Abstract
Background
Particulate matter (PM) < 2.5 μm (PM2.5) or fine PM is a serious public health concern. It affects DNA methylation and heightens carcinogenesis. Deleted in lung and esophageal cancer 1 (DLEC1) is a tumor suppressor gene. However, aberrant methylation of the gene is associated with several cancers. We evaluated the association between PM2.5 and DLEC1 promoter methylation in Taiwanese adults based on regular outdoor exercise.
Methods
We obtained DNA methylation and exercise data of 496 participants (aged between 30 and 70 years) from the Taiwan Biobank (TWB) database. We also extracted PM2.5 data from the Air Quality Monitoring Database (AQMD) and estimated participants’ exposure using residential addresses.
Results
DLEC1 methylation and PM2.5 were positively associated: beta coefficient (β) = 0.114 × 10−3; p value = 0.046. The test for interaction between exercise and PM2.5 on DLEC1 methylation was significant (p value = 0.036). After stratification by exercise habits, PM2.5 and DLEC1 methylation remained significantly associated only among those who exercised regularly (β = 0.237 × 10−3; p value = 0.007). PM2.5 quartile-stratified analyses revealed an inverse association between regular exercise and DLEC1 methylation at PM2.5 < 27.37 μg/m3 (β = − 5.280 × 10−3; p value = 0.009). After combining exercise habits and PM2.5 quartiles, one stratum (i.e., regular exercise and PM2.5 < 27.37 μg/m3) was inversely associated with DLEC1 methylation (β = -5.160 × 10−3, p value = 0.007).
Conclusions
We found significant positive associations between PM2.5 and DLEC1 promoter methylation. Regular exercise at PM2.5 < 27.37 μg/m3 seemingly regulated DLEC1 promoter methylation.
Similar content being viewed by others
Introduction
PM2.5 induces the generation of reactive oxygen species (ROS) which have detrimental effects [1], like immune response stress, inflammatory injury, DNA damage, and oxidative stress that enhance cancer formation [1,2,3]. PM2.5 is a critical public health issue that accounts for most air pollution-related global deaths [1, 4]. It accounted for approximately 3.5 and 4.2 million global deaths in 1990 and 2015, respectively. Moreover, it was the fifth top cause of global mortality in 2015 [5]. While PM2.5 could enhance inflammation and oncogenesis [3], exercise, on the other hand, could curb inflammation and related complications [6,7,8,9]. All mechanisms by which PM2.5 and exercise affect tumorigenesis are not yet delineated.
DNA methylation is an epigenetic change that integrates the interactions between genes and the environment [10]. Abnormal patterns of this epigenetic marker are promising diagnostic and prognostic tumor markers because they are frequently detected even at the earliest stages of tumor formation [11, 12]. DNA methylation is one of the epigenetic mechanisms underlying the incidence of air pollution-induced allergic diseases [10] and human tumors [13, 14]. PM2.5-induced DNA methylation alterations aggravate the risk of cancer by repressing tumor suppressor genes and activating oncogenes [15]. DNA methylation is believed to be an epigenetic indicator of exercise intervention [16, 17]. For instance, in a study where exercise-induced immunologic benefits attenuated the detrimental effects of air pollution on the lungs, DNA methylation was a marker of such benefits [18].
Deleted in lung and esophageal cancer 1 (DLEC1) is an important element in head and neck tumorigenesis [13, 19,20,21,22]. It is a bona fide tumor suppressor gene located on chromosome 3p22.3 [13, 19,20,21,22,23,24]. Its tumor-suppressing potentials were initially identified in lung, renal, and esophageal carcinomas [21]. Abnormalities in DLEC1 methylation are potential diagnostic and prognostic epigenetic tumor markers [25]. For instance, DLEC1 promoter methylation is a prognostic biomarker for gastric, lung, advanced ovarian, and endometrial cancer [11, 26,27,28,29,30,31]. It is also a diagnostic biomarker for prostate, breast, ovarian, colorectal, gastric, nasopharyngeal, and lung cancer [11, 25, 30, 32,33,34,35,36,37,38,39].
Findings on the relationship between air pollution and DNA methylation warrant critical replication and validation [40]. Exposure to smoky coals was associated with higher DLEC1 methylation in plasma and tissue samples from Chinese lung cancer patients [32], implying that air pollution-induced DLEC1 methylation could influence cancer etiology. Even though both DLEC1 methylation and PM2.5 play prominent roles in carcinogenesis, their relationship has received limited research attention. Moreover, studies on DLEC1 methylation among Taiwanese are lacking. Furthermore, the combined effects of exercise and PM2.5 exposure on health, alongside the underlying mechanisms require more investigations [8, 16, 41]. We evaluated the association between PM2.5 and DLEC1 promoter methylation in relation to exercise among TWB participants.
Methods
Data sources and study population
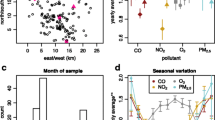
We obtained data from two sources, namely the TWB database and AQMD. Recruitment of participants into the TWB project is restricted to Taiwanese aged between 30 and 70 years who have never been diagnosed with cancer. The TWB has 29 recruitment centers throughout Taiwan [42]. The TWB database (2008 to 2015) has data on methylation, exercise, sex, age, cigarette smoking, weight, height, secondhand smoke exposure, and alcohol/betel nut intake. Five DLEC1 promoter CpG sites (cg04833533, cg16150706, cg11542528, cg20684180, and cg23881725) were available in the TWB database. It is worth stating that this dataset does not have air pollution data. So, we obtained annual averages (2006–2011) of daily concentrations of PM2.5 (μg/m3), SO2 (ppb), CO (ppm), O3 (ppb), and NOx (ppb) from the AQMD. The Environmental Protection Administration (EPA) in Taiwan monitors air quality through the Air Quality Monitoring Network (AQMN) [43, 44]. To date, the EPA has set up about 77 fully automated air quality monitoring stations nationwide for daily monitoring of air pollution [44]. We included 496 individuals with complete data in the final analyses.
Main exposure and outcome
The main exposure was PM2.5 while the outcome was DLEC1 promoter methylation. We used the residential addresses of participants and estimated their PM2.5 exposure. Health GeneTech Corp. performed all the DNA methylation experiments. In brief, a trained and qualified researcher with a medical background collected about 9 ml of venous blood from each participant into sodium citrate tubes. The blood samples were kept at 4 °C and transported to the laboratory for further experiments. DNA isolation and purification was done using an automated chemical extraction instrument called Chemagic™ Prime™. Purified DNA samples were treated with sodium bisulfite using EZ DNA Methylation Kit (Zymo Research, CA, USA). DNA methylation was assessed with the Infinium® MethylationEPIC BeadChipEPIC array (Illumina Inc.) and presented as beta values, which range from 0 to 1 [45, 46]. Lower and higher beta values indicate lower and higher methylation levels, respectively. The Infinium® MethylationEPIC BeadChipEPIC array targets more than 850,000 CpG sites across the genome. Quality control measures for methylation data were untaken as previously stated [47, 48]. Ethical approval for this study was given by the Institutional Review Board of Chung Shan Medical University Hospital (CS2-20007).
Covariates
We used participants’ residential addresses and estimated the annual average exposure levels of SO2 (ppb), CO (ppm), O3 (ppb), and NOx (ppb). Self-filled TWB questionnaires contained data on exercise, sex, age, cigarette smoking, betel nut chewing, alcohol intake, and secondhand smoke exposure. Detailed descriptions of these variables and BMI are found somewhere else [47, 48]. Summarily, we defined outdoor exercise as engaging in any outdoor activities (e.g., Chinese martial arts, “Wai-Tan-Kung,” “Neidan-Kung,” “Falun Dafa,” “Taijiquan,” “Xiang Kong,” “Yuan Chin Dance,” “Qigong,” strolling, jogging, hiking, rope jumping, arm swing, soccer, golf, croquet, tennis, basketball, other ball games, biking, mountain climbing, and hula hoop) lasting over 30 min, at least three times per week.
Statistical analyses
We stratified the basic characteristics of the participants into regular and no regular exercise. DLEC1 promoter methylation (mean ± standard error) was the average of the beta values of the 5 DLEC1 promoter CpG sites. We evaluated the differences in continuous variables (DLEC1 methylation levels, PM2.5, SO2, CO, O3, NOx, and age) between the two exercise groups with the T test and the differences in the categorical variables (sex, cigarette smoking, alcohol/betel nut intake, BMI, and exposure to secondhand smoke) between the two groups with the chi-square test. Moreover, we determined the association between PM2.5 and DLEC1 promoter methylation by employing multivariate linear regression analysis and adjusted for covariates (consisting of exercise, SO2, CO, O3, NOx, sex, age, cigarette smoking, BMI, secondhand smoke exposure, and alcohol/betel nut intake). We also used multivariate linear regression and determined the interaction between PM2.5 and exercise on DLEC1 promoter methylation. We adjusted for cell-type composition in whole blood using the Reference-Free Adjustment for Cell-Type composition (ReFACTor) method [49]. For all analyses, a p value < 0.05 was considered statistically significant. All statistical analyses were executed with the SAS software; version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
We included a total of 496 participants (i.e., 211 with regular and 285 with no regular exercise habits). DLEC1 promoter methylation, age, cigarette smoking, BMI, and secondhand smoke exposure were significantly different (p value < 0.050) between the exercise groups (Table 1).
PM2.5 was significantly associated with hypermethylation or higher levels (β = 0.114 × 10−3; p value = 0.046) while age was significantly associated with hypomethylation or lower levels of DLEC1 promoter methylation: β = − 0.206 × 10−3; p value = < 0.001 (Table 2).
Exercise and PM2.5 had a significant interaction on DLEC1 methylation (p value = 0.036). When we stratified participants by regular exercise habits, PM2.5 and DLEC1 methylation remained positively associated. However, this association was significant only in the regular exercise group (β = 0.237 × 10−3, p value = 0.007). The inverse association between age and DLEC1 methylation remained significant in both exercise groups. The β coefficient was − 0.212 × 10−3 (p value < 0.001) for regular exercise and − 0.144 × 10−3 (p value = 0.042) for no regular exercise (Table 3). There was a significant association between obesity and DLEC1 methylation in those who exercised regularly: β = 3.870 × 10−3, p value = 0.029.
When we stratified PM2.5 concentrations into quartiles (Table 4), DLEC1 methylation in participants who exercised regularly was significant only at PM2.5 < 27.37 μg/m3 (β = − 5.280 × 10−3; p value = 0.009). The methylation was also significant at PM2.5 levels 31.82 ≤ PM2.5 < 38.73 μg/m3 (β = 3.980 × 10−3, p value = 0.009) for SO2, 27.37 ≤ PM2.5 < 31.82, and PM2.5 ≥ 38.73 μg/m3 (β = 3.850 × 10−3; p value = 0.003, β = − 1.700 × 10−3; p value = 0.035, respectively) for O3, and 31.82 ≤ PM2.5 < 38.73 and PM2.5 ≥ 38.73 μg/m3 (β = − 0.310 × 10−3; p value < 0.001 and β = − 0.320 × 10−3; p value = 0.003, respectively) for age.
Further stratification by exercise habits and PM2.5 quartiles revealed significant lower DLEC1 promoter methylation levels in one stratum (regular exercise at PM2.5 levels < 27.37 μg/m3): β = − 5.160 × 10−3; p value = 0.007 (Table 5).
Discussion
Based on the available literature, there are gaps in research focusing on the relationship between DLEC1 methylation and PM2.5 exposure. To our utmost knowledge, the current study is the first to investigate such a relationship. PM2.5 exposure was significantly associated with DLEC1 hypermethylation. Exercise seemingly modulated this relationship. That is, in relation to exercise, DLEC1 methylation was significantly associated with PM2.5 at levels < 27.37 μg/m3. In other words, the relationship between DLEC1 methylation and regular exercise disappeared as PM2.5 levels increased, implying that exercise might significantly influence DLEC1 methylation only at lower levels of PM2.5. This suggests that taking exercise when PM2.5 levels are high might expose people to more PM2.5 pollution, thereby abating the benefits of regular exercise. That is, the benefits of regular exercise might disappear when PM2.5 concentrations increase. Thus, people should not be encouraged to exercise when PM2.5 levels are high.
PM2.5 induces the generation of ROS [1], which when unbalanced, could have oncogenic consequences [1], like immune response stress, inflammatory injury, DNA damage, and oxidative stress [1,2,3]. ROS imbalance results from improperly regulated ROS production [2]. It also occurs when free radicals do not properly neutralize or detoxify oxidative effects [2]. Oxidative stress resulting from ROS is believed to be the main driving force for most air pollution-related adverse health effects [40]. PM2.5 is a critical issue that has increased oral, lung, breast, ovarian, and hepatic cancer morbidity and mortality in Taiwan [4, 50,51,52].
DLEC1 exhibits its cancer-inhibiting potentials by decreasing the invasiveness and metastasis of tumor cells [21, 22, 53] and also by enhancing apoptosis and arresting the G1 phase of the cell cycle [54, 55]. However, an abnormal methylation profile (like DLEC1 hypermethylation) is significantly linked to head and neck, ovarian, lung, renal, nasopharyngeal, oral, adrenocortical, hepatocellular, esophageal, gastric, and squamous cell carcinoma [11, 28, 30,31,32, 38, 39, 53, 54, 56,57,58,59,60,61,62,63,64]. In light of this, we believe that the PM2.5-related DLCE1 hypermethylation observed in our study could also heighten the risk of cancer.
Transcriptional suppression of DLEC1 by promoter hypermethylation is also believed to be an early event in carcinogenesis [62]. This is evident in adrenocortical, lung, nasopharyngeal, and esophageal squamous cell carcinoma [26, 30, 31, 57, 61]. Upregulation of DLEC1 is associated with reduced growth and invasiveness while downregulation is associated with cell proliferation, invasiveness, and poor disease prognosis [22, 31, 53, 58, 62]. DLEC1 expression could be restored with demethylating agents when downregulated, as demonstrated in lung, prostate, oral, nasopharyngeal, renal, ovarian, adrenocortical, and uterine tumors [11, 22, 25, 31, 53, 60,61,62, 65]. This reversible nature of DLEC1 suggests that it could be a potential treatment target for these cancers.
The detrimental effects of air pollution on health could be attenuated by exercise [18]. This could be achieved, in part, through protective immunologic responses and DNA methylation [18]. In our study, DLEC1 methylation was inversely associated with exercise, suggesting that exercise could attenuate PM2.5-related DLEC1 hypermethylation and the subsequent adverse effects. The possible mechanism through which exercise could modulate PM2.5-induced DLEC1 methylation is unclear. As previously stated, PM2.5 exacerbates inflammation and oxidative stress that could induce epigenetic alterations, especially DNA methylation [3, 66, 67]. Conversely, exercise regulates inflammation [6,7,8,9] and protects against air pollution-related health outcomes through altered DNA methylation profiles [18]. Inflammation is suggested as a possible contributor to epigenetic changes resulting from exercise interventions [66]. For instance, available literature corroborates the idea that exercise-associated inflammatory effects could be among the mechanisms that regulate DNA methylation [68]. Therefore, exercise might affect DLEC1 methylation by suppressing PM2.5-induced inflammation.
Previously, smoking and DLCE1 methylation were not significantly related [11, 28, 34]. It is important to note that in our study, smoking, SO2, CO, O3 showed significant associations with DLEC1 only after PM2.5 was stratified into quartiles. The attainment of a significant association between DLEC1 methylation and smoking after stratification into quartiles implies that PM2.5 might aggravate smoking-related DLEC1 methylation effects. Therefore, smoking which is already a harmful lifestyle habit could be more detrimental in air polluted areas. So far, the relation of age with DLEC1 methylation has not been concordant. For example, in esophageal cancer, age was significantly associated with DLCE1 methylation and expression [57]. On the other hand, in lung and gastric cancer, both factors had no significant association [11, 29]. In the current study, we found significant inverse associations between DLEC1 methylation and age, suggesting that DLEC1 methylation might decrease with increasing age. Age was also inversely associated with DNA methylation in a genome-wide DNA methylation study [69]. In the context of the current study, the underpinning mechanism cannot be clearly stated.
DNA methylation is a reliable molecular predictor of cancer because it is the most common epigenetic variation that could be detected even at premalignant or early malignant stages [13, 70]. Moreover, in noncancerous tissues, it could reveal previous exposure to carcinogens and so, is a possible indicator of disease risk [71]. Hence, the observed DLEC1 hypermethylation due to PM2.5 and smoking might serve as an early predictor of adverse health conditions. Moreover, hypomethylation of DLEC1 due to regular exercise at low PM2.5 levels shows that exercise intervention could help reverse the methylation status of DLEC1 and possibly upregulate the gene. As a limitation, we could not adjust for occupational exposure because we did not have related information. Moreover, we did not evaluate DLEC1 expression due to the unavailability of data in the TWB dataset. Nevertheless, many studies found significant associations between DLEC1 hypermethylation and downregulation [26, 30, 31, 57, 61].
Conclusions
This study shows that PM2.5 might affect DLEC1 methylation in individuals with no personal history of cancer. Exercise might regulate the effect of PM2.5 on DLEC1 methylation, especially at low concentrations of PM2.5. Our findings support prior reports that DNA methylation in noncancerous tissues could reveal previous exposure to carcinogens. Hence, DLEC1 promoter hypermethylation might elucidate the epigenetic mechanism through which PM2.5 enhances disease onset and might be a possible biomarker of disease risk.
Availability of data and materials
The data that support the findings of this study are available from Taiwan Biobank but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Taiwan Biobank.
Abbreviations
- DLEC1:
-
Deleted in lung and esophageal cancer 1
- PM:
-
Particulate matter
- PM2.5 :
-
Particulate matter (PM) < 2.5 μm
- SO2 :
-
Sulfur dioxide
- CO:
-
Carbon monoxide
- O3 :
-
Ozone
- NOx:
-
Nitrogen oxides
- β :
-
Beta coefficient
- DNA:
-
Deoxyribonucleic acid
- ROS:
-
Reactive oxygen species
- CpG:
-
Cytosine-phosphate-guanine
- SE:
-
Standard error
- BMI:
-
Body mass index
- AQMD:
-
Air Quality Monitoring Database
- TWB:
-
Taiwan Biobank
References
Gangwar RS, Bevan GH, Palanivel R, Das L, Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: recent insights. Redox Biol. 2020. p. 101545.
Mydin RBS, Okekpa SI. Reactive oxygen species, cellular redox homeostasis and cancer. IntechOpen: Homeostasis-An Integrated Vision; 2018.
Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C. 2008;26:339–62.
Su S-Y, Liaw Y-P, Jhuang J-R, Hsu S-Y, Chiang C-J, Yang Y-W, et al. Associations between ambient air pollution and cancer incidence in Taiwan: an ecological study of geographical variations. BMC Public Health. 2019;19:1–8.
Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. 2017;389:1907–18.
Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr Physiol. 2012;2:2775–809.
Nimmo M, Leggate M, Viana J, King J. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab. 2013;15:51–60.
Zhang Z, Hoek G, L-y C, Chan T-C, Guo C, Chuang YC, et al. Particulate matter air pollution, physical activity and systemic inflammation in Taiwanese adults. Int J Hyg Environ Health. 2018;221:41–7.
You T, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of exercise training on chronic inflammation in obesity. Sports Med. 2013;43:243–56.
Clifford RL, Jones MJ, MacIsaac JL, McEwen LM, Goodman SJ, Mostafavi S, et al. Inhalation of diesel exhaust and allergen alters human bronchial epithelium DNA methylation. J Allergy Clin Immunol. 2017;139:112–21.
Zhang Y, Miao Y, Yi J, Wang R, Chen L. Frequent epigenetic inactivation of deleted in lung and esophageal cancer 1 gene by promoter methylation in non–small-cell lung cancer. Clin Lung Cancer. 2010;11:264–70.
Wang Z, Li L, Su X, Gao Z, Srivastava G, Murray PG, et al. Epigenetic silencing of the 3p22 tumor suppressor DLEC1 by promoter CpG methylation in non-Hodgkin and Hodgkin lymphomas. J Transl Med. 2012;10:209.
Demokan S, Dalay N. Role of DNA methylation in head and neck cancer. Clin Epigenetics. 2011;2:123.
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28.
Li R, Zhou R, Zhang J. Function of PM2. 5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol Lett. 2018;15:7506–14.
Barrón-Cabrera E, Ramos-Lopez O, González-Becerra K, Riezu-Boj JI, Milagro FI, Martínez-López E, et al. Epigenetic modifications as outcomes of exercise interventions related to specific metabolic alterations: a systematic review. Lifestyle genomics. 2019;12:25–44.
Denham J, Marques FZ, Bruns EL, O’Brien BJ, Charchar FJ. Epigenetic changes in leukocytes after 8 weeks of resistance exercise training. Eur J Appl Physiol. 2016;116:1245–53.
Lovinsky-Desir S, Jung KH, Jezioro JR, Torrone DZ, de Planell-Saguer M, Yan B, et al. Physical activity, black carbon exposure, and DNA methylation in the FOXP3 promoter. Clin Epigenetics. 2017;9:65.
Peng H, Zhao T, Yao K. Study of DLC1 gene expression in nasopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2002;37:454–7.
Smith IM, Mithani SK, Liu C, Chang SS, Begum S, Dhara M, et al. Novel integrative methods for gene discovery associated with head and neck squamous cell carcinoma development. Arch Otolaryngol–Head Neck Surg. 2009;135:487–95.
Daigo Y, Nishiwaki T, Kawasoe T, Tamari M, Tsuchiya E, Nakamura Y. Molecular cloning of a candidate tumor suppressor gene, DLC1, from chromosome 3p21. 3. Cancer Res. 1999;59:1966–72.
Kwong J, Chow LSN, Wong AYH, Hung WK, Chung GTY, To KF, et al. Epigenetic inactivation of the deleted in lung and esophageal cancer 1 gene in nasopharyngeal carcinoma. Genes Chromosomes Cancer. 2007;46:171–80.
Zhang Q, Ying J, Li J, Fan Y, Poon FF, Ng KM, et al. Aberrant promoter methylation of DLEC1, a critical 3p22 tumor suppressor for renal cell carcinoma, is associated with more advanced tumor stage. J Urol. 2010;184:731–7.
Al Sarakbi W, Reefy S, Jiang WG, Roberts T, Newbold R, Mokbel K. Evidence of a tumour suppressor function for DLEC1 in human breast cancer. Anticancer Res. 2010;30:1079–82.
Zhang L, Zhang Q, Li L, Wang Z, Ying J, Fan Y, et al. DLEC1, a 3p tumor suppressor, represses NF-κB signaling and is methylated in prostate cancer. J Mol Med. 2015;93:691–701.
Chen C-A, Chiang Y-C, Chang M-C, Hu Y-H, You S-L, Cheng Y-YK, et al. Gene methylation profiles as prognostic markers in ovarian clear cell and endometrioid adenocarcinomas. Am J Transl Res. 2015;7:139.
Lin H-W, Fu C-F, Chang M-C, Lu T-P, Lin H-P, Chiang Y-C, et al. CDH1, DLEC1 and SFRP5 methylation panel as a prognostic marker for advanced epithelial ovarian cancer. Epigenomics. 2018;10:1397–413.
Sasaki H, Hikosaka Y, Kawano O, Moriyama S, Yano M, Fujii Y. Methylation of the DLEC1 gene correlates with poor prognosis in Japanese lung cancer patients. Oncol Lett. 2010;1:283–7.
Ye X, Feng G, Jiao N, Pu C, Zhao G, Sun G. Methylation of DLEC1 promoter is a predictor for recurrence in Chinese patients with gastric cancer. Dis Markers. 2014;2014:804023.
Pastuszak-Lewandoska D, Kordiak J, Antczak A, Migdalska-Sęk M, Czarnecka KH, Górski P, et al. Expression level and methylation status of three tumor suppressor genes, DLEC1, ITGA9 and MLH1, in non-small cell lung cancer. Med Oncol. 2016;33:75.
Seng T, Currey N, Cooper WA, Lee CS, Chan C, Horvath L, et al. DLEC1 and MLH1 promoter methylation are associated with poor prognosis in non-small cell lung carcinoma. Br J Cancer. 2008;99:375–82.
Huang X, Wu C, Fu Y, Guo L, Kong X, Cai H. Methylation analysis for multiple gene promoters in non-small cell lung cancers in high indoor air pollution region in China. Bull Cancer. 2018;105:746–54.
Tian F, Yip SP, Kwong DLW, Lin Z, Yang Z, Wu VWC. Promoter hypermethylation of tumor suppressor genes in serum as potential biomarker for the diagnosis of nasopharyngeal carcinoma. Cancer Epidemiol. 2013;37:708–13.
Nawaz I, Qiu X, Wu H, Li Y, Fan Y, Hu L-F, et al. Development of a multiplex methylation specific PCR suitable for (early) detection of non-small cell lung cancer. Epigenetics. 2014;9:1138–48.
Park SY, Kwon HJ, Lee HE, Ryu HS, Kim S-W, Kim JH, et al. Promoter CpG island hypermethylation during breast cancer progression. Virchows Arch. 2011;458:73–84.
Wang G, Zhang W, Zhou B, Jin C, Wang Z, Yang Y, et al. The diagnosis value of promoter methylation of UCHL1 in the serum for progression of gastric cancer. Biomed Res Int. 2015;2015:741030.
Zhang Y, Ye X, Geng J, Chen L. Epigenetic inactivation of deleted in lung and esophageal cancer 1 gene by promoter methylation in gastric and colorectal adenocarcinoma. Hepato-gastroenterology. 2010;57:1614–9.
Swellam M, Ramadan A, Mahmoud MS, Hashim M, Sobeih ME. Diagnostic role of aberrant DNA promoter methylation in ovarian cancer. In: Annual Research & Review in Biology; 2017. p. 1–13.
Li X, Mao W, Guo D, Xu H. Clinicopathological significance and diagnostic value of DLEC1 hypermethylation in lung cancer: a meta-analysis. J Nippon Med Sch. 2019;86:62–9.
Rider CF, Carlsten C. Air pollution and DNA methylation: effects of exposure in humans. Clin Epigenetics. 2019;11:131.
Matt F, Cole-Hunter T, Donaire-Gonzalez D, Kubesch N, Martínez D, Carrasco-Turigas G, et al. Acute respiratory response to traffic-related air pollution during physical activity performance. Environ Int. 2016;97:45–55.
Biobank T. Purpose of Taiwan Biobank; 2015.
Lin C-H, Wu Y-L, Lai C-H, Watson JG, Chow JC. Air quality measurements from the southern particulate matter supersite in Taiwan. Aerosol Air Qual Res. 2008;8:233–64.
Environmental Protection Administration T. Air quality observation and forecast network. Taiwan; 2020.
Moran S, Arribas C, Esteller M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics. 2016;8:389–99.
Smyth L, Kilner J, Maxwell A, McKnight A. Comparison of methylation patterns generated from genomic and cell-line derived DNA using the Illumina Infinium MethylationEPIC BeadChip array. BMC Res Notes. 2019;12:1–8.
Tantoh DM, Wu M-F, Ho C-C, Lung C-C, Lee K-J, Nfor ON, et al. SOX2 promoter hypermethylation in non-smoking Taiwanese adults residing in air pollution areas. Clin Epigenetics. 2019;11:46.
Tantoh DM, Lee K-J, Nfor ON, Liaw Y-C, Lin C, Chu H-W, et al. Methylation at cg05575921 of a smoking-related gene (AHRR) in non-smoking Taiwanese adults residing in areas with different PM 2.5 concentrations. Clin Epigenetics. 2019;11:69.
Rahmani E, Zaitlen N, Baran Y, Eng C, Hu D, Galanter J, et al. Sparse PCA corrects for cell type heterogeneity in epigenome-wide association studies. Nat Methods. 2016;13:443.
Tseng C-H, Tsuang B-J, Chiang C-J, Ku K-C, Tseng J-S, Yang T-Y, et al. The relationship between air pollution and lung cancer in nonsmokers in Taiwan. J Thorac Oncol. 2019;14:784–92.
Pan W-C, Wu C-D, Chen M-J, Huang Y-T, Chen C-J, Su H-J, et al. Fine particle pollution, alanine transaminase, and liver cancer: a Taiwanese prospective cohort study (REVEAL-HBV). JNCI: J Natl Cancer Inst. 2016;108.
Hung L-J, Tsai S-S, Chen P-S, Yang Y-H, Liou S-H, Wu T-N, et al. Traffic air pollution and risk of death from breast cancer in Taiwan: fine particulate matter (PM2. 5) as a proxy marker. Aerosol Air Qual Res. 2012;12:275–82.
Kwong J, Lee J-Y, Wong K-K, Zhou X, Wong DT, Lo K-W, et al. Candidate tumor-suppressor gene DLEC1 is frequently downregulated by promoter hypermethylation and histone hypoacetylation in human epithelial ovarian cancer. Neoplasia (New York, NY). 2006;8:268.
Seven D, Yavuz E, Kilic E, Baltaci E, Karaman E, Ulutin T, et al. DLEC1 is not silenced solely by promoter methylation in head and neck squamous cell carcinoma. Gene. 2015;563:83–6.
Ying J, Poon F, Yu J, Geng H, Wong AHY, Qiu G, et al. DLEC1 is a functional 3p22. 3 tumour suppressor silenced by promoter CpG methylation in colon and gastric cancers. Br J Cancer. 2009;100:663–9.
Haizhu S, Jun Y, ZHANG Y, Rui W, Longbang C. DNA methylation of tumor suppressor genes located on chromosome 3p in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2011;14.
Li L, Xu J, Qiu G, Ying J, Du Z, Xiang T, et al. Epigenomic characterization of a p53-regulated 3p22. 2 tumor suppressor that inhibits STAT3 phosphorylation via protein docking and is frequently methylated in esophageal and other carcinomas. Theranostics. 2018;8:61.
Montavon C, Gloss BS, Warton K, Barton CA, Statham AL, Scurry JP, et al. Prognostic and diagnostic significance of DNA methylation patterns in high grade serous ovarian cancer. Gynecol Oncol. 2012;124:582–8.
Qiu G-H, Salto-Tellez M, Ross JA, Yeo W, Cui Y, Wheelhouse N, et al. The tumor suppressor gene DLEC1 is frequently silenced by DNA methylation in hepatocellular carcinoma and induces G1 arrest in cell cycle. J Hepatol. 2008;48:433–41.
Ricketts CJ, Morris MR, Gentle D, Brown M, Wake N, Woodward ER, et al. Genome-wide CpG island methylation analysis implicates novel genes in the pathogenesis of renal cell carcinoma. Epigenetics. 2012;7:278–90.
Fonseca AL, Kugelberg J, Starker LF, Scholl U, Choi M, Hellman P, et al. Comprehensive DNA methylation analysis of benign and malignant adrenocortical tumors. Genes Chromosomes Cancer. 2012;51:949–60.
Chan W-H, Chang K-P, Yang S-W, Yao T-C, Ko T-Y, Lee Y-S, et al. Transcriptional repression of DLEC1 associates with the depth of tumor invasion in oral squamous cell carcinoma. Oral Oncol. 2010;46:874–9.
Loyo M, Brait M, Kim MS, Ostrow KL, Jie CC, Chuang AY, et al. A survey of methylated candidate tumor suppressor genes in nasopharyngeal carcinoma. Int J Cancer. 2011;128:1393–403.
Ayadi W, Karray-Hakim H, Khabir A, Feki L, Charfi S, Boudawara T, et al. Aberrant methylation of p16, DLEC1, BLU and E-cadherin gene promoters in nasopharyngeal carcinoma biopsies from Tunisian patients. Anticancer Res. 2008;28:2161–7.
Navarro A, Yin P, Monsivais D, Lin SM, Du P, Wei J-J, et al. Genome-wide DNA methylation indicates silencing of tumor suppressor genes in uterine leiomyoma. PLoS One. 2012;7.
Hartnett L, Egan LJ. Inflammation, DNA methylation and colitis-associated cancer. Carcinogenesis. 2012;33:723–31.
Abu-Remaileh M, Bender S, Raddatz G, Ansari I, Cohen D, Gutekunst J, et al. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 2015;75:2120–30.
Horsburgh S, Robson-Ansley P, Adams R, Smith C. Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev. 2015;21.
Smith JA, Zagel AL, Sun YV, Dolinoy DC, Bielak LF, Peyser PA, et al. Epigenomic indicators of age in African Americans. Hereditary Genet : Curr Res. 2014;3.
Guo M, Peng Y, Gao A, Du C, Herman JG. Epigenetic heterogeneity in cancer. Biomark Res. 2019;7:23.
Nakajima T, Enomoto S, Ushijima T. DNA methylation: a marker for carcinogen exposure and cancer risk. Environ Health Prev Med. 2008;13:8.
Acknowledgements
We are thankful to Chung Shan Medical University Hospital for funding this work.
Funding
This work was supported by funds from the Chung Shan Medical University Hospital Project (CSH-2020-C-010).
Author information
Authors and Affiliations
Contributions
Conceptualization, Ying-Hsiang Chou, Disline Manli Tantoh, Ming-Chi Wu, Yeu-Sheng Tyan, Pei-Hsin Chen, Oswald Ndi Nfor, Shu-Yi Hsu, Chao-Yu Shen, Chien-Ning Huang, and Yung-Po Liaw; formal analysis, Pei-Hsin Chen and Yung-Po Liaw; methodology, Ying-Hsiang Chou, Disline Manli Tantoh, Ming-Chi Wu, Yeu-Sheng Tyan, Pei-Hsin Chen, Oswald Ndi Nfor, Shu-Yi Hsu, Chao-Yu Shen, Chien-Ning Huang, and Yung-Po Liaw; supervision, Chao-Yu Shen, Chien-Ning Huang, and Yung-Po Liaw; validation, Ying-Hsiang Chou, Disline Manli Tantoh, Ming-Chi Wu, Yeu-Sheng Tyan, Pei-Hsin Chen, Oswald Ndi Nfor, Shu-Yi Hsu, Chao-Yu Shen, Chien-Ning Huang, and Yung-Po Liaw; writing—original draft, Ying-Hsiang Chou and Disline Manli Tantoh; writing—review and editing, Ying-Hsiang Chou, Disline Manli Tantoh, Ming-Chi Wu, Yeu-Sheng Tyan, Pei-Hsin Chen, Oswald Ndi Nfor, Shu-Yi Hsu, Chao-Yu Shen, Chien-Ning Huang, and Yung-Po Liaw. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Chung Shan Medical University Institutional Review Board (CS2-20007) granted ethical approval for this study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chou, YH., Tantoh, D.M., Wu, MC. et al. PM2.5 exposure and DLEC1 promoter methylation in Taiwan Biobank participants. Environ Health Prev Med 25, 68 (2020). https://doi.org/10.1186/s12199-020-00909-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12199-020-00909-x