Abstract
Survival of glioblastoma patients varies and prognostic markers are important in the clinical setting. With digital pathology and improved immunohistochemical multiplexing becoming a part of daily diagnostics, we investigated the prognostic value of the Ki-67 labelling index (LI) in glioblastomas more precisely than previously by excluding proliferation in non-tumor cells from the analysis. We investigated the Ki-67 LI in a well-annotated population-based glioblastoma patient cohort (178 IDH-wildtype, 3 IDH-mutated). Ki-67 was identified in full tumor sections with automated digital image analysis and the contribution from non-tumor cells was excluded using quantitative double-immunohistochemistry. For comparison of the Ki-67 LI between WHO grades (II-IV), 9 IDH-mutated diffuse astrocytomas and 9 IDH-mutated anaplastic astrocytomas were stained. Median Ki-67 LI increased with increasing WHO grade (median 2.7%, 6.4% and 27.5%). There was no difference in median Ki-67 LI between IDH-mutated and IDH-wildtype glioblastomas (p = 0.9) and Ki-67 LI was not associated with survival in glioblastomas in neither univariate (p = 0.9) nor multivariate analysis including MGMT promoter methylation status and excluding IDH-mutated glioblastomas (p = 0.2). Ki-67 may be of value in the differential diagnostic setting, but it must not be over-interpreted in the clinico-pathological context.
Similar content being viewed by others
Introduction
Patients with gliomas have different survival outcome and efforts are made to distinguish between patients with more favorable versus poor prognosis1,2,3,4. Methylation of the O6-methylguanine-DNA-methyltransferase (MGMT) promoter may be used to decide whether elderly glioblastoma patients will benefit from treatment with Temozolomide alone instead of radiotherapy5,6,7. Other important prognostic biomarkers for patients with diffuse astrocytic and oligodendroglial tumors include isocitrate dehydrogenase (IDH) mutation, 1p/19q codeletion and histone 3 K27M mutation8. These three molecular alterations have since 2016 been used as strong defining diagnostic markers for the distinction of biologically and clinically distinct glioma entities in the revised World Health Organization (WHO) classification of central nervous system tumors 20169. Nevertheless, additional reliable biomarkers are urgently needed for better assessment of prognosis for the individual glioma patient—including patients having the most frequent glioma—the IDH-wildtype glioblastoma.
Ki-67 is a nuclear protein that directly reflects a specific physiological proliferative state of the cell expressing this marker10. Association of the Ki-67 labelling index (LI) in tumour tissues with patient survival has been reported for several cancer types11,12,13,14,15,16,17. In gliomas, immunohistochemical assessment of the Ki-67 labeling index has become the most widely used method for measuring proliferation in the diagnostic setting. Different studies have investigated the prognostic potential of Ki-67 in gliomas as reviewed by Chen et al.18. The Ki-67 LI increases with increasing WHO grade19,20,21,22,23 and high Ki-67 LI is associated with poor overall survival (OS) in patients with lower grade gliomas or ependymomas24,25,26. In the glioblastoma patients, the prognostic role of Ki-67 LI is less clear. Some groups showed that a high Ki-67 LI was associated with improved OS27,28,29, whereas other groups reported that high levels of Ki-67 were associated with poor OS20,21,23,30. Yet other groups reported that Ki-67 LI was not associated with OS3,19,31,32,33,34. These previous studies on Ki-67 did not include MGMT promoter methylation status and post-surgical treatment in the survival analysis although these parameters have significant prognostic impact on the outcome in the glioblastoma patients.
The reported results may also be influenced by observer-based scoring, which is prone to intra- and inter-observer variation, e.g. due to different counting methods and subjective assessment of staining positivity. Vörös et al.35 reported that a semi-quantitative assessment of the reproducibility of the Ki-67 LI in breast cancer is an acceptable and reproducible method. Similar approaches based on automated quantification have also been used to assess Ki-67 LI in tumors in the brain; indicating that automated digital quantification may be more robust than semi-quantitative scoring performed by pathologists36,37,38.
Another aspect influencing the results in previous studies is the Ki-67-positive non-neoplastic cells within the tumor tissue. A considerable number of microglia and macrophages, with a certain proliferative potential, can be found in brain tumors, and especially high-grade gliomas are characterized by dense infiltration with activated microglial cells and macrophages, lymphocytic infiltrates, reactive astrocytic gliosis and vascular proliferation39,40. Thus, non-neoplastic cells and in particular activated microglia and macrophages contribute to the overall proliferative activity and Ki-67 labeling in these tumors and may influence results obtained in earlier studies41. We expected that we, by exclusion of the Ki-67 contribution from proliferating non-tumor cells would be able to assess the prognostic value of the true Ki-67 level in tumor cells in glioblastomas more precisely than previously. Therefore, we used a double immunohistochemical staining approach combining Ki-67 immunostaining with a cocktail of antibodies against CD45 (immune cells), CD31 (vascular structures), Iba-1 (microglia and macrophages) and smooth muscle actin (ASMA) (vascular structures), which made it possible to identify non-neoplastic Ki-67 positive cells in the investigated glioma tissues. This allowed us to obtain a more precise evaluation of the Ki-67 LI in the actual tumor cell fraction of the tumor tissue. Because we investigated central tumor tissue only, it was decided not to stain for glial cells and neurons. To obtain a representative Ki-67 LI for each tumor we used full sections—like in daily diagnostics—instead of tissue microarrays consisting of tissue cores being only 1–3 mm in diameter.
The aim of this study was to investigate the prognostic value of Ki-67 LI restricted to glial tumor cells using a digital computer-based quantification method and a double immunohistochemical staining approach for exclusion of non-neoplastic cells. This approach was used on a well-annotated population-based cohort of astrocytic tumors. Importantly, critical prognostic parameters such as IDH mutation, MGMT promoter methylation as well as post-surgical therapy were taken into account.
Materials and methods
Patients
From a population-based cohort of 433 patients in the Region of Southern Denmark we identified 181 glioblastoma patients (178 IDH-wildtype, 3 IDH-mutated) with a sufficient amount of viable tumor tissue for immunohistochemical analyses. For the purpose of comparison, tissue from 9 IDH-mutated diffuse astrocytomas and 9 IDH-mutated anaplastic astrocytomas were stained. Tissue were obtained from the local pathological department, where it was ruinously stored. All patients underwent initial surgery between 01.01.2005 and 31.12.2009, and no treatment was received prior to surgery. This cohort has been described thoroughly and has been used in previous biomarker studies40,42,43,44,45,46,47,48. Patient characteristics are listed in Table 1.
Immunohistochemistry
Immunohistochemical staining was carried out using the BenchMark Ultra IHC/ISH staining system (Ventana Medical Systems, Inc, AZ, USA). The primary antibody was Ki-67/ MIB-1 (monoclonal mouse antibody, Dako, no. M7240). To detect Ki-67 labeling in non-neoplastic cells a cocktail of antibodies against CD45 (monoclonal mouse antibody, Dako, no. M0701), CD31 (monoclonal mouse antibody, Dako, no. M0823), Alpha-smooth muscle actin (ASMA) (monoclonal rabbit antibody, Spring Bioscience no. M4710) and ionized calcium-binding adaptor molecule-1 (Iba1) (polyclonal rabbit antibody, Wako) was used for double immunohistochemical staining. For Ki-67 a detection system UVDAB-HRP plus amplification followed by application of the uDAB-kit (ref. no. 760–500) was used. For the other antibodies, we used the detection system UVRed-AP followed by application of the uRed-kit (ref. no. 760–501). Nuclear counter staining was performed using Hematoxylin II (Ventana Medical Systems, Tucson, AZ) at the BenchMark Ultra instrument. Finally, slides were washed, dehydrated, and coverslipped using a Tissue-Tek Film coverslipper (Sakura, Alphen aan den Rijn, The Netherlands). The slides were scanned on the Hamamatsu whole-slide scanner (Hamamatsu, Hamamatsu City, Japan).
MGMT assessment
The MGMT promoter status was determined using pyrosequencing (MGMT Pyro kit; Qiagen, Hilden, Germany) as described by the manufacturer. Briefly; DNA was purified from 10 lm paraffin slides using QIAamp DNA FFPE Tissue kit (Qiagen), and MGMT pyrosequencing was performed according to the kit instructions. Methylation percentages at four CpG sites were measured, if one or more sites had a methylation of 10% or higher, the tumor was considered as MGMT methylated.
Image analysis
The digital images were analyzed using the Visiopharm software module (Visiopharm, Hørsholm, Denmark). The regions of interest were manually outlined, excluding areas containing staining-artifacts, brain parenchyma, necrosis or large vessels. Sampling was performed using systematic uniform random sampling at 20 × magnification; all images were reviewed and non-tumor areas were manually outlined. Images were analyzed using an algorithm developed in the Tissuemorph APP-Control system. The algorithm identified all nuclei expressing Ki-67 and a 2.5 μm perimeter was subsequently grown around all detected nuclei. The exclusion cocktail identified non-tumor cells by a red-labelled cytoplasm, and nuclei surrounded by red-labelled cytoplasm were subsequently excluded from further analyses. It was pre-defined that only images including more than 50% viable tumor tissue were included in further analysis and that more than 5 useable images should be obtained for each tumor. Based on a sample fraction study it was shown that sampling in 10% or more of the tissue did not provide further information than sampling in 5% of the tissue (data not shown). As sampling in 10% or more was time-consuming, sampling in 5% of the tissue was chosen. Due to small amount of tissue, 27 tumors were re-sampled at a 20% fraction.
Statistical analyses
The non-parametric Kruskal–Wallis test was used for testing the difference in Ki-67 LI between the histological grades. Overall survival (OS) was defined as time from primary surgery until death or censoring (April 1st 2018). OS wasillustrated by Kaplan–Meier plots and differences were evaluated by log-rank tests. The median Ki-67 LI was pre-defined as the cut-off value. Due to the high number of tumors, immunostainings were performed in nine runs. Each run included a positive control for Ki-67 LI estimation. Run-to-run variations were analyzed; no significant variation was detected (p > 0.05).
Ethics
The study was approved by the local Committee on Health Research Ethics in Southern Denmark and the Danish Data Protection Agency. A dispensation was given by the local Committee on Health Research Ethics in Southern Denmark allowing colletion of data without informed consent from the patients. Use of the tissue was not prohibited in any of the patients according to the Danish Tissue Application Register. All methods were carried out in accordance with relevant guidelines and regulations.
Ethics approval
The local Committee on Health Research Ethics and the Danish Data Protection Agency approved the study.
Results
Staining patterns and software classifier
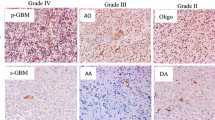
The immunohistochemical double staining identified Ki-67 positive nuclei in both neoplastic and non-neoplastic cells (Fig. 1A-F). IDH-mutated diffuse astrocytomas (WHO grade II) with low cellularity had only a few scattered Ki-67 positive tumor cells and moderate expression of exclusion-marker (Fig. 1A), whereas IDH-mutated anaplastic astrocytomas (WHO grade III) with moderate cellularity showed moderate expression of the exclusion-marker (Fig. 1B). In glioblastomas (WHO grade IV) the frequency of non-neoplastic cells varied from moderate (Fig. 1C) to high (Fig. 1D).
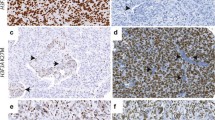
Immunohistochemical expression of Ki-67 (brown) and the exclusion-markers CD45, CD31, Iba1 and ASMA (red)—identified by a double immunohistochemical staining cocktail in astrocytic brain tumors. (A) IDH-mutated diffuse astrocytoma (DA) with low cellularity showing only a few scattered Ki-67 positive tumor cells not labeled by red (see insert). (B) IDH-mutated anaplastic astrocytoma (AA) with moderate cellularity showing higher presence of Ki-67 positive tumor cells not labeled by red. (C) IDH-mutated glioblastoma (GBM) and (D) IDH-wildtype glioblastoma with high cellularity and increased number of Ki-67 positive tumor cells not labeled by red. An increased fraction of non-tumor cells also expressed Ki-67 (see insert in C). The appearance of pseudopalisading necrosis with pink positive staining of microglia/macrophages (black asterix) and microvascular proliferations with pink positive staining of endothelial/smooth muscle cells (black arrow) are shown respectively in (C) and (D). (E + F) The staining was quantified by a software-based classifier identifying tumor cells having Ki-67 positive nuclei and not being labeled by red (blue arrowhead, blue label), non-tumor cells having Ki-67 positive nuclei and red cytoplasm (turquoise arrowhead, gray label) and supposed Ki-67 negative tumor cells not being labeled by red (green label). Magnification by × 20 (A-D) and × 80 (E–F). Scale bar indicates 100 µm (A-D), 25 µm (E–F).
Morphologically and immunohistochemically most non-neoplastic cells resembled activated microglia and tumor-infiltrating macrophages (Fig. 1A-D), but both small and large vessels were also identified (Fig. 1A-D). The trained software-based classifier easily detected Ki-67 positive and negative nuclei in both neoplastic and non-neoplastic cells (Fig. 1E–F).
Ki-67 LI
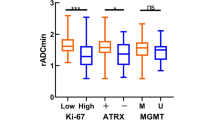
In astrocytic IDH-mutated tumors median Ki-67 LI was 2.7%, 6.4% and 27.5% in WHO grade II, III and IV tumors. There was a significant difference between WHO grade II and IV tumors (p = 0.004) and between WHO grade III and IV tumors (p = 0.04). In IDH-wildtype glioblastomas median Ki-67 was 24.4%. This was not significantly different from IDH-mutated glioblastomas (p = 0.9) (Fig. 2).
In patients with IDH-wildtype glioblastomas, median OS was 10 months in patients with low Ki-67 LI and 8 months in patients with high Ki-67 LI (p = 0.9). This was not significant in neither univariate (HR = 1.0, p = 0.9) nor multivariate analysis (HR = 1.29, p = 0.15) (Fig. 3A). Similar results were obtained when MGMT status was included (Fig. 3B) and when IDH-mutated glioblastomas were excluded (HR = 1.23, p = 0.23) (Table 2).
An optimal cut-point analysis was performed. It was not possible to identify a cut-point that provided more information than the median (data not shown).
In the subgroup of patients receiving treatment with curative intend (n = 97) median Ki-67 LI was 25% (0.01–50%). Ki-67 was not associated with OS in these patients in univariate (HR = 0.98, p = 0.9) or multivariate analyses (Table 2, Fig. 3C-D).
Discussion
In this study we investigated the prognostic value of the Ki-67 LI in glioblastoma patients from a population-based cohort including MGMT promoter methylation and post-surgical treatment in the survival analysis. As an important methodological aspect we excluded the contribution of non-neoplastic cells to the Ki-67 LI and used digital quantification of full sections in order to minimize intra-observer bias and bias from evaluating small tumor areas, both being known pitfalls in biomarker studies38,49,50. Moreover, the use of full sections reflects daily diagnostics for possible glioblastomas, where most biomarkers are evaluated on full sections.
In accordance with previous results, we found that the Ki-67 LI increases with increasing WHO grade19,20,21,22,23,51. As expected, we also found that cells with microglial cell and macrophage morphology expressed Ki-67 and that the fraction of these cells increased with increasing WHO grade. This is in line with a study by Klein el al.41, who reported on a immunohistochemical double-labeling study with Ki-67 as proliferation-marker and Ki-M1P (CD68) as microglia marker in 40 astrocytomas WHO grade I-IV. The authors showed that proliferating microglia cells are present in all WHO grades and that the proliferative activity in microglia increased with increasing WHO grade. As the degree of microglial cell infiltration may differ between individual gliomas, we speculate that the Ki-67 contribution from microglial cells may have contributed to the divergent results reported for Ki-67 LI and outcome of glioma patients in previous studies. Therefore, we excluded the proliferative microglial cells, macrophages and other non-neoplastic cells in the tumor tissue in the present study, and specifically focused on the evaluation of proliferative tumor cells only. This is supposed to provide a more accurate estimate of the proliferative capacity of the tumor cells per se and thereby also the patient outcome.
In patients with glioblastomas, we found no correlation between Ki-67 LI and OS. This is in accordance with several other reports3,19,31,32,33,34, but in contrast to other studies20,21,23,27,28,29,30. We divided our cohort of glioblastoma patients at the median Ki-67 LI (24%), which is similar to other groups who included the contribution of Ki-67 from non-tumour cells21,23. Interestingly; Moskowitz et al.31 used the Ki-67 LI as a continuous parameter and Kuriyama et al.34 used quartiles, but neither of these groups identified an association between the Ki-67 LI and OS. To address a potential bias due to different cutoff values, we performed an explorative optimal cutoff analysis in our cohort; however, we were unable to identify an association between Ki-67 LI and outcome for glioblastoma patients despite the use of different cutoffs.
A strength in our study is the inclusion of MGMT promoter methylation status and information on post-surgical treatment in the multivariate analyses, as both parameters are known to influence survival. Other groups investigating the prognostic value of Ki-67 LI in glioblastomas included only MGMT status29, adjuvant chemotherapy27,30,33 or none of these important parameters in their analyses3,20,21,23,28,31,32. Yang et al. was the only group to33 include both MGMT status and post-surgical treatment in the multivariate analyses. They reported on 254 glioblastoma patients, however; no information regarding IDH-status was reported. Further; although the Ki-67 immunohistochemical stainings were performed on whole slides—scoring was performed by pathologists using a 5-point scale. Despite these methodological differences compared to our study, the authors reported that Ki-76 LI was not associated with OS, a result similar to ours. It should be noticed that all studies reporting that Ki-67 LI is associated with OS, a finding that we could not validate in our population-based patient cohort, lack information of MGMT promoter methylation status and post-surgical treatment in the multivariate analyses. Therefore, the prognostic value of Ki-67 LI in glioblastomas is most likely very limited.
Several groups, including our group38, have reported that the reproducibility of Ki-67 LI varies between studies. This may be due to inter- and intra-observer variability when detecting Ki-67 LI in tumors35,50. Polley et al. investigated intra- and inter-laboratory variability in 100 breast cancer patients between 8 different laboratories. Each laboratory scored Ki-67 as percentage of positively stained invasive tumor cells using its own method. The authors found high intra-laboratory reproducibility, whereas the inter-laboratory reproducibility was modest. In contrast to the present study, Polley et al. investigated manual scoring only. Our group has recently reported that manual scoring has limited reproducibility in glioma patients38, and we thus decided to use digital quantification in the present study.
Conclusion
We found no association of Ki-67 LI with overall survival in IDH-wildtype glioblastomas, independent of the employed cutoff values and even when MGMT promoter methylation status and postsurgical treatment were included in the survival analysis. Due to the small number of IDH-mutated glioblastomas it is not possible to assess the prognostic value of Ki-67 in these patients. For the first time the prognostic value of Ki-67 was addressed using digital quantification of full sections excluding Ki-67 positive nuclei from non-neoplastic cells. Over-interpretation of the prognostic potential of Ki-67 LI in the clinico-pathological setting should therefore be avoided.
Data availability
Data is available from the corresponding author upon reasonable request.
References
Zhou, Y. H. et al. Establishment of prognostic models for astrocytic and oligodendroglial brain tumors with standardized quantification of marker gene expression and clinical variables. Biomark. Insights 5, 153–168. https://doi.org/10.4137/bmi.s6167 (2010).
Wrensch, M. et al. Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro Oncol. 8, 12–26. https://doi.org/10.1215/s1522851705000268 (2006).
Tsidulko, A. Y. et al. Prognostic relevance of NG2/CSPG4, CD44 and Ki-67 in patients with glioblastoma. Tumour Boil. 39, 1010428317724282. https://doi.org/10.1177/1010428317724282 (2017).
Hegi, M. E. et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 26, 4189–4199 (2008).
Malmstrom, A. et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 13, 916–926. https://doi.org/10.1016/s1470-2045(12)70265-6 (2012).
Reifenberger, G. et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int. J Cancer 131, 1342–1350. https://doi.org/10.1002/ijc.27385 (2012).
Wick, W. et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol. 13, 707–715. https://doi.org/10.1016/s1470-2045(12)70164-x (2012).
Reifenberger, G., Wirsching, H. G., Knobbe-Thomsen, C. B. & Weller, M. Advances in the molecular genetics of gliomas—implications for classification and therapy. Nat. Rev. Clin. Oncol. 14, 434–452. https://doi.org/10.1038/nrclinonc.2016.204 (2017).
Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol 131, 803–820. https://doi.org/10.1007/s00401-016-1545-1 (2016).
Gerdes, J., Schwab, U., Lemke, H. & Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31, 13–20 (1983).
Artigiani Neto, R., Logullo, A. F., Stavale, J. N. & Lourenco, L. G. Ki-67 expression score correlates to survival rate in gastrointestinal stromal tumors (GIST). Acta Cir. Bras. 27, 315–321 (2012).
Fisher, G. et al. Prognostic value of Ki-67 for prostate cancer death in a conservatively managed cohort. Br. J. Cancer 108, 271–277. https://doi.org/10.1038/bjc.2012.598 (2013).
Krabbe, L. M. et al. Prospective analysis of Ki-67 as an independent predictor of oncologic outcomes in patients with high grade upper tract urothelial carcinoma. J. Urol. 191, 28–34. https://doi.org/10.1016/j.juro.2013.07.012 (2014).
Ma, X. et al. Ki67 Proliferation index as a histopathological predictive and prognostic parameter of oral mucosal melanoma in patients without distant metastases. J. Cancer 8, 3828–3837. https://doi.org/10.7150/jca.20935 (2017).
Pascale, M. et al. The proliferation marker Ki67, but not neuroendocrine expression, is an independent factor in the prediction of prognosis of primary prostate cancer patients. Radiol. Oncol. 50, 313–320. https://doi.org/10.1515/raon-2016-0033 (2016).
Preusser, M. et al. Ki67 index in intracranial ependymoma: A promising histopathological candidate biomarker. Histopathology 53, 39–47. https://doi.org/10.1111/j.1365-2559.2008.03065.x (2008).
Yan, J. et al. Relation between Ki-67, ER, PR, Her2/neu, p21, EGFR, and TOP II-alpha expression in invasive ductal breast cancer patients and correlations with prognosis. Asian Pac. J. Cancer Prev. APJCP 16, 823–829 (2015).
Chen, W. J., He, D. S., Tang, R. X., Ren, F. H. & Chen, G. Ki-67 is a valuable prognostic factor in gliomas: Evidence from a systematic review and meta-analysis. Asian Pac. J. Cancer Prev. APJCP 16, 411–420 (2015).
Arshad, H., Ahmad, Z. & Hasan, S. H. Gliomas: Correlation of histologic grade, Ki67 and p53 expression with patient survival. Asian Pac. J. Cancer Prev. APJCP 11, 1637–1640 (2010).
Shen, C., Yang, L. & Yuan, X. Endothelin B receptor expression in human astrocytoma: Association with clinicopathological variables and survival outcomes. Int. J. Neurosci. 121, 626–631. https://doi.org/10.3109/00207454.2011.602808 (2011).
Yoshida, Y. et al. The expression level of sphingosine-1-phosphate receptor type 1 is related to MIB-1 labeling index and predicts survival of glioblastoma patients. J. Neurooncol. 98, 41–47. https://doi.org/10.1007/s11060-009-0064-5 (2010).
Wang, X. et al. Histopathological findings in the peritumoral edema area of human glioma. Histol. Histopathol. 30, 1101–1109. https://doi.org/10.14670/hh-11-607 (2015).
Watanabe, Y. et al. Expression of phosphoprotein enriched in astrocytes 15 kDa (PEA-15) in astrocytic tumors: A novel approach of correlating malignancy grade and prognosis. J. Neurooncol. 100, 449–457. https://doi.org/10.1007/s11060-010-0201-1 (2010).
Duregon, E. et al. Ki-67 proliferation index but not mitotic thresholds integrates the molecular prognostic stratification of lower grade gliomas. Oncotarget 7, 21190–21198. https://doi.org/10.18632/oncotarget.8498 (2016).
Armstrong, T. S., Vera-Bolanos, E., Bekele, B. N., Aldape, K. & Gilbert, M. R. Adult ependymal tumors: Prognosis and the M. D. Anderson Cancer Center experience. Neuro-oncology 12, 862–870. https://doi.org/10.1093/neuonc/noq009 (2010).
Shaffrey, M. E. et al. The Ki-67 labeling index as a prognostic factor in Grade II oligoastrocytomas. J. Neurosurg. 102, 1033–1039. https://doi.org/10.3171/jns.2005.102.6.1033 (2005).
Wong, E. et al. Cut-point for Ki-67 proliferation index as a prognostic marker for glioblastoma. Asia Pac. J. Clin. Oncol. https://doi.org/10.1111/ajco.12826 (2018).
Bredel, M. et al. High expression of DNA topoisomerase IIalpha and Ki-67 antigen is associated with prolonged survival in glioblastoma patients. Eur. J Cancer (Oxford, England: 1990) 38, 1343–1347 (2002).
Yue, Q. et al. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J. Neurooncol. 116, 251–259. https://doi.org/10.1007/s11060-013-1314-0 (2014).
Li, S. W. et al. Prognostic factors influencing clinical outcomes of glioblastoma multiforme. Chin. Med. J. 122, 1245–1249 (2009).
Moskowitz, S. I., Jin, T. & Prayson, R. A. Role of MIB1 in predicting survival in patients with glioblastomas. J. Neurooncol. 76, 193–200. https://doi.org/10.1007/s11060-005-5262-1 (2006).
Okita, Y. et al. Pathological findings and prognostic factors in recurrent glioblastomas. Brain Tumor Pathol. 29, 192–200. https://doi.org/10.1007/s10014-012-0084-2 (2012).
Yang, P. et al. Management and survival rates in patients with glioma in China (2004–2010): A retrospective study from a single-institution. J. Neurooncol. 113, 259–266. https://doi.org/10.1007/s11060-013-1103-9 (2013).
Kuriyama, H. et al. Prognostic significance of an apoptotic index and apoptosis/proliferation ratio for patients with high-grade astrocytomas. Neuro Oncol. 4, 179–186 (2002).
Voros, A. et al. The use of digital images improves reproducibility of the ki-67 labeling index as a proliferation marker in breast cancer. Pathol Oncol. Res. POR 20, 391–397. https://doi.org/10.1007/s12253-013-9708-z (2014).
Lopez, X. M. et al. Clustering methods applied in the detection of Ki67 hot-spots in whole tumor slide images: An efficient way to characterize heterogeneous tissue-based biomarkers. Cytometry Part A 81, 765–775. https://doi.org/10.1002/cyto.a.22085 (2012).
Slodkowska, J. et al. Accuracy of a remote quantitative image analysis in the whole slide images. Diagn. Pathol. 6(1), 20. https://doi.org/10.1186/1746-1596-6-s1-s20 (2011).
Nielsen, L. A. G. et al. Evaluation of the proliferation marker Ki-67 in gliomas: Interobserver variability and digital quantification. Diagn. Pathol. 13, 38. https://doi.org/10.1186/s13000-018-0711-2 (2018).
Charles, N. A., Holland, E. C., Gilbertson, R., Glass, R. & Kettenmann, H. The brain tumor microenvironment. Glia 60, 502–514 (2012).
Sorensen, M. D., Dahlrot, R. H., Boldt, H. B., Hansen, S. & Kristensen, B. W. Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathol. Appl. Neurobiol. https://doi.org/10.1111/nan.12428 (2017).
Klein, R. & Roggendorf, W. Increased microglia proliferation separates pilocytic astrocytomas from diffuse astrocytomas: A double labeling study. Acta Neuropathol. 101, 245–248 (2001).
Dahlrot, R. H. et al. Prognostic value of Musashi-1 in gliomas. J. Neurooncol. 115, 453–461. https://doi.org/10.1007/s11060-013-1246-8 (2013).
Dahlrot, R. H. et al. Clinical value of CD133 and nestin in patients with glioma: A population-based study. Int. J. Clin. Exp. Pathol. 7, 3739–3751 (2014).
Dahlrot, R. H., Kristensen, B. W., Hjelmborg, J., Herrstedt, J. & Hansen, S. A population-based study of high-grade gliomas and mutated isocitrate dehydrogenase 1. Int. J. Clin. Exp. Pathol. 6, 31–40 (2013).
Dahlrot, R. H., Kristensen, B. W., Hjelmborg, J., Herrstedt, J. & Hansen, S. A population-based study of low-grade gliomas and mutated isocitrate dehydrogenase 1 (IDH1). J. Neurooncol. 114, 309–317. https://doi.org/10.1007/s11060-013-1186-3 (2013).
Hermansen, S. K., Dahlrot, R. H., Nielsen, B. S., Hansen, S. & Kristensen, B. W. MiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomas. J. Neurooncol. 111, 71–81. https://doi.org/10.1007/s11060-012-0992-3 (2013).
Music, D. et al. Expression and prognostic value of the WEE1 kinase in gliomas. J. Neurooncol. https://doi.org/10.1007/s11060-015-2050-4 (2016).
Petterson, S. A. et al. High levels of c-Met is associated with poor prognosis in glioblastoma. J. Neurooncol. 122, 517–527. https://doi.org/10.1007/s11060-015-1723-3 (2015).
Grzybicki, D. M. et al. Interobserver variability associated with the MIB-1 labeling index: High levels suggest limited prognostic usefulness for patients with primary brain tumors. Cancer 92, 2720–2726 (2001).
Polley, M. Y. et al. An international Ki67 reproducibility study. J. Natl Cancer Inst. 105, 1897–1906. https://doi.org/10.1093/jnci/djt306 (2013).
Abd El Atti, R. M., Abou Gabal, H. H., Osman, W. M. & Saad, A. S. Insights into the prognostic value of DJ-1 and MIB-1 in astrocytic tumors. Diagn. Pathol. 8, 126. https://doi.org/10.1186/1746-1596-8-126 (2013).
Acknowledgements
We acknowledge the excellent laboratory work by the technicians Helle Wohlleben and Tanja Dreehsen Højgaard.
Funding
Region of Southern Denmark Research grant.
Author information
Authors and Affiliations
Contributions
Study design: J.A.B., R.H.D., B.W.K. Acquisition of data: J.A.B., R.H.D., M.D.S., A.M.R., J.K.P. Analysis and interpretation of data: J.A.B., R.H.D., G.R., S.H., B.W.K. Drafting of manuscript: J.A.B., R.H.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dahlrot, R.H., Bangsø, J.A., Petersen, J.K. et al. Prognostic role of Ki-67 in glioblastomas excluding contribution from non-neoplastic cells. Sci Rep 11, 17918 (2021). https://doi.org/10.1038/s41598-021-95958-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95958-9
- Springer Nature Limited
This article is cited by
-
Radiomics and visual analysis for predicting success of transplantation of heterotopic glioblastoma in mice with MRI
Journal of Neuro-Oncology (2024)
-
An initial study on the predictive value using multiple MRI characteristics for Ki-67 labeling index in glioma
Journal of Translational Medicine (2023)
-
Cardiovascular complications in a diabetes prediction model using machine learning: a systematic review
Cardiovascular Diabetology (2023)
-
Low expression of Ki-67/MIB-1 labeling index in IDH wild type glioblastoma predicts prolonged survival independently by MGMT methylation status
Journal of Neuro-Oncology (2023)
-
A circular RNA derived from GLIS3 accelerates the proliferation of glioblastoma cells through competitively binding with miR-449c-5p to upregulate CAPG and GLIS3
BMC Neuroscience (2022)