Abstract
Catharanthus roseus (L.) G. Don (Apocynaceae) is a well-studied herb renowned for its in vitro culture as a source of the anti-cancer alkaloid, vincristine. However, despite the recognized advantages of triploid cells over diploid cells in terms of productivity, the triploid endosperm tissue of this important medicinal plant has not been utilized for in vitro culture initiation. In this investigation, zygotic embryos and endosperm tissues were cultured on Murashige and Skoog (MS) medium supplemented with various combinations of auxins and cytokinins. The medium containing 2.50 µM 6-Benzyladenine (BA) and 1.25 µM 2,4-Dichlorophenoxyacetic acid (2,4-D) proved to be the most effective for callus and cell culture formation. Ploidy analysis using ploidy analyzer confirmed that the endosperm-derived callus exhibited mixoploid, while the embryo-derived callus remained diploid. Liquid Chromatography Mass Spectrometry (LC–MS) analysis of callus and cell cultures grown on MS media with different combinations of auxins, cytokinins, elicitors, and precursors (both biotic and abiotic) revealed the accumulation of vincristine. Notably, treatment with a biotic elicitor derived from Aspergillus niger (300 mg/l) demonstrated superior efficacy in promoting the maximum accumulation of vincristine in endosperm-derived callus and cell biomass. These findings hold promise for the sustainable production of the anti-cancer alkaloid vincristine from endosperm-derived callus and cell cultures of Catharanthus roseus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, the global population has seen exponential growth leading to increased reliance on plants for sustenance, medication, and textile production. One crucial defense against various perilous diseases worldwide lies in the therapeutic properties of plants [1]. Globally, over 85% of traditional medicinal treatments rely on plants or their extracts [2, 3]. However, the intricate structures and high cost of bioactive compounds found in plants pose challenges for chemical synthesis [4, 5]. Several factors contribute to the complexity of synthesizing bioactive compounds in plants, including cultivation duration, seasonal variations, variability in medicinal content, insufficient techniques for extraction and standardization, and a limited understanding of plant physiology [6, 7].
The demand for raw materials to extract phytochemicals has risen steadily in response to the growing global need for plant-derived medicines and formulations [6]. To address the persistent issue of availability of medicinal plants, alternative approaches are needed to ensure a steady supply of high-quality raw materials from sustainable sources. Plant cell and tissue culture technology offer promising avenues for producing these phytochemicals in vitro, essentially functioning as living chemical resource factories [8, 9]. Micro-propagated plants provide uniform, sterile, and compatible plant material for identifying and characterizing active constituents [9,10,11]. Moreover, compounds produced from tissue cultures may be easier to purify through simple extraction methods due to the absence of significant pigment content, potentially reducing production and processing costs [12, 13]. In plant tissue culture technology, various methods such as callus cultures, cell cultures, hairy root cultures, and genetic modifications can be employed to extract potentially beneficial metabolic components [14]. The cell culture technique provides a straightforward means to produce haploid and triploid cells, which are crucial systems for enhancing the in vitro production of medicinal components [15].
Consequently, in the current study, we focused on the triploid nature of the explant to enhance the production of secondary metabolites. Compared to their diploid counterparts, triploid plants and triploid cell biomass exhibit faster growth rates and accumulate more metabolites [16]. Triploid plants and triploid cell biomass have more vigorous growth and accumulation of metabolites than their diploid counterparts [16, 17]. Traditionally, triploids are produced by crossing-induced superior tetraploids and diploids. This approach is tedious and lengthy (especially for tree species). In contrast, the regeneration of plants from the endosperm (a naturally occurring triploid tissue) offers a direct, single-step approach to triploid production [18].
Catharanthus roseus (L.) G. Don (Family: Apocynaceae) (2n = 16) commonly called as Madagascar Periwinkle native to Madagascar. The plant is mainly considered as the rich source of various alkaloids, which are distributed in all parts of the plant. Total of about 130 alkaloids are reported from Catharanthus roseus, of which, 25 alkaloids belong to the indole group [19]. All the alkaloids showed diverse array of activities against large number of diseases. Of the 25 indole alkaloids, two namely vinblastine and vincristine are mainly present in the aerial parts. These alkaloids are reported to have found extensive application in the treatment of human neoplasma. Vincristine sulphate is very effective for treating acute leukaemia in children and lymphocytic leukemia [20]. It is also effective against Hodgkin’s disease, Wilkins’s tumor, neuroblastoma and reticulum cell sarcoma [21]. Our current efforts aimed to enhance the production of anti-cancer indole alkaloid vincristine by cultivating Catharanthus roseus endosperm and embryo cultures in vitro.
2 Materials and methods
2.1 Plant materials, surface sterilization, and culture conditions
Pods of the Catharanthus roseus (L.) G. Don plant (1-year-old) were collected from Botanical Garden, Department of Botany, Savitribai Phule Pune University, Pune at immature, mature, and fully mature stages. The collection of the plants used in study complies with local or national guidelines. The pods were washed in running tap water and sterile distilled water for 10 min followed by surface sterilization using 0.1% HgCl2 solution for 7 min. Then the endosperm and embryo were excised from the seeds (Fig. 1A–C) and were cultured in test tubes (25 × 150 cm) each containing 10 ml MS medium [22]. The pH of the MS medium was adjusted to 5.7 ± 0.1 prior to autoclaving at 121 °C for 15 min containing 0.8% (w/v) of agar (Hi Media, Mumbai). The cultures were maintained at 25 ± 2 °C under an 8 h dark/16 h light photoperiod (30 μmol m−2 s−1) at 60–70% relative humidity.
Excision of embryo and endosperm from Catharanthus roseus seeds and callus induction. A: Seed excised from pod; B: Endosperm excised from seed; C: Embryo excised from seed; D: Endosperm derived callus on MS + 1.25 µM 2,4-D + 2.50 µM BA; E: Embryo derived callus on MS + 1.25 µM 2,4-D + 2.50 µM Kin; F: Cell culture on MS + 1.25 µM BA + 2.50 µM 2,4-D
2.2 Callus induction and cell culture
White or milky endosperm and green shiny embryos were dissected out from surface sterilized pods and were cultured separately on MS medium with 3% sucrose, coconut milk (10, 15, 20, 25%), casein hydrolysate (100 mg/l), and auxins (NAA, IAA, and 2,4-D; 0.5–2.5 µM) and cytokinins (BA, Kin, and TDZ; 2.5–15.0 µM) alone and in combinations. The embryo-derived calli began to proliferate on eighth day of inoculation, while the endosperm explants induced callus on fifteenth day of inoculation. After 21 days, both cultures were maintained on the parental MS media. Fresh and dry weights were used to calculate the growth rate of callus.
Two g of 8-week-old endosperm and embryo-derived callus were chopped and inoculated in 250 ml conical flasks with 40 ml MS liquid medium supplemented with combinations of cytokinins and auxins. Cultures were maintained on a rotary shaker at 80 rpm in controlled conditions for 21 days. The growth of cultures was measured based on fresh weight and dry weight.
2.3 Ploidy level analysis
About 56-day-old callus, obtained from embryo and endosperm explants were subjected to ploidy analysis using a ploidy analyzer (Partec PA-II, GmbH, Münster, Germany). The endosperm and embryo-derived callus (0.5 g) were chopped separately in triplicates. To this cell biomass, 0.2 ml of nuclei extraction buffer (CyStain PI Absolute P) was added and allowed to stand for 1 min. Then 0.8 ml of Precise P staining buffer (CyStain UV) was added to make the final volume 1.0 ml. Then this one ml solution with cell biomass was filtered through a 0.22 µ membrane filter and placed in a cuvette for ploidy analysis using a Ploidy analyzer.
2.4 Elicitors and precursors feeding
Pure cultures of Aspergillus niger (NCIM 545), Helminthosporium sp. (NCIM 1280), and Alternaria sp. (NCIM 1079) were procured from The National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory (NCL), Pune, India. Fungal strains obtained were cultivated for 28 days on a liquid potato-dextrose medium and were autoclaved for 20 min at 121 °C. Then the mycelial mats were collected and dried for 48 h in a hot air oven at 60 °C and ground to fine powder employed as biotic elicitors. Yeast extract powder (Type I) and chitin [poly (N-acetyl-1,4-D-glucopyranosamine)] were obtained from Hi Media, Mumbai, India. The concentrations of all the biotic elicitors were added at 100, 200, 300, 400, and 500 mg/l in the medium.
Potassium chloride (KCl); polyethylene glycol-6000 (PEG-6000), mannitol, sorbitol, and signaling agent salicylic acid were used as abiotic elicitors. The callus and cell cultures were exposed to different concentrations of salicylic acid (0–250 µM); mannitol and sorbitol (0–500 mM); PEG-6000 (0–5 mM) and KCl (0–200 mM) in the respective medium. Callus and cell cultures were exposed to precursors like pyruvic acid, tryptophan, and α-Keto-glutaric acid (0–500 mg/l) in liquid MS medium fortified with 1.25 µM BA in conjunction with 2.50 µM 2,4-D.
2.5 Extraction and estimation of vincristine
The dried, powdered sample (1000 mg) was placed in a test tube and extracted three times over 12 h with 20 ml of methanol each time. After that, the test tube was placed in a DL-60D ultrasonic bath running at 80 W for 60 min. Using a rotary evaporator, the extracted mixture was concentrated to 2 ml. The concentrate was then filtered through a sterile 0.22 µM membrane filter and the filtrate was stored at -80 °C for further use.
Quantitative analysis of vincristine in the samples was done using the LC–MS model Agilent 1290 Infinity Series RRLC-MS interfaced to an Agilent 6538 LC/MS Q-TOF (Agilent Technologies, USA). A volume of 20 µl of each sample was injected into ZORBAX Eclipse plus reversed-phase column (C18, 2.1 mm × 50 mm) of 1.8 µm particle size. The column temperature was maintained at 40 °C. The mobile phase comprised solvent A [Water: 0.1% Trifluoroacetic acid (TFA)] and Solvent B acetonitrile: TFA (ACN: 0.1% TFA). The vincristine (Fig. 2A) content was quantified by comparing them with standard vincristine (Fig. 2B) expressed in terms of ng g−1 DW.
2.6 Statistical analysis
Each experiment had a minimum of three repetitions and was conducted using a completely randomized design. To find significant differences between means, analysis of variance (ANOVA) was used to evaluate the data. The software package SPSS (version 9.1) was used to compare means that differed significantly using Duncan's Multiple Range Test (DMRT) at the 5% probability level. Data variation is represented as mean ± SE.
3 Results
3.1 Callus induction and ploidy analysis
Various permutations and combinations of auxins and cytokinins were tried for callus induction. Endosperm derived from immature and fully mature seeds when treated with auxins and cytokinins did not respond to callus induction. Among the different concentrations of auxins and cytokinins, MS medium fortified with 2.50 µM Kin + 1.25 µM 2,4-D was found superior for callus induction and growth from endosperm and embryo explants (Fig. 1D, E; Supplementary Table 1). The ploidy analysis showed that the endosperm-derived callus was found to be mixoploid and the embryo-derived callus was diploid.
3.2 Effect of plant growth regulators on growth and alkaloid accumulation in callus cultures
The addition of cytokinins (BA, Kin, and TDZ) enhanced the proliferation and growth of the endosperm as well as the embryo-derived callus (Supplementary Table 2). Among the different types and concentrations of cytokinins, 5.0 µM BA was found to be optimum for the growth of callus. The growth of an embryo and endosperm-derived callus varies among the types and concentrations of auxins used in the MS medium (Supplementary Table 3). Among the different concentrations of auxins, the maximum dry weight (0.27 ± 0.1 gm DW) of endosperm-derived callus was obtained on MS medium fortified with 7.5 µM 2,4-D. The addition of cytokinins in combination with auxins resulted in a declined growth of the embryo and endosperm-derived callus (Supplementary Table 4). Among the different treatments, Kin in combination with IAA was found to be better for the growth of callus derived from both types of explants.
Callus cultured on medium without plant growth regulator did not show accumulation of vincristine (Supplementary Table 2). Increasing concentration of BA and Kin in the medium resulted in increased accumulation of vincristine content in both the calli. Among the different types and concentrations of cytokinins, MS medium fortified with 10 µM Kin was found to be superior for accumulation of vincristine in the embryo (44.4 ± 0.6 ng g−1 DW) and endosperm (55.3 ± 1.7 ng g−1 DW) derived callus (Supplementary Table 2; Fig. 3). Supplementation of TDZ was found to be inhibitory for the accumulation of vincristine.
Increasing concentration of auxins resulted in increased accumulation of vincristine up to 7.5 µM concentrations. Further higher concentrations of auxin-containing medium resulted in a lower accumulation of vincristine in the callus. Among the different auxins, the addition of 7.5 µM NAA greatly enhanced the production of vincristine in the endosperm (87.7 ± 0.7 ng g−1 DW) and embryo (57.1 ± 2.3 ng g−1 DW) derived callus as compared to control (Supplementary Table 3). Medium fortified with NAA showed lower biomass accumulation but the accumulation of vincristine was more as compared to IAA and 2,4-D containing medium.
Medium fortified with cytokinins and auxins showed an accumulation of vincristine in both the calli. Among the different combinations, MS medium with 1.25 µM BA and 2.50 µM 2,4-D was found to be superior for accumulation of vincristine content (90.0 ± 2.9 ng g−1 DW) in the endosperm-derived callus and 81.3 ± 1.6 ng g−1 DW in embryo-derived callus culture (Supplementary Table 4).
3.3 Cell culture
3.3.1 Production of cell cultures
MS + 1.25 µM BA + 2.50 µM 2,4-D was found to be optimum for the accumulation of vincristine in the callus obtained from endosperm and embryo explants (Fig. 1F; Supplementary Table 4). Therefore, this medium was used as a parental medium to produce cell culture and for further treatments using cell culture.
3.3.2 Effect of plant growth regulators on growth and alkaloid accumulation in cell cultures
Among the different types and concentrations of cytokinins, 7.5 µM Kin was found to be optimum for cell culture growth. The DW of endosperm-derived cell biomass on MS basal medium was 0.45 ± 0.02 g. Similarly, embryo-derived cell culture showed about 0.41 ± 0.01 gm DW per culture (Supplementary Table 5). The addition of BA in the medium supported the growth of cell cultures but the response was found to be less than that of Kin, whereas the TDZ-containing medium showed an inhibitory effect (Supplementary Table 5). Cell culture grown on medium without plant growth regulator did not show accumulation of alkaloids. Among the different concentrations of cytokinins, MS medium fortified with 7.5 µM BA was found superior for accumulation of vincristine in the embryo (67.4 ± 1.2 ng g−1 DW) and endosperm (86.3 ± 2.9 ng g−1 DW) derived cell biomass (Supplementary Table 5). Kin (7.5 µM) was also found beneficial in enhancing the accumulation of vincristine in endosperm-derived cell suspension culture (63.7 ± 1.2 ng g−1 DW) and in embryo-derived cell biomass (58.4 ± 1.1 ng g−1 DW).
Among the different concentrations of auxins, the maximum biomass (0.79 ± 0.01 g) was obtained on MS medium fortified with 7.5 µM 2,4-D in endosperm-derived cell culture. Similarly, embryo-derived cell culture showed maximum biomass accumulation on the same medium. Among auxin-supplemented medium, the IAA-containing medium (7.5 µM) showed maximum accumulation of vincristine in endosperm-derived cell suspension culture (75.1 ± 1.2 ng g−1 DW) and embryo-derived cell biomass (70.7 ± 1.6 ng g−1 DW). The addition of 7.5 µM NAA was also found effective for enhancing the production of vincristine in the cell biomass derived from the endosperm (74.2 ± 0.5 ng g−1 DW) and embryo (72.3 ± 1.2 ng g−1 DW). Addition of 2,4-D in the medium promotes the biomass production but failed to enhance the vincristine synthesis (Supplementary Table 6).
The application of cytokinins in combination with auxins resulted in a decline in the growth of the cell culture derived from both explants (Supplementary Table 7). Among the different treatments, BA in combination with 2,4-D was found to be better for the growth of cell cultures derived from both types of explants (Supplementary Table 7). Among the different combinations, MS + 1.25 µM BA + 2.50 µM 2,4-D was found to be superior for the accumulation of vincristine in the cell suspension culture of endosperm-derived cell biomass (98.1 ± 1.3 ng g−1 DW) and embryo-derived cell biomass (93.4 ± 1.2 ng g−1 DW) (Supplementary Table 7). Among the different combinations, BA in conjunction with 2,4-D was found to be effective for the growth of endosperm as well as embryo-derived cell suspension culture.
3.3.3 Effect of abiotic elicitors
The effect of abiotic elicitors on growth of cell culture and alkaloid accumulation is depicted in Table 1. The results revealed that the addition of abiotic elicitors (supplementary Fig. 1) such as different concentrations of salicylic acid (50 – 250 µM), mannitol and sorbitol (100 – 500 mM), and KCl (40 – 200 mM) resulted in a decline in the growth of cell culture with increased concentrations. The addition of increasing PEG-6000 (1—5 mM), increased the growth of cell culture as compared to the other treatments. Among the different concentrations of salicylic acid, medium fortified with 150 mM showed a higher accumulation of vincristine (106.3 ± 1.3 ng g−1 DW). Among the different abiotic elicitors addition of salicylic acid was found to be more effective for the promotion of vincristine accumulation followed by the addition of 300 mg/l mannitol in the medium.
3.3.4 Effect of biotic elicitors
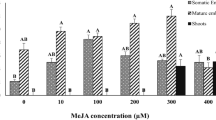
The growth of the cell suspension culture was negatively impacted by the addition of various biotic elicitors (Table 2, Supplementary Fig. 2). When compared to the control, a sharp decrease in the dry biomass formation was seen for all types and concentrations of biotic elicitor used. Among the different biotic elicitors used in the present study, the elicitor derived from Aspergillus niger showed maximum accumulation of vincristine (108.7 ± 1.0 ng g−1 DW) at 300 mg/l as compared with the control. Yeast extract also showed a significant increase (107.9 ± 1.3 ng g−1 DW) in vincristine content in the cell biomass. Supplementation of elicitor derived from Alternaria sp. negatively affects the vincristine accumulation in the cell biomass because as the concentration of the elicitor increased in the medium, vincristine accumulation decreased.
3.3.5 Effect of precursor feeding
Precursors feeding in the medium showed a negative effect on cell biomass production. The biomass production was decreased with the addition of an increased concentration of precursors in the medium (Table 3). About a 1.75-fold decrease in cell biomass production was observed with the addition of precursors in the medium. The addition of tryptophan was found beneficial for the accumulation of vincristine in the cell suspension cultures of Catharanthus roseus. Vincristine content increased with increasing concentration of tryptophan in the medium up to 300 mg/l concentration and decreased thereafter. Maximum vincristine (106.5 ± 1.7 ng g−1 DW) accumulation was observed in the medium fortified with 300 mg/l tryptophan. In the present study, supplementation of pyruvic acid improves the accumulation of vincristine (Table 3). The addition of 300 mg/l pyruvic acid resulted in about 1.1-fold increased accumulation of vincristine in the cell biomass of Catharanthus roseus. The addition of α-Keto-glutaric acid was not found suitable for promoting vincristine content in the cell suspension culture. In the present study, supplementation of different concentrations of abiotic elicitors, biotic elicitors, and precursors was not found to be promotive for the accumulation of indole alkaloids.
4 Discussion
In modern medicine, plants play multiple roles. They not only form the basis for therapeutic agents and serve as taxonomic markers for discovering new compounds but also preserve the traditional herbal medicine culture of human civilization [23, 24]. In vitro propagated plants provide a convenient source of homogeneous, sterile, and suitable plant material for biochemical analysis and identification of active ingredients [10, 12, 25]. Calluses or cells can be used to cultivate on a large scale, from which active compounds can be extracted [26]. In vitro cultures of Catharanthus roseus have been extensively studied as alternatives for producing therapeutically important alkaloids [27,28,29].
The endosperm, with its unique genesis, development, and ploidy level, holds special significance [30]. In our results, endosperm exhibited an adverse response in medium lacking plant growth regulators. This finding is consistent with Kiong et al. [31], who observed brownish discoloration and death of Cycas revoluta endosperm. Similar outcomes were reported earlier in Catharanthus roseus by Smith et al. [32] and in C. albus [33, 34]. Sustaining callus culture often requires various combinations of auxin with cytokinin in many medicinal plants, where the auxin-to-cytokinin ratio plays a crucial role in determining the type of culture established [35].
In our investigation, combination treatments (MS + 1.25 µM BA + 2.50 µM 2,4-D) produced superior outcomes in terms of callus induction and growth. These findings highlight the specific growth regulator requirements of plants and their endogenous availability. While embryo explants exhibited callus formation with several other combinations, the rate of callus induction and growth was notably faster with MS + 1.25 µM BA + 2.50 µM 2,4-D. Callus cultivated on MS basal medium did not demonstrate alkaloid accumulation. Different plant growth regulator regimes resulted in distinct effects on alkaloid biosynthesis. Prior studies demonstrate that replacing 2,4-D with IAA promoted callus to synthesize more ajmalicine and serpentine but no detectable vindoline and catharanthine [36]. Non-embryogenic callus obtained on MS + 1 mg/l NAA + 0.5 mg/l BA from nodal explants exhibited late (after 20 weeks) synthesis of vinblastine content in Catharanthus roseus [19].
In our investigation, among various permutations and combinations, 5.0 µM BA and 7.5 µM 2,4-D were found to be optimum for callus growth. Maximum vincristine content was obtained on MS supplemented with 1.25 µM BA and 2.50 µM 2,4-D. Datta and Srivastava [37] initiated callus cultures from different explants of different ages of Catharanthus roseus and noted only a detectable quantity of vinblastine compared to in vivo plants. Soleimani et al. [38] indicated that the maximum concentration of vincristine was produced at 1 mg/l 2,4-D + 0.5 mg/l Kin in Catharanthus roseus. Our results align with these findings. An interesting observation is that vincristine accumulation was consistently higher in endosperm-derived callus compared to embryo-derived callus.
Among different auxins, 2,4-D was found to be more effective for cell culture growth. Similar results were reported earlier in Catharanthus roseus [39,40,41]. Addition of cytokinins in combination with auxins resulted in a decline in cell culture growth derived from both embryo and endosperm explants. Among different treatments, BA in combination with 2,4-D was found to be better for cell culture growth established using callus derived from both explant types. Linh et al. [42] reported maximum callus growth on 2,4-D in combination with Kin from stem explant in Catharanthus roseus var. Quang Ninh. In contrast, earlier cell culture studies in Catharanthus roseus were conducted using MS medium fortified with Kin in combination with IAA [43,44,45].
Suspension cultures grown on MS basal medium did not exhibit alkaloid accumulation. MS medium supplemented with 7.5 µM Kin proved superior for vincristine accumulation in both endosperm-derived and embryo-derived calli of Catharanthus roseus. Additionally, MS + 1.25 µM BA + 2.50 µM 2,4-D demonstrated superior vincristine accumulation (98.1 ± 1.3 ng g−1 DW) in endosperm-derived cell suspension culture. The application of growth-limiting conditions has occasionally resulted in greater secondary metabolite accumulation [46, 47]. Earlier studies achieved growth limitations and increased alkaloid accumulation through low temperatures in Catharanthus roseus [48, 49], changes in phytohormone composition [50], or special induction media [51, 52]. Vincristine accumulation was significantly higher in cell culture compared to callus culture on medium containing cytokinin in combination with auxins as opposed to medium fortified with cytokinin or auxin alone. These results suggest that the growth and accumulation of alkaloids are influenced by the combination and concentrations of cytokinins and auxins in the medium.
Abiotic elicitors, typically represented by inorganic salts and physical conditions, play a critical role in the plant stress response [53,54,55]. Plants react to both abiotic and biotic stresses by triggering the accumulation of osmolytes, antioxidants, and antioxidative enzyme machinery to mitigate further damage [56,57,58]. Stress acts as an external constraint on cultured cells, impeding the normal synthesis of secondary metabolites [59, 60]. Our study demonstrates that abiotic elicitors such as salicylic acid, KCl, mannitol, sorbitol, and PEG-6000 significantly inhibit the growth of cell suspension cultures in Catharanthus roseus. Particularly noteworthy is the relatively higher growth observed in PEG-6000-treated cultures, possibly due to cellular absorption of PEG-6000. This suggests that stress factors exert an unfavourable impact on cell growth.
In our investigation, the addition of various types and concentrations of abiotic elicitors led to a higher accumulation of indole alkaloids in the cell culture of Catharanthus roseus. Among the different abiotic elicitors tested, medium supplemented with 150 mM salicylic acid exhibited the highest vincristine accumulation (106.3 ± 1.3 ng g−1 DW). Similar findings were reported by Vazquez-Flota et al. [61] in cell suspension or hairy root and rootless shoot cultures of Catharanthus roseus treated with SA. Salicylic acid activates genes in plants involved in the biosynthesis and production of specific types of secondary metabolites [62]. Acetylsalicylic acid, an analog of SA, has been shown to induce the production of indole alkaloids in cell cultures of Catharanthus roseus [43]. Additionally, Zhao et al. [36] observed that compact callus initiated from stem explants, when treated with 250 mM mannitol, demonstrated increased ajmalicine production (42.3 mg/l) in Catharanthus roseus. However, Gantet et al. [63] found that Catharanthus roseus cell suspension cultures treated with 2,4-D + Methyl jasmonate produced the highest levels of ajmalicine.
Plants are known to respond to biotic elicitors by increasing the production of secondary metabolites [64, 65]. One significant approach involves stimulating secondary metabolite production with elicitors produced by microbes, which can enhance the accumulation of secondary metabolites in plant cell culture [53, 66]. Among the biotic elicitors tested, the elicitor derived from Aspergillus niger demonstrated the highest accumulation of vincristine (108.7 ± 1.0 ng g−1 DW). Yeast extract also notably increased vincristine content in the cell biomass (107.9 ± 1.3 ng g−1 DW). Although chitin powder supplementation slightly induced vincristine content, it was not significantly different from the control. Interestingly, the elicitor derived from Alternaria sp. had a negative impact on vincristine accumulation in the cell biomass. Ramezani et al. [67] observed increased vincristine production after treatment with Priformospora fungal extract (250 mg/l) in cell suspension culture of Catharanthus roseus. However, Alternaria sesami fungal culture extract was utilized in callus culture to enhance vincristine content (21.72%) after 105 days in Catharanthus roseus [68]. Maqsood and Abdul [69] reported that treatment with 1.5 mg/l yeast extract effectively induced vinblastine and vincristine content in Catharanthus roseus.Introducing precursors to differentiated cell cultures, resembling organized tissue in whole plants, provides new avenues for the development of secondary metabolites. Precursor feeding in plant cell cultures has been widely employed as a method to boost secondary metabolite synthesis [70, 71]. In the current study, tryptophan was discovered to enhance vincristine accumulation. Similar findings were reported by Zhao et al. [36], who observed increased vincristine accumulation in compact callus clusters of Catharanthus roseus when fed with tryptamine or tryptophan.
The addition of precursors such as tryptamine, tryptophan, and loganin to cell suspension cultures has demonstrated potential in increasing ajmalicine levels by up to six-fold [72]. However, supplementation of transgenic cell line cultures solely with tryptamine and tryptophan did not lead to any observable production of indole alkaloids in Catharanthus roseus [44]. Morgan and Shanks [73] investigated the combined impact of elicitors and precursors on the serpentine and ajmalicine content in the hairy root culture of Catharanthus roseus, but their study did not yield significant results. Guo et al. [74] discovered that treatment with 30 mg/l of α-Keto-glutaric acid induced tabersonine content, indicating a complex relationship between precursor supplementation and alkaloid production in Catharanthus roseus.
5 Conclusion
This study marks a significant breakthrough in the field by introducing an innovative method for exclusively producing vincristine from mixoploid callus and cell cultures of Catharanthus roseus. The enhanced yield of vincristine holds particular importance for its clinical applications and extraction, as larger quantities are required to meet the demand for natural products in medical treatments. By concentrating on mixoploid callus and cell cultures originating from the endosperm of Catharanthus roseus, this research presents a promising approach to optimizing vincristine production. Overall, the findings of this study pave the way for future research endeavors aimed at enhancing the production efficiency of vincristine and other valuable secondary metabolites in Catharanthus roseus, thus contributing to advancements in therapeutic medicine.
Data availability
All data generated or analysed during this study are included in this article and its supplementary information files.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BA:
-
6-Benzyladenine
- IAA:
-
Indole-3-acetic acid
- KCl:
-
Potassium chloride
- Kin:
-
Kinetin
- LC-MS:
-
Liquid Chromatography Mass Spectrometry
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthaleneacetic acid
- PEG:
-
Polyethylene glycol
- TDZ:
-
Thidiazuron
References
Mintah SO, Asafo-Agyei T, Archer MA, Atta-Adjei P Jr, Boamah D, Kumadoh D, Appiah A, Ocloo A, Boakye D, Agyare C. Medicinal plants for treatment of prevalent diseases, medicinal plants. In: Perveen S, Al-Taweel A, editors. Pharmacognosy: Medicinal plants. London, UK: InTechOpen; 2019. p. 177–96.
Odey MO, Iwara IA, Udiba UU, Johnson JT, Inekwe UV, Asenye ME, Victor O. Preparation of plant extracts from indigenous medicinal plants. Int J Sci and Technol. 2012;1:688–92.
Niazi P, Monib AW. The role of plants in traditional and modern medicine. J Pharmacog Phytochem. 2024;13:643–7.
Zang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13:1–26.
Atanasov AG, Zotchev SB, Dirsch VM, the International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:1–17.
Chen SL, Yu H, Luo HM, Wu Q, Li CF, Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med. 2016;11:37.
Balekundri A, Mannur V. Quality control of the traditional herbs and herbal products: a review. Fut J Pharma Sci. 2020;6:67.
Ahire ML, Ghane SG, Lokhande VH, Suprasanna P, Nikam TD. Micropropagation of Uraria picta through adventitious bud regeneration and antimicrobial activity of callus. In Vitro Cell. and Dev. Biol Plants. 2011;47:488–95.
Espinosa-Leal CA, Puente-Garza CA, García-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248:1–18.
Sajc L, Grubisic D, Vunjan-Novakovic G. Bioreactors for plant engineering: an outlook for further research. Biochem Eng J. 2000;4:89–99.
Fazili MA, Bashir I, Ahmad M, Yaqoob U, Geelani SN. In vitro strategies for the enhancement of secondary metabolite production in plants: a review. Bull Natl Res Cent. 2022;46:35.
Patil JG, Ahire ML, Nitnaware KM, Panda S, Bhatt VP, Kavi Kishor PB, Nikam TD. In vitro propagation and production of cardiotonic glycosides in shoot cultures of Digitalis purpurea L. by elicitation and precursor feeding. Appl Microbiol And Biotechnol. 2013;97:2379–93.
Chandran H, Meena M, Barupal T, Sharma K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep. 2020;26: e00450.
Efferth T. Biotechnology applications of plant callus cultures. Engineering. 2019;5:50–9.
Akgol M, Simsek O, Donmez S, Kacar YA. An overview of in vitro haploid plant production in Citrus. Am J Plant Biol. 2017;2:19–23.
Wang X, Cheng ZM, Zhi S, Xu F. Breeding triploid plants: a review. Czech J Genet Plant Breed. 2016;52:41–54.
Hu Y, Sun D, Hu H, Zuo X, Xia T, Xie J. A comparative study on morphological and fruit quality traits of diploid and polyploid carambola (Averrhoa carambola L.) genotypes. Sci Horticult. 2021;277:109843.
Thomas TD, Chaturvedi R. Endosperm culture: a novel method for triploid plant production. Plant Cell Tiss Organ Cult. 2008;93:1–14.
Aslam J, Mujib A, Fatima Z, Sharma MP. Variations in vinblastine production at different stages of somatic embryogenesis, embryo, and field-grown plantlets of Catharanthus roseus L. (G) Don, as revealed by HPLC. In Vitro Cell. and Dev. Biol Plants. 2010;46:348–53.
Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7: e577.
Ahmed MB, Islam SU, Alghamdi AAA, Kamran M, Ahsan H, Lee YS. Phytochemicals as chemo-preventive agents and signaling molecule modulators: current role in cancer therapeutics and inflammation. Int J Mol Sci. 2022;23:15765.
Murashige T, Skoog FA. revised medium for rapid growth and bioassay of tobacco tissue cultures. Physiol Plant. 1962;15:473–97.
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–614.
Chaachouay N, Zidane L. Plant-derived natural products: a source for drug discovery and development. Drugs Drug Candidates. 2024;3:184–207.
Otari SS, Devkar SP, Patel SB, Ghane SG. Micropropagation and elicited production of triterpenoid saponin glycosides and stigmasterol via precursor and elicitor feeding in Bacopa floribunda (R.Br.) Wettst. - A potential nootropic herb. Front Plant Sci. 2023;14:1096842.
Phillipson JD. Plants as source of valuable products. In: Charwood BV, Rhods MJC, editors. Secondary products from plant tissue culture. New York: Oxford Press; 1990. p. 1–23.
Aslam J, Mujib A, Nasim SA, Sharma MP. Screening of vincristine yield in ex vitro and in vitro somatic embryos derived plantlets of Catharanthus roseus L. (G) Don. Sci Horticult. 2009;119:325–9.
Mekky H, Al-Sabahi J, Abdel Kareem MFM. Potentiating biosynthesis of the anticancer alkaloids vincristine and vinblastine in callus cultures of Catharanthus roseus. S Afr J Bot. 2018;114:29–31.
Das A, Sarkar S, Bhattacharyya S, Gantait S. Biotechnological advancements in Catharanthus roseus (L.) G. Don Appl Microbiol Biotechnol. 2020;104:4811–35.
Chaturvedi R, Razdan MK, Bhojwani SS. An efficient protocol for the production of triploid plants from endosperm callus of neem, Azadirchta indica A. Juss J Plant Physiol. 2003;160:557–64.
Kiong APL, Thing YS, Gansau JA, Hussein S. Induction of multiplication of callus from endosperm of Cycas revoluta. Afr J Biotechnol. 2008;7:4279–84.
Smith JI, Smart NJ, Misawa M, Kurz WGW, Tallevi SG, DiCosmo F. Increased accumulation indole alkaloids by some cell lines of Catharanthus roseus in response to addition of vanadyl sulfate. Plant Cell Rep. 1987;6:142–5.
Abou MA. Ein Standardnährmedium für die Anzucht von Kalluskulturen einiger Arzneipflanzen. Z Pflanzenphysiol. 1977;85:273–7.
Constabel F, Gaudet-La Prairie P, Kurz WGW, Kutney JP. Alkaloid production in Catharanthus roseus cell cultures. XII. Biosynthetic capacity of callus from original explants and regenerated shoots. Plant Cell Rep. 1982;1:139–42.
Slater A, Scott N, Fowler M. Plant Biotechnology: The Genetic Manipulation of Plants. Oxford: Oxford University Press; 2003.
Zhao J, Hu Q, Guo YQ, Zhu WH. Effects of stress factors, bioregulators, and synthetic precursors on indole alkaloid production in compact callus clusters cultures of Catharanthus roseus. Appl Microbiol Biotechnol. 2001;55:693–8.
Datta A, Srivastava PS. Variation in vinblastine production by Catharanthus roseus during in vivo and in vitro differentiation. Phytochemistry. 1997;46:136–7.
Soleimani F, Zarghami R, Ebrahimzadeh M. Effects of 2,4-D and Kin concentrations on vinblastine and vincristine alkaloid contents in callus of periwinkle (Catharanthus roseus). Int J Agr Sci. 2013;3:759–65.
Zheng Z, Wu M. Cadmium treatments enhances the production of alkaloids secondary metabolites in Catharanthus roseus. Plant Sci. 2004;166:507–14.
Seoussi MM, Creche J, Rideau M. Relation between hypoxia and alkaloid accumulation in Catharanthus roseus cell suspension. J Appl Sci Res. 2007;3:287–90.
Verma AK, Singh RR, Singh S. Improved alkaloid content in callus culture of Catharanthus roseus. Bot Serbica. 2012;36:123–30.
Linh TM, Mai NC, Hoe PT, Ngoc NT, Thao PTH, Ban NK, Van NT. Development of a cell suspension culture system for promoting alkaloid and vinca alkaloid biosynthesis using endophytic fungi isolated from local Catharanthus roseus. Plants. 2021;10:672.
Zhao J, Zhu WH, Hu Q. Effects of light and plant growth regulators on the biosynthesis of vindoline and other indole alkaloids in Catharanthus roseus callus cultures. Plant Growth Regul. 2000;33:43–9.
Whitmer S, Van der Heijden R, Verpoorte R. Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tissue Organ Cult. 2002;69:85–93.
Xu M, Dong J. Nitric oxide stimulates indole alkaloid production in Catharanthus roseus cell suspension cultures through a protein kinase-dependant signal pathways. Enzyme Microb Technol. 2005;37:49–53.
Gonçalves S, Romano A. 2018. Production of plant secondary metabolites by using biotechnological tools, In Vijayakumar R, Raja SS, eds, Secondary Metabolites - Sources and Applications. IntechOpen. https://doi.org/10.5772/intechopen.76414
Khan T, Ullah MA, Garros L, Hano C, Abbasi BH. Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J Photochem Photobiol B: Biol. 2019;190:163–71.
Tiovonen L, Laakso S, Rosenqvist H. The effect of temperature on growth, indole alkaloid accumulation and lipid composition of Catharanthus roseus cell suspension cultures. Plant Cell Rep. 1992;11:390–4.
Ratnadewi D. 2017. Alkaloids in plant cell cultures. In Georgiev V, Pavlov A, eds, Alkaloids - Alternatives in Synthesis, Modification and Application. IntechOpen; 2017. https://doi.org/10.5772/66288
Zenk MH, El-Shagi H, Arens H, Stoeckigt J, Weiler EW, Deus B. Formation of the indole alkaloids serpentine and ajmalicine in cell suspension cultures of Catharanthus roseus. In: Barz W, Reinhard E, Zenk MH, editors. Plant Tissue Culture and its Biotechnological Application. Heidelberg, New York: Springer Verlag Berlin; 1977. p. 27–43.
Mujib A, Ilah A, Gandotra N, Abdin MZ. In vitro application to improve alkaloid yield in Catharanthus roseus. In: Govil JN, Kumar PA, Singh VK, editors. Biotechnology and Genetic Engineering of Recent Progress in Medicinal Plants, vol. IV. Sci Tech Publication. USA: Houston; 2002. p. 415–40.
Mishra MR, Srivastava RK, Akhtar N. Enhanced alkaloid production from cell culture system of Catharanthus roseus in combined effect of nutrient salts, sucrose and plant growth regulators. J Biotechnol Biomed Sci. 2018;1:14–34.
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J. Elicitation, an effective strategy for the biotechnological production of the bioactive high-added value compounds in plant cell factories. Molecules. 2016;21:182.
Isah T, Umar S, Mujib A, Sharma MP, Rajasekharan PE, Zafar D, Frukh A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue and Organ Cult. 2018;132:239–65.
Petrova M, Miladinova-Georgieva K, Geneva M. Influence of abiotic and biotic elicitors on organogenesis, biomass accumulation, and production of key secondary metabolites in Asteraceae plants. Int J Mol Sci. 2024;25:4197.
Ahire ML, Walunj PR, Kavi Kishor PB, Nikam TD. Effect of sodium chloride induced stress on growth, proline, glycine betaine accumulation, antioxidative defense and bacoside A content in in vitro regenerated shoots of Bacopa monnieri (L.) Pennell. Acta Physiol Plant. 2013;35:1943–53.
Ahire ML, Laxmi S, Walunj PR, Kavi Kishor PB, Nikam TD. Effect of potassium chloride and calcium chloride induced stress on in vitro cultures of Bacopa monnieri (L.) Pennell. J Plant Biochem and Biotechnol. 2014;23:366–78.
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681.
Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal and Behav. 2011;6:1720–31.
Anjitha KS, Sameena PP, Puthur JT. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress. 2021;2: 100038.
Vazquez-Flota F, Hernández-Domínguez E, Miranda-Ham ML, Monforte-González M. A differential response to chemical elicitors in Catharanthus roseus in vitro cultures. Biotechnol Lett. 2009;31:591–5.
Taguchi G, Yazawa T, Hayashida N, Okazaki M. Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrate specificity and are induced by salicylic acid and auxin. Eur J Biochem. 2001;268:4086–94.
Gantet P, Imbault N, Thiersault M, Doireu P. Necessity of functional octadecanoic pathway for indole alkaloid synthesis by Catharanthus roseus cell suspension cultured in an auxin-starved medium. Plant Cell Physiol. 1998;39:220–5.
Esmaelzadeh BS, Sharifi M. Increasing the production of plant secondary metabolites using biotic elicitors. J Cell Tissue. 2013;4:119–28.
Al-Khayri JM, Rashmi R, Toppo V, Chole PB, Banadka A, Sudheer WN, Nagella P, Shehata WF, Al-Mssallem MQ, Alessa FM, Almaghasla MI, Rezk AAS. Plant secondary metabolites: the weapons for biotic stress management. Metabolites. 2023;13:716.
Bajwa MN, Bibi A, Idrees MZ, Zaman G, Farooq M, Bhatti TT. Elicitation, a mechanistic approach to change the metabolic pathway of plants to produce pharmacological important compounds in in-vitro cell cultures. Global J Eng Sci. 2021;8:2021.
Ramezani A, Haddad R, Sedaghati B, Jafari D. Effects of fungal extracts on vinblastine and vincristine production and their biosynthesis pathway genes in Catharanthus roseus. S Afr J Bot. 2018;119:163–71.
Birat K, Siddiqi TO, Mir SR, Aslan J, Bansal R, Khan W, Dewangan RP, Panda BP. Enhancement of vincristine under in vitro culture of Catharanthus roseus supplemented with Alternaria sesami endophytic fungal extract as a biotic elicitor. Int Microbiol. 2022;25:275–84.
Maqsood M, Abdul M. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Rev Bras Farmacogn. 2017;27:549–56.
Zhao J, Verpoorte R. Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev. 2007;6:435–57.
Almagro L, Fernández-Pérez F, Pedreño MA. Indole alkaloids from Catharanthus roseus: bioproduction and their effect on human health. Molecules. 2015;20:2973–3000.
El-Sayed M, Verpoorte R. Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tissue Organ Cult. 2002;68:265–70.
Morgan JH, Shanks JV. Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol. 2000;79:137–45.
Guo ZG, Liu Y, Gong MZ, Chen W, Li WY. Regulation of vinblastine biosynthesis in cell suspension cultures of Catharanthus roseus. Plant Cell Tissue Organ Cult. 2013;112:43–54.
Acknowledgements
Financial support from UGC, SAP-DRS III, DST-FIST, and DST-PURSE program, Government of India to the Department of Botany, University of Pune, Pune is gratefully acknowledged. JGP is thankful to the UGC for the Junior Research Fellowship under the meritorious student program and Principal, and Head, Department of Botany, MGSM's Dadasaheb Dr. S. G. Patil College, Chopda, Dist. Jalgaon for continuous support.
Author information
Authors and Affiliations
Contributions
J.G.P. and M.L.A. contributed to data acquisition and formal analysis. R.A.S. contributed to writing—review & editing. T.D.N. and M.L.A. contributed to the conceptualization, funding acquisition, supervision, and writing of—the original draft. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patil, J.G., Nikam, T.D., Shinde, R.A. et al. Effect of abiotic and biotic elicitors on vincristine accumulation in endosperm derived in vitro cultures in Catharanthus roseous (L.) G. Don. Discov. Plants 1, 8 (2024). https://doi.org/10.1007/s44372-024-00009-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44372-024-00009-y