which can cause a resultant modification in the process of plant growth and development and can facilitate tolerance to stress [45, 46]. Table 2 shows potential candidate genes responsible for tolerance to salt stress in various vegetable crops.
It was documented that many genes encode regulative proteins, such as TFs, which adjust the pathways of salt sensing and transduction of signals and the expression of a range of salinity stress-reactive genes, while some of these up-regulated or down-regulated genes code proteins that act as the main function in stress-related growth or metabolic changes [1, 47].
In a previous study, in the Cucumis melo L. genome, 82 genes of NAC (CmNAC) were discovered [48]. In that study, the researchers identified that CmNAC genes are implicated significantly in the response to salinity stress. Also, the expression analysis demonstrated that under salinity stress, about 12 genes of CmNAC genes were overexpressed in the roots of melon and they induced tolerance to salinity in melon. In a study, the WRKY transcription factor of sweet potato was cloned in Arabidopsis and the transgenic seedlings of Arabidopsis showed tolerance to salinity and drought stress [49]. In pepper, Zhang et al. [46] demonstrated that CaNAC035 is affected by abiotic stress, such as salinity. To comprehend the function of CaNAC035 under abiotic stress, they applied virus-induced gene silencing in pepper to observe the knockdown and overexpression of CaNAC035 in pepper and Arabidopsis, respectively. Their results revealed that seedlings of pepper in which CaNAC035 was silenced, exhibited more sensitivity than the control groups under salinity stress. Also, lower chlorophyll content was found in CaNAC035-silenced seedlings, but a higher symptom of tolerance to salinity stress was found in CaNAC035-overexpressed Arabidopsis. They also identified 18 proteins that potentially interact with CaNAC035 and can partake in functions, such as response to salinity stress, tolerance, and photosynthetic activities. Their results indicated that CaNAC035 is a positive controller of tolerance to abiotic tension in capsicum, which performs via various signaling pathways.
Apart from NAC transcriptome factors, the responsible genes for cellular homeostasis, phytohormone modulation, and osmotic adjustment perform the main functions in deriving tolerance to environmental stress [48]. In the transgenic seedlings of Solanum lycopersicum L. cv. MicroTom overexpression of LeNHX2 and SlSOS2 genes led to overexpression of K+, Na+/H+ antiporter, and the regulatory kinase SlSOS2 under salinity stress. All transgenic plants demonstrate better tolerance to salinity than wild-type and the seedlings overexpressing both SlSOS2 and LeNHX2 outperformed those overexpressing only one of these genes in terms of plant yield. Furthermore, higher K+ content was observed in the fruits of all transgenic plants relative to the wild type, and also higher Na+ content was observed in the fruits of plants that expressed SlSOS2 under excessive salinity stress. The transgenic seedlings overexpressing LeNHX2 had higher K+ levels in their leaves, stems, and roots than wild-type and single transgenic seedlings overexpressing SlSOS2. The plants with SlSOS2 overexpressing grown under salinity had a higher content of Na+ in stems and leaves compared to the wild-type and LeNHX2 single transgenic plants. A higher LRWC and water use efficiency were observed in all transgenic lines than in wild-type (control) under salt conditions. The results of this study suggest that the combined overexpression of LeNHX2 and SlSOS2 enhances growth and LRWC under salinity stress, and also impacts K+ and Na+ homeostasis, and improves the yield of tomato plants [50].
Genes of the calcium signaling pathway and the pathway of mitogen-activated protein kinase (MAPKs) play a crucial function in tolerance to salinity by triggering salinity-responsive genes. For potatoes, the genes of the Ca+ signaling pathway (CBLs) and MAPKs signaling pathway genes (MAPKK9, MAPK4, and MAPK6), were overexpressed in salinity-tolerant cultivars, illustrating their function under salinity [51]. Similarly, RNA sequencing analysis revealed that WRKY22 and other hormone signal transduction pathways perform a significant function in providing tolerance to salinity stress in the tolerant genotype of wild-type sweet potato (Ipomoea pescaprae) [52].
Additionally, transcriptome analysis demonstrated that the genes of the SOS pathway (SOS2, NHX protein genes, V-ATPase genes, and PM-ATPase genes) play an important role in the growth and performance of potato under salinity stress. Also, it was shown that in sweet potato, jasmonic acid plays a crucial performance under salinity conditions. Under 200 mM NaCl, transcriptome analysis indicated that jasmonic acid biosynthesis and signaling genes, including IbLOX, IbAOS, IbAOC, IbOPR3, IbCOI1, and IbPDF1.2 were overexpressed in the salinity-tolerant line of sweet potato [46]. Also, it was demonstrated that genes of ABA signaling pathways (PYL, SnRK2, and MPK kinase) were up-regulated in tomato demonstrating their role in inducing tolerance to salinity [53].
Heat shock proteins (HSPs) accumulate under abiotic/biotic stress in plants. The autophagy of non-useful proteins is done by HSPs under salinity and drought stresses. Watermelon is susceptible to environmental stresses and, in a previous study, the role of HSP20 proteins was explored in watermelon. In this respect, the qRT-PCR analysis and GUS staining demonstrated that exogenous treatment of ABA, and salinity stress markedly decreased the expression of ClHSP22.8 (a member of the HSP20 family). However, in transgenic lines of Arabidopsis, the over-expression of ClHSP22.8 caused hypersensitivity to ABA, and tolerance reduction to salinity stress. The results showed that overexpression of ClHSP22.8 has a negative role in the plant defense mechanism system under salinity stress [54].
The comparison and analysis of transcriptome data in salt-tolerant and salt-susceptible genotypes in vegetables can make very evident the function of the signaling pathway, the integrity of the membrane, transcription factors, and the synthesis of phytohormones. Thus, these are potential targets for breeding salinity-tolerant genotypes in vegetable crops.
Breeding for salinity tolerance: conventional and modern approaches
To develop salinity tolerance genotypes, smart breeding is important in vegetables [55, 56]. Sequencing of whole genome plays a significant role in the genomic study, proteomics, transcriptomics, metabolomics, and QTLs studies for increasing tolerance to salinity in vegetables. Collecting germplasm, including wild genotypes has supplied significant genetic diversity for tolerance to salt, and the prosperity of breeding techniques relies mainly on the germplasm selections. Screening of plant germplasms to identify salinity-tolerant genotypes has accelerated the production of salinity-resistant cultivars through gene transfer [34, 57].
In conventional breeding methods, the breeders have applied hybridization, recurrent selection, pedigree selection, backcrossing, and induced mutation approaches for developing salinity-tolerant genotypes in vegetables. However, new approaches to breeding, such as CRISPR/Cas9, CRISPR/Cpf1, prime and base editing, dCas9 epigenetic changes, and various further transgene-free genome editing approaches exist to supply the lacuna of selection cycles and the restricted genetic diversity [58].
Additionally, polyploidy can play a vital role in species invasion success through a combined application of (1) ‘pre-adaptation’, whereas polyploid lineages are primed to conditions in the new range and, consequently, have higher rates of survival and fitness in the earliest establishment stage; and (2) the possibility for successive acclimation due to a larger genetic variation that may assist the ‘evolving of invasiveness’. Polyploidization, on the other hand, may play a significant role in (3) restoring sexual reproduction after hybridization or, conversely, (4) asexual reproduction in the absence of appropriate individuals [59]. Polyploidy is an important evolutionary factor in both wild and domesticated plants. Polyploid organisms frequently have more vigor and, in certain situations, exceed their diploid relative in a variety of ways. Polyploids’ exceptional superiority has been the goal of many plant breeders over the last century, who have induced polyploidy and/or utilized natural polyploids in a variety of methods to produce ever-enhanced plant cultivars [60]. The expansion of plant organs (“gigas” impact), buffering of harmful mutations, increased heterozygosity, and heterosis are some of the most significant implications of polyploidy for plant breeding (hybrid vigor). In terms of this technique, the genotypes with high performance and growth, and increased resistance to both biotic and abiotic stressors have been developed. When crossing between two species is not possible due to ploidy level discrepancies, polyploids can be employed as a bridge for gene transfer between them. Furthermore, polyploidy frequently causes lower fertility due to meiotic mistakes, permitting the development of seedless cultivars. In contrast, the genome doubling in a newly generated sterile hybrid offers the recovery of its fertility [60].
Nonetheless, the MAS is known as a molecular marker related to the agronomics character of interest controlled by single genes or QTL. This is the maximum efficient and effective way to create new salinity-tolerant vegetable genotypes. With the application of MAS, the breeding process can be shortened by screening crops with the target genes at earlier growth stages, saving time and material, and agronomic inputs, such as fertilizers, herbicides, and irrigation, and abiotic factors have no effect.
For salinity tolerance to introgress/transfer-related genes, marker-assisted backcross (MABC) breeding is a more accurate and efficient technique. It allows for plant selection throughout each breeding season to ensure the presence of introgressed target genes, as well as the restoration of the parental genome (RPG) and decreased linkage drag [61,62,63]. To specify different QTLs and the introgression of Quantitative characters in vegetables by MAS, several molecular markers are accessible. Various DNA markers, including SNP, restriction fragment length polymorphisms (RFLP), amplified fragment length polymorphisms (RFLP), sequence-tagged sites (STS), and SSR, can be utilized for genetic analysis in molecular identification and characterization and MAS [64, 65]. GWAS was used to discover the Or gene on chromosome 3, as well as the link between terpenoid biosynthesis pathway genes and volatile terpenoid odor ingredients in carrot [66, 67]. Whether candidate genes were specified using GWAS in various germplasm collections or bi-parental populations, carrots were screened for salt tolerance using plant introductions (wild germplasms and commercialized hybrids) [68], and five developed carrot genotypes (PI 509,433, PI 652,374, PI 652,402, PI 652,403, and PI 652,405) were shown to have greater tolerance to saline.
Kompetitive Allele-Specific PCR (KASP™) is a novel and fast genotyping method of SNP genotyping which needs only a few markers of SNP to genotype different samples. In a previous study, the genetic variety of 73 onion genotypes, including wild type, commercial, and native variants, from around the world was investigated using KASP genotyping technique. With the evaluation of The SNP dataset, 375 polymorphic loci with a very low percentage of non-calling sites (0.03%) were retrieved [68]. 89% of the genotypes of onions amplified all polymorphic loci and were contemplated for structural analysis of the population. The ΔK approach allowed for the discovery of gene pools and proposed four populations, each representing the geographical source of the genotypes. Based on their statistical analysis, their SNP set was successful in introducing potential genotypes for use in successful breeding projects and revealing population-specific alleles. It is worth noting that seventy-four loci were linked with phenotypical characteristics, such as bulbing photoperiod, bulb shape, or bulb color, and tree loci were specified as potential selections target for onion improvements [69].
A wild type of tomato (Solanum pimpinellifolium) presents a breeding potential source for desired features, including abiotic and biotic stress tolerance. The genome sequencing of S. pimpinellifolium ‘LA0480’ was performed by Razali et al. [70]. Furthermore, they presented phenotypic results from a field study that showed S. pimpinellifolium had a high tolerance to salinity relative to cultivated tomato. The results showed that annotated gene numbers (25,970) and the ‘LA0480’ genome assembly size (811 Mb) were within the range identified for other sequenced tomato genotypes. Additionally, they advanced and used the Dragon Eukaryotic Analysis (DEAP) platform to functionally annotate genes encoding the ‘LA0480’ protein. Their findings revealed that genes involved in biotic and abiotic stress responses were overrepresented. For understanding the genetic basis for such variations in S. pimpinellifolium and S. lycopersicum, they explored fifteen genes that have formerly been demonstrated to promote tolerance to salinity. They discovered that S. pimpinellifolium had higher homologs of the inositol-3-phosphate synthase and phosphatase genes, which are both key enzymes in the biosynthesis of inositol and its byproducts. Nonetheless, their findings suggested that changes in the inositol phosphate chain may lead to greater salt tolerance [70].
CRISPR is a gene-editing technology commonly utilized in numerous species. The desired plant phenotype has been achieved in numerous crops by specific genetic tinkering utilizing CRISPR-CAS systems [71, 72]. The CRISPR/Cas9 technique consists of a single guide RNA (sgRNA) molecule with a specific target sequence that is homologous to a target gene and the DNA endonuclease Cas9. The utilization of the CRISPR/Cas9 system necessitates the construction of a reliable and ubiquitous technique for transferring CRISPR/Cas9 reagents in cells [73]. Effective delivery techniques involve protoplast transfection, Agrobacterium (Agrobacterium tumefaciens)-mediated transformation, and particle bombardment [74,75,76,77]. The most extensively utilized delivery strategy for producing crop varieties and achieving successful targeted mutagenesis is Agrobacterium-mediated transformation [77]. CRISPR/Cas9 success has been documented in Arabidopsis and several significant vegetables, such as a tomato (Solanum lycopersicum), broccoli (Brassica oleracea var. italic), melon (Cucumis melo), lettuce, and carrot [78,79,80,81,82].
The genes of auxin response factors (ARF) perform a key function in plant responses to environmental stresses. Both OsARF11 and OsARF15 are expressed differentially in plants under salt stress conditions [83]. A CRISPR/Cas9-induced SlARF4 mutant showed tolerance to salinity compared to the control [78].
Conclusion
As reported by different studies, vegetables have varying degrees of tolerance and susceptibility to salt. Today, salt is a major issue for food security since it reduces the quality and quantity of vegetables during their growth and development stages. Salinity causes osmotic, water, and nutrient imbalances, as well as cellular interference and morphological interference, which eventually impact plant physiochemical processes. Tolerance to salt is the outcome of a complex system, including phytohormones, antioxidants, phytoprotectants, and the co-regulation of numerous genes and their relationships. While it is a complex mechanism, numerous genes related to tolerance in some genotypes of vegetables were identified, and salinity-tolerant genotypes can be generated by transferring them via breeding methods into susceptible genotypes. However, a conventional breeding method demands exhaustive research work on vegetables and depends on mixing characteristics from different populations within a species and then selecting from the plant’s natural complement of genetic elements. Therefore, new techniques of molecular breeding, such as cutting-edge molecular tools, and CRISPR technology are also now available in economically important vegetables and give fair chance for the development of genetically modified organisms (GMOs).
Data availability
Not applicable.
References
Gupta B, Huang B. (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. International journal of genomics 2014: 701596
Machado RMA, Serralheiro RP. Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae. 2017;3(2):30.
Shams M, Yildirim E. Variations in response of CaPAO and CaATG8c genes, hormone, photosynthesis and antioxidative system in pepper genotypes under salinity stress. Sci Hort. 2021;282:110041.
Ansari HH, Siddiqui A, Wajid D, Tabassum S, Umar M, Siddiqui ZS. Profiling of energy compartmentalization in photosystem II (PSII), light harvesting complexes and specific energy fluxes of primed maize cultivar (P1429) under salt stress environment. Plant Physiol Biochem. 2022;170:296–306.
Dhokne K, Pandey J, Yadav RM, Ramachandran P, Rath JR, Subramanyam R. Change in the photochemical and structural organization of thylakoids from pea (Pisum sativum) under salt stress. Plant Physiol Biochem. 2022;177:46–60. https://doi.org/10.1016/j.plaphy.2022.02.004
Chourasia KN, More SJ, Kumar A, Kumar D, Singh B, Bhardwaj V, Kumar A, Das SK, Singh RK, Zinta G, Tiwari RK, Lal MK. Salinity responses and tolerance mechanisms in underground vegetable crops: an integrative review. Planta. 2022;255(3):68. https://doi.org/10.1007/s00425-022-03845-y
Fang S, Hou X, Liang X. Response mechanisms of plants under saline-alkali stress. Front Plant Sci. 2021;12:1049.
Shams M, Yildirim E, Ekinci M, Turan M, Dursun A, Parlakova F, Kul R. Exogenously applied glycine betaine regulates some chemical characteristics and antioxidative defence systems in lettuce under salt stress. Hort Environ Biotech. 2016b;57(3):225–31. https://doi.org/10.1007/s13580-016-0021-0
Shah AN, Tanveer M, Abbas A, Fahad S, Baloch MS, Ahmad MI, Saud S, Song Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: a research perspective. Plant Physiol Biochem. 2021;158:53–64. https://doi.org/10.1016/j.plaphy.2020.11.044
Giordano M, Petropoulos SA, Cirillo C, Rouphael Y. Biochemical, physiological, and molecular aspects of ornamental plants adaptation to deficit irrigation. Horticulturae. 2021;7(5):107.
Wei Z, Fang L, Li X, Liu J, Liu F. Endogenous ABA level modulates the effects of CO2 elevation and soil water deficit on growth, water and nitrogen use efficiencies in barley and tomato plants. Agric Water Manage. 2021;249:106808.
Shams M, Yildirim E, Ekinci M, Turan M, Dursun A, Parlakova F, Kul R. Exogenously applied glycine betaine regulates some chemical characteristics and antioxidative defence systems in lettuce under salt stress. Hortic Environ Biotechnol. 2016a;57(3):225–31. https://doi.org/10.1007/s13580-016-0021-0
Dong X, Sun L, Guo J, Liu L, Han G, Wang B. Exogenous boron alleviates growth inhibition by NaCl stress by reducing cl – uptake in sugar beet (Beta vulgaris). Plant Soil. 2021;464(1):423–39.
Azizi F, Farsaraei S, Moghaddam M. Application of exogenous ascorbic acid modifies growth and pigment content of Calendula officinalis L. flower heads of plants exposed to NaCl stress. J Soil Sci Plant Nutr. 2021;21(4):2803–14.
Dadasoglu E, Ekinci M, Kul R, Shams M, Turan M, Yildirim E. Nitric oxide enhances salt tolerance through regulating antioxidant enzyme activity and nutrient uptake in pea. Legume Research-An International Journal. 2021;44(1):41–5.
Rubio F, Nieves-Cordones M, Horie T, Shabala S. Doing ‘business as usual’comes with a cost: evaluating energy cost of maintaining plant intracellular K + homeostasis under saline conditions. New Phytol. 2020;225(3):1097–104.
Shams M, Yildirim E, Arslan E, Agar G. Salinity induced alteration in DNA methylation pattern, enzyme activity, nutrient uptake and H2O2 content in pepper (Capsicum annuum L.) cultivars. Acta Physiol Plant. 2020;42(4):59. https://doi.org/10.1007/s11738-020-03053-9
Khalil R, Elsayed N, Khan TA, Yusuf M. (2022) Potassium: A Potent Modulator of Plant Responses Under Changing Environment. Role of Potassium in Abiotic Stress. Springer, pp. 221–247
Nieves-Cordones M, Azeem F, Long Y, Boeglin M, Duby G, Mouline K, Hosy E, Vavasseur A, Chérel I, Simonneau T. Non-autonomous stomatal control by pavement cell turgor via the K + channel subunit AtKC1. Plant Cell. 2022;34(5):2019–37.
Yang X, Han Y, Hao J, Qin X, Liu C, Fan S. Exogenous spermidine enhances the photosynthesis and ultrastructure of lettuce seedlings under high-temperature stress. Sci Hort. 2022;291:110570.
Breś W, Kleiber T, Markiewicz B, Mieloszyk E, Mieloch M. The effect of NaCl stress on the response of lettuce (Lactuca sativa L). Agronomy. 2022;12(2):244.
Semida WM, El-Mageed A, Taia A, Abdelkhalik A, Hemida KA, Abdurrahman HA, Howladar SM, Leilah AA, Rady MO. Selenium modulates antioxidant activity, osmoprotectants, and photosynthetic efficiency of onion under saline soil conditions. Agronomy. 2021;11(5):855.
Faizan M, Bhat JA, Chen C, Alyemeni MN, Wijaya L, Ahmad P, Yu F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem. 2021;161:122–30.
Shams M, Ekinci M, Ors S, Turan M, Agar G, Kul R, Yildirim E. Nitric oxide mitigates salt stress effects of pepper seedlings by altering nutrient uptake, enzyme activity and osmolyte accumulation. Physiol Mol Biology Plants. 2019;25(5):1149–61. https://doi.org/10.1007/s12298-019-00692-2
Ranjan A, Sinha R, Sharma TR, Pattanayak A, Singh AK. Alleviating aluminum toxicity in plants: implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol Plant. 2021;173(4):1765–84.
Sahin U, Ekinci M, Ors S, Turan M, Yildiz S, Yildirim E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci Hort. 2018;240:196–204.
Moradbeygi H, Jamei R, Heidari R, Darvishzadeh R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci Hort. 2020;272:109537.
Liang W, Ma X, Wan P, Liu L. Plant salt-tolerance mechanism: a review. Biochem Biophys Res Commun. 2018;495(1):286–91. https://doi.org/10.1016/j.bbrc.2017.11.043
Kaya C, Higgs D, Ashraf M, Alyemeni MN, Ahmad P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol Plant. 2020;168(2):256–77.
Smith LP, Bitler BG, Richer JK, Christenson JL. Tryptophan catabolism in epithelial ovarian carcinoma. Trends in cancer research. 2019;14:1.
Kulma A, Szopa J. Catecholamines are active compounds in plants. Plant Sci. 2007;172(3):433–40.
Campos MD, Félix MdR, Patanita M, Materatski P, Varanda C. High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding. Hortic Res. 2021;8:171.
Aslam A, Zhao S, Lu X, He N, Zhu H, Malik AU, Azam M, Liu W. High-throughput LC-ESI-MS/MS Metabolomics Approach reveals regulation of Metabolites related to diverse functions in mature fruit of grafted Watermelon. Biomolecules. 2021;11(5):628.
Razzaq A, Kaur P, Akhter N, Wani SH, Saleem F. Next-generation breeding strategies for climate-ready crops. Front Plant Sci. 2021;12. https://doi.org/10.3389/fpls.2021.620420
Ranc N, Muños S, Xu J, Le Paslier M-C, Chauveau A, Bounon R, Rolland S, Bouchet J-P, Brunel D, Causse M. (2012) Genome-Wide Association Mapping in Tomato (Solanum lycopersicum) Is Possible Using Genome Admixture of Solanum lycopersicum var. cerasiforme. G3 Genes|Genomes|Genetics 2 (8):853–864. https://doi.org/10.1534/g3.112.002667
Sim S-C, Durstewitz G, Plieske J, Wieseke R, Ganal MW, Van Deynze A, Hamilton JP, Buell CR, Causse M, Wijeratne S, Francis DM. Development of a large SNP genotyping array and generation of high-density genetic maps in Tomato. PLoS ONE. 2012;7(7):e40563. https://doi.org/10.1371/journal.pone.0040563
Solis J, Baisakh N, Brandt SR, Villordon A, La Bonte D. Transcriptome profiling of beach morning glory (Ipomoea imperati) under salinity and its comparative analysis with sweetpotato. PLoS ONE. 2016;11(2):e0147398.
Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun J-H, Bancroft I, Cheng F. The genome of the mesopolyploid crop species Brassica rapa. Nat Genet. 2011;43(10):1035–9.
Yu J, Zhao M, Wang X, Tong C, Huang S, Tehrim S, Liu Y, Hua W, Liu S. Bolbase: a comprehensive genomics database for Brassica oleracea. BMC Genomics. 2013;14(1):1–7.
Sharma R, Mishra M, Gupta B, Parsania C, Singla-Pareek SL, Pareek A. De novo assembly and characterization of stress transcriptome in a salinity-tolerant variety CS52 of Brassica juncea. PLoS ONE. 2015;10(5):e0126783.
Liu N, Yang J, Guo S, Xu Y, Zhang M. Genome-wide identification and comparative analysis of conserved and novel microRNAs in grafted watermelon by high-throughput sequencing. PLoS ONE. 2013;8(2):e57359.
Ram C, Danish S, Kesawat MS, Panwar BS, Verma M, Arya L, Yadav S, Sharma V. Genome-wide comprehensive characterization and expression analysis of TLP gene family revealed its responses to hormonal and abiotic stresses in watermelon (Citrullus lanatus). Gene. 2022;844:146818. https://doi.org/10.1016/j.gene.2022.146818
Alexis AC. Determination of saline tolerance under in vitro conditions of lettuce (Lactuca sativa L. var. crespa) induced polyploidy. Agricultural Res Technol. 2019;23(3):277–9.
Kreps JA, Wu Y, Chang H-S, Zhu T, Wang X, Harper JF. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130(4):2129–41.
Yagci S, Yildirim E, Yildirim N, Shams M, Agar G. Nitric oxide alleviates the effects of copper-induced DNA methylation, genomic instability, LTR retrotransposon polymorphism and enzyme activity in lettuce. Plant Physiol Rep. 2019;24(3):289–95.
Zhang H, Ma F, Wang X, Liu S, Saeed UH, Hou X, Zhang Y, Luo D, Meng Y, Zhang W. Molecular and functional characterization of CaNAC035, an NAC transcription factor from pepper (Capsicum annuum L). Front Plant Sci. 2020;11:14.
Mann A, Kumar N, Lata C, Kumar A, Kumar A, Meena B. Functional annotation of differentially expressed genes under salt stress in Dichanthium annulatum. Plant Physiol Rep. 2019;24(1):104–11.
Wei S, Gao L, Zhang Y, Zhang F, Yang X, Huang D. Genome-wide investigation of the NAC transcription factor family in melon (Cucumis melo L.) and their expression analysis under salt stress. Plant Cell Rep. 2016;35(9):1827–39. https://doi.org/10.1007/s00299-016-1997-8
Zhu H, Zhou Y, Zhai H, He S, Zhao N, Liu Q. A novel sweetpotato WRKY transcription factor, IbWRKY2, positively regulates drought and salt tolerance in transgenic Arabidopsis. Biomolecules. 2020b;10(4):506.
Baghour M, Gálvez FJ, Sánchez ME, Aranda MN, Venema K, Rodríguez-Rosales MP. Overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production in double transgenic tomato plants. Plant Physiol Biochem. 2019;135:77–86. https://doi.org/10.1016/j.plaphy.2018.11.028
Zaynab M, Hussain A, Sharif Y, Fatima M, Sajid M, Rehman N, Yang X, Khan KA, Ghramh HA, Li S. Mitogen-activated protein kinase expression profiling revealed its role in regulating stress responses in potato (Solanum tuberosum). Plants. 2021;10(7):1371.
Jiang J, Ma S, Ye N, Jiang M, Cao J, Zhang J. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. 2017;59(2):86–101.
Wang W-R, Liang J-H, Wang G-F, Sun M-X, Peng F-T, Xiao Y-S. Overexpression of PpSnRK1α in tomato enhanced salt tolerance by regulating ABA signaling pathway and reactive oxygen metabolism. BMC Plant Biol. 2020;20(1):1–15.
He Y, Yao Y, Li L, Li Y, Gao J, Fan M. A heat-shock 20 protein isolated from watermelon (ClHSP22.8) negatively regulates the response of Arabidopsis to salt stress via multiple signaling pathways. PeerJ. 2021;9:e10524. https://doi.org/10.7717/peerj.10524
Pinar H, Bulbul C, Simsek D, Shams M, Mutlu N, Ercisli S. Development of molecular markers lınked to QTL/genes controllıng Zn effıcıency. Mol Biol Rep. 2021. https://doi.org/10.1007/s11033-021-06736-9
Amombo E, Ashilenje D, Hirich A, Kouisni L, Oukarroum A, Ghoulam C, El Gharous M, Nilahyane A. Exploring the correlation between salt tolerance and yield: research advances and perspectives for salt-tolerant forage sorghum selection and genetic improvement. Planta. 2022;255(3):1–16.
Das A, Singh S, Islam Z, Munshi A, Behera T, Dutta S, Weng Y, Dey S. Current progress in genetic and genomics-aided breeding for stress resistance in cucumber (Cucumis sativus L). Sci Hort. 2022;300:111059.
Haroon M, Wang X, Afzal R, Zafar MM, Idrees F, Batool M, Khan AS, Imran M. Novel plant breeding techniques shake hands with cereals to increase production. Plants. 2022;11:8.
Te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubešová M, Pyšek P. The more the better? The role of polyploidy in facilitating plant invasions. Ann Botany. 2012;109(1):19–45.
Sattler MC, Carvalho CR, Clarindo WR. The polyploidy and its key role in plant breeding. Planta. 2016;243(2):281–96. https://doi.org/10.1007/s00425-015-2450-x
Singh R, Singh Y, Xalaxo S, Verulkar S, Yadav N, Singh S, Singh N, Prasad K, Kondayya K, Rao PR. From QTL to variety-harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Sci. 2016;242:278–87.
Tiwari DN. A critical review of submergence tolerance breeding beyond sub 1 gene to mega varieties in the context of climate change. Int J Adv Sci Res Eng. 2018;4:140–8.
Mani B, Kumar B, Krishnamurthy S. Genetic variability and diversity of rice landraces of South Western India based on morphological traits. ORYZA-An Int J Rice. 2014;51(4):261–6.
Khan M, Kamal S, Saeed M, Iqbal J. Identification of quantitative trait loci for Na+, K + and ca + + accumulation traits in rice grown under saline conditions using F2 mapping population. Brazilian J Bot. 2015;38(3):555–65.
Haque MA, Rafii MY, Yusoff MM, Ali NS, Yusuff O, Datta DR, Anisuzzaman M, Ikbal MF. Advanced breeding strategies and future perspectives of salinity tolerance in rice. Agronomy. 2021;11:8.
Keilwagen J, Lehnert H, Berner T, Budahn H, Nothnagel T, Ulrich D, Dunemann F. (2017) The terpene synthase gene family of carrot (Daucus carota L.): identification of QTLs and candidate genes associated with terpenoid volatile compounds. Frontiers in plant science 8:1930
Ellison SL, Luby CH, Corak KE, Coe KM, Senalik D, Iorizzo M, Goldman IL, Simon PW, Dawson JC. Carotenoid presence is associated with the Or gene in domesticated carrot. Genetics. 2018;210(4):1497–508.
Bolton A, Simon P. (2019) Variation for salinity tolerance during seed germination in diverse carrot (Daucus carota L.) germplasm. HortScience 54 (1):38–44
Villano C, Esposito S, Carucci F, Iorizzo M, Frusciante L, Carputo D, Aversano R. High-throughput genotyping in onion reveals structure of genetic diversity and informative SNPs useful for molecular breeding. Mol Breeding. 2018;39(1):5. https://doi.org/10.1007/s11032-018-0912-0
Razali R, Bougouffa S, Morton MJL, Lightfoot DJ, Alam I, Essack M, Arold ST, Kamau AA, Schmockel SM, Pailles Y, Shahid M, Michell CT, Al-Babili S, Ho YS, Tester M, Bajic VB, Negrao S. The genome sequence of the wild Tomato Solanum pimpinellifolium provides insights into salinity tolerance. Front Plant Sci. 2018;9. https://doi.org/10.3389/fpls.2018.01402
Wan, L., Wang, Z., Tang, M., Hong, D., Sun, Y., Ren, J., ... & Zeng, H. CRISPR-Cas9 gene editing for fruit and vegetable crops: strategies and prospects. Horticulturae, 2021;7(7):193.
Kaur H, Pandey DK, Goutam U, Kumar V. CRISPR/Cas9-mediated genome editing is revolutionizing the improvement of horticultural crops: recent advances and future prospects. Sci Hort. 2021;289:110476.
Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, Xie Y. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Molecular plant. 2015 Aug 3;8(8):1274-84.
Zhu H, Li C, Gao C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat Rev Mol Cell Biol. 2020a;21(11):661–77.
Ran Y, Liang Z, Gao C. Current and future editing reagent delivery systems for plant genome editing. Sci China Life Sci. 2017;60(5):490–505.
Xin T, Tian H, Ma Y, Wang S, Yang L, Li X, Zhang M, Chen C, Wang H, Li H, Xu J, Huang S, Yang X. Targeted creation of new mutants with compact plant architecture using CRISPR/Cas9 genome editing by an optimized genetic transformation procedure in cucurbit plants. Hortic Res. 2022;9:uhab086. https://doi.org/10.1093/hr/uhab086
Hu B, Li D, Liu X, Qi J, Gao D, Zhao S, Huang S, Sun J, Yang L. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant. 2017;10(12):1575–8.
Bouzroud S, Gasparini K, Hu G, Barbosa MA, Rosa BL, Fahr M, Bendaou N, Bouzayen M, Zsögön A, Smouni A, Zouine M. Down Regulation and loss of Auxin Response factor 4 function using CRISPR/Cas9 alters Plant Growth, Stomatal function and improves Tomato Tolerance to Salinity and osmotic stress. Genes. 2020;11(3). https://doi.org/10.3390/genes11030272
Kim Y-C, Ahn WS, Cha A, Jie EY, Kim SW, Hwang B-H, Lee S. (2022) Development of glucoraphanin-rich broccoli (Brassica oleracea var. italica) by CRISPR/Cas9-mediated DNA-free Bol MYB 28 editing. Plant Biotechnology Reports:1–10
Giordano A, Santo Domingo M, Quadrana L, Pujol M, Martín-Hernández AM, Garcia-Mas J. CRISPR/Cas9 gene editing uncovers the role of CTR1 and ROS1 in melon fruit ripening and epigenetic regulation. J Exp Bot. 2022;73:4022–33.
Choi SH, Ahn WS, Jie EY, Cho H-S, Kim SW. (2022) Development of late-bolting plants by CRISPR/Cas9-mediated genome editing from mesophyll protoplasts of lettuce. Plant Cell Reports:1–4
Oleszkiewicz T, Klimek-Chodacka M, Kruczek M, Godel-Jędrychowska K, Sala K, Milewska-Hendel A, Zubko M, Kurczyńska E, Qi Y, Baranski R. Inhibition of carotenoid biosynthesis by CRISPR/Cas9 triggers cell wall remodelling in carrot. Int J Mol Sci. 2021;22(12):6516.
Jain M, Khurana JP. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J. 2009;276(11):3148–62.
Kashyap SP, Kumari N, Mishra P, Moharana DP, Aamir M. Tapping the potential of Solanum lycopersicum L. pertaining to salinity tolerance: perspectives and challenges. Genet Resour Crop Evol. 2021;68(6):2207–33. https://doi.org/10.1007/s10722-021-01174-9
Jaime-Pérez N, Pineda B, García‐Sogo B, Atares A, Athman A, Byrt CS, Olías R, Asins MJ, Gilliham M, Moreno V. The sodium transporter encoded by the HKT1; 2 gene modulates sodium/potassium homeostasis in tomato shoots under salinity. Plant Cell Environ. 2017;40(5):658–71.
Li D, Fu F, Zhang H, Song F. Genome-wide systematic characterization of the bZIP transcriptional factor family in tomato (Solanum lycopersicum L). BMC Genomics. 2015;16(1):1–18.
Alshareef NO, Wang JY, Ali S, Al-Babili S, Tester M, Schmöckel SM. Overexpression of the NAC transcription factor JUNGBRUNNEN1 (JUB1) increases salinity tolerance in tomato. Plant Physiol Biochem. 2019;140:113–21.
Yactayo-Chang JP, Acosta-Gamboa LM, Nepal N, Lorence A. (2017) The role of plant high-throughput phenotyping in the characterization of the response of high ascorbate plants to abiotic stresses. Ascorbic acid in plant growth, development and stress tolerance:321–354
Akbudak MA, Filiz E, Çetin D. Genome-wide identification and characterization of high-affinity nitrate transporter 2 (NRT2) gene family in tomato (Solanum lycopersicum) and their transcriptional responses to drought and salinity stresses. J Plant Physiol. 2022;272:153684.
Du C, Si Y, Wang Z, Guo Y, Li Y, Liu C, Fan H. Overexpression of CsPP2-A1 in cucumber enhanced salt tolerance by participating ABA-JA signaling pathway and antioxidant system. Environ Exp Bot. 2022;204:105095.
Hou K, Wang Y, Tao M-Q, Jahan MS, Shu S, Sun J, Guo S-R. Characterization of the CsPNG1 gene from cucumber and its function in response to salinity stress. Plant Physiol Biochem. 2020;150:140–50.
Zhou Y, Hu L, Jiang L, Liu H, Liu S. Molecular cloning and characterization of an ASR gene from Cucumis sativus. Plant Cell Tissue and Organ Culture (PCTOC). 2017;130:553–65.
Yang X, Li H, Yang Y, Wang Y, Mo Y, Zhang R, Zhang Y, Ma J, Wei C, Zhang X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE. 2018;13(1):e0191308.
Huang Y, Du J, Liu Y, Cao X, Liu Z, Li M. MAPK Gene Family in Lactuca sativa: genome-wide identification, Regulatory Network, and expression patterns in Stem Development and stress responses. Horticulturae. 2022;8(11):1087.
Shah IH, Manzoor MA, Sabir IA, Ashraf M, Haq F, Arif S, Abdullah M, Niu Q, Zhang Y. Genome-wide identification and comparative analysis of MATE gene family in Cucurbitaceae species and their regulatory role in melon (Cucumis melo) under salt stress. Hortic Environ Biotechnol. 2022;63(4):595–612.
Zhao Y-H, Deng Y-J, Wang Y-H, Lou Y-R, He L-F, Liu H, Li T, Yan Z-M, Zhuang J, Xiong A-S. Changes in carotenoid concentration and expression of carotenoid biosynthesis genes in Daucus carota taproots in response to increased salinity. Horticulturae. 2022;8(7):650.
Li M-Y, Xu Z-S, Tian C, Huang Y, Wang F, Xiong A-S. Genomic identification of WRKY transcription factors in carrot (Daucus carota) and analysis of evolution and homologous groups for plants. Sci Rep. 2016;6(1):23101.
Zhou L, Li J, He Y, Liu Y, Chen H. Functional characterization of SmCBF genes involved in abiotic stress response in eggplant (Solanum melongena). Sci Hort. 2018;233:14–21.
Chen J, Wang S, Wu F, Wei M, Li J, Yang F. Genome–wide identification and functional characterization of auxin response factor (ARF) genes in eggplant. Int J Mol Sci. 2022;23(11):6219.
Chiang C-M, Chen C-C, Chen S-P, Lin K-H, Chen L-R, Su Y-H, Yen H-C. Overexpression of the ascorbate peroxidase gene from eggplant and sponge gourd enhances flood tolerance in transgenic Arabidopsis. J Plant Res. 2017;130:373–86.
Wang B, Zhai H, He S, Zhang H, Ren Z, Zhang D, Liu Q. A vacuolar Na+/H + antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweetpotato. Sci Hort. 2016;201:153–66.
Wang Y, Zhan Y, Wu C, Gong S, Zhu N, Chen S, Li H. Cloning of a cystatin gene from sugar beet M14 that can enhance plant salt tolerance. Plant Sci. 2012;191–192:93–9. https://doi.org/10.1016/j.plantsci.2012.05.001
Ma C, Wang Y, Gu D, Nan J, Chen S, Li H. Overexpression of S-adenosyl-L-methionine synthetase 2 from sugar beet M14 increased Arabidopsis tolerance to salt and oxidative stress. Int J Mol Sci. 2017;18(4):847.
Chaudhry UK, Gökçe ZNÖ, Gökçe AF. Drought and salt stress effects on biochemical changes and gene expression of photosystem II and catalase genes in selected onion cultivars. Biologia. 2021;76(10):3107–21. https://doi.org/10.1007/s11756-021-00827-5
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MS compiled the literature and wrote the manuscript. AK edited and reviewed the article. Both authors approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
Not applicable.
Research involving Human Participants and/or Animals
Not applicable.
Informed consent
Not applicable.
Statement specifying permissions
Not applicable.
Statement on experimental research and field studies on plants
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shams, M., Khadivi, A. Mechanisms of salinity tolerance and their possible application in the breeding of vegetables. BMC Plant Biol 23, 139 (2023). https://doi.org/10.1186/s12870-023-04152-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04152-8