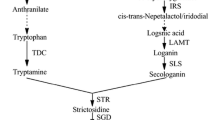
Abstract
Various elicitors of hydroxylase, peroxidase, acetyltransferase and inhibitors of oxygenase were added to a Catharanthus roseus cell culture medium to investigate the regulatory effects on tabersonine, vindoline and vinblastine biosynthesis. Hydrogen peroxide was found to be the most effective agent for enhancing the biosynthesis of tabersonine. By adding 20 μg/L hydrogen peroxide, the tabersonine concentration reached 9.02 mg/g dry weight (DW) after culturing cell suspensions for 7 days. With the addition of 30 μg/L acetyl CoA, the most vindoline (final cell content of 0.33 mg/g DW) was produced. By effective inhibition of lochnericine biosynthesis with the addition of 0.5 μmol/L benzotriazole, the cell content of vindoline was increased to 0.42 mg/g DW. An orthogonal experiment consisting of multiple regulation factors was carried out to optimize vinblastine biosynthesis. It was shown that optimal vinblastine biosynthesis was achieved by addition of 5 mg/L acetyl CoA, 20 μg/L hydrogen peroxide, 0.5 μmol/L benzotriazole, 100 mg/L tryptophan, 100 mg/L loganin and 30 mg/L cerium chloride. Under these conditions, the cell content of vinblastine reached 0.81 mg/g DW. Simultaneous changes in cell content and enzyme activities of Cytochrome P-450 monooxygenases, Deacetylvindoline-O-acetyltransferase and Peroxidase enzyme indicated that these enzymes were closely linked to vinblastine biosynthesis.


Similar content being viewed by others
Abbreviations
- T16H:
-
Tabersonine-16-hydroxylase
- DAT:
-
Deacetylvindoline-O-acetyltransferase
- STR:
-
Strictosidine synthase
- LCO:
-
Lochnericine cyclooxygenase
- POD:
-
Peroxidase enzyme
- P-450:
-
Cytochrome P-450 monooxygenases
- G10H:
-
Geraniol-10-hydroxylase
- D4H:
-
Deacetoxyvindoline 4-hydroxylase
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- TDC:
-
Tryptophan decarboxylase
- DTT:
-
Dithiothreitol
- HA:
-
Hydroxyphenylpyruvic acid
References
Balsevich J, Deluca V, Kurz WGW (1986) Altered alkaloid pattern in dark-grown seedlings of catharanthus-roseus—the isolation and characterization of 4-desacetoxyvindoline—a novel indole alkaloid and proposed precursor of vindoline. Heterocycles 24:2415–2421
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Meth Enzymol 11:764–775
Collu G, Garcia AA, van der Heijden R, Verpoorte R (2002) Activity of the cytochrome P450 enzyme geraniol 10-hydroxylase and alkaloid production in plant cell cultures. Plant Sci 162:165–172
Decarolis E, Chan F, Balsevich J, Deluca V (1990) Isolation and characterization of a 2-oxoglutarate dependent dioxygenase involved in the 2nd-to-last step in vindoline biosynthesis. Plant Physiol 94:1323–1329
Echevarría-Machado I, Escobedo-G MRM, Larqué-Saavedra A (2007) Responses of transformed Catharanthus roseus roots to ferntomolar concentrations of salicylic acid. Plant Physiol Biochem 45:501–507
El-Sayed M, Verpoorte R (2005) Methyljasmonate accelerates catabolism of monoterpenoid indole alkaloids in Catharanthus roseus during leaf processing. Fitoterapia 76:83–90
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6:277–305
Grosman N (2007) Comparison of the influence of NSAIDs with different COX-selectivity on histamine release from mast cells isolated from naive and sensitized rats. Int Immunopharmacol 7:532–540
Guo Z-G, Liu Y, Xing X-H (2011) Enhanced catharanthine biosynthesis through regulation of cyclooxygenase in the cell suspension culture of Catharanthus roseus (L.) G. Don. Process Biochem 46:783–787
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004) Metabolic engineering of the indole pathway in Catharanthus roseus hairy roots and increased accumulation of tryptamine and serpentine. Metab Eng 6:268–276
Jung KH, Kwak SS, Choi CY, Liu JR (1994) Development of 2-stage culture process by optimization of inorganic salts for improving catharanthine production in hairy root cultures of Catharanthus-Roseus. J Ferment Bioeng 77:57–61
Kumar S, Dutta A, Sinha AK, Sen J (2007) Cloning, characterization and localization of a novel basic peroxidase gene from Catharanthus roseus. FEBS J 274:1290–1303
Levine A, Tenhaken R, Dixon R (1994) Lamb C H2O2 from the oxidative burst orchestrate the plant hypersensitive disease resistance response. Cell 79:583–593
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. Meth Biochem Anal 1:357–424
Magnotta M, Murata J, Chen JX, De Luca V (2007) Expression of deacetylvindoline-4-O-acetyltransferase in Catharanthus roseus hairy roots. Phytochem 68:1922–1931
Mishra P, Kumar S (2000) Emergence of periwinkle Catharanthus roseus as a model system for molecular biology of alkaloids: Phytochemistry, pharmacology, plant biology and in vivo and in vitro cultivation. J Med Aromat Plant Sci 22(2–3):306–337
Morgan JA, Shanks JV (1999) Inhibitor studies of tabersonine metabolism in C-roseus hairy roots. Phytochem 51:61–68
Moreno PRH, Vanderheijden R, Verpoorte R (1995) Cell and tissue-cultures of catharanthus-roseus: a literature survey. 2. updating from 1988 to 1993. Plant Cell Tissue Organ Cult 42:1–25
Murashige T, Skoog F (1962) Arevised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. Ii. Solubilization, purification, and properties. J Biol Chem 239:2379–2385
Peebles CAM, Hong SB, Gibson SI, Shanks JV, San KY (2006) Effectss of terpenoid precursor feeding on Catharanthus roseus hairy roots over-expressing the alpha or the alpha and beta subunits of anthranilate synthase. Biotechnol Bioeng 93:534–540
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406:731–734
Pratap Kumar P, Jaspreet K, Pritika S (2011) A liquid culture system for shoot proliferation and analysis of pharmaceutically active constituents of Catharanthus roseus (L.) G. Don. Plant Cell Tiss Organ Cult 105:299–307
Rajagopalan PTR, Datta A, Pei DH (1997) Purification, characterization, and inhibition of peptide deformylase from Escherichia coli. Biochemistry (US) 36:13910–13918
Salzer P, Corbiere H, Boller T (1999) Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 208:319–325
Sanchez-Sampedro MA, Fernandez-Tarrago J, Corchete P (2005) Yeast extract and methyl jasmonate-induced silymarin production in cell cultures of Silybum marianum (L.) Gaertn. J Biotech 119:60–69
Singh G, Gavrieli J, Oakey JS et al (1998) Interaction of methyl jasmonate, wounding and fungal elicitation during sesquiterpene induction in Hyoscyamus muticus in root cultures. Plant Cell Rep 17:391–395
Smith JI, Smart NJ, Kurz WGW, Misawa M (1987) The use of organic and inorganic-compounds to increase the accumulation of indole alkaloids in catharanthus-roseus (l) g-don cell-suspension cultures. J Exp Bot 38:1501–1506
Stafford A, Fowler MW (1983) Effects of carbon and nitrogen growth limitation upon nutrient-uptake and metabolism in batch cultures of catharanthus-roseus (l) g-don. Plant Cell Tissue Organ Cult 2:239–251
St-Pierre B, Laflamme P, Alarco AM, De Luca V (1998) The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J 14:703–713
Stpierre B, Deluca V (1995) A cytochrome-p-450 monooxygenase catalyzes the first step in the conversion of tabersonine to vindoline in catharanthus-roseus. Plant Physiol 109:131–139
van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:607–628
Vazquez-Flota FA, De Luca V (1998) Jasmonate modulates development- and light-regulated alkaloid biosynthesis in Catharanthus roseus. Phytochem 49:395–402
Wang Y, Dai CC, Zhao YW (2011) Fungal endophyte-induced volatile oil accumulation in Atractylodes lancea plantlets is mediated by nitric oxide, salicylic acid and hydrogen peroxide. Process Biochem 46(3):730–735
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11240_2012_213_MOESM1_ESM.tif
LC image of Catharanthus roseus cells’ extraction; The retention times of catharanthine, vindoline, vinblastine, and tabersonine were 4.07, 10.36, 11.34 and 14.76 min, respectively. Below was the MS of the extraction when retention time is 4.06 min. We indicate that it should be catharanthine (TIFF 1053 kb)
11240_2012_213_MOESM2_ESM.tif
MS of the extraction when retention time was 7.66 min, we indicate that it was catharanthine (molecular weight, 336.43), below was the MS of the extraction when retention time was 10.36 min, we indicate that it was vindoline (molecular weight, 456.54) (TIFF 1068 kb)
11240_2012_213_MOESM3_ESM.tif
MS of the extraction when retention time was 11.34 min, we indicate that it was vinblastine (molecular weight, 810.9); below is the MS of the extraction when retention time was 14.76 min, we indicated that it was tabersonine (molecular weight, 336.43) (TIFF 909 kb)
Rights and permissions
About this article
Cite this article
Guo, ZG., Liu, Y., Gong, MZ. et al. Regulation of vinblastine biosynthesis in cell suspension cultures of catharanthus roseus . Plant Cell Tiss Organ Cult 112, 43–54 (2013). https://doi.org/10.1007/s11240-012-0213-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0213-y