Abstract
This study presents a comprehensive analysis of phytoplankton diversity and optical properties in the coastal waters of Ratnagiri, India. Ratnagiri, located on the southern coast of Maharashtra, is one of the major centre for fishing activities in India as well as a developing hub for industrial activities. Given its ecological significance and the pressures it faces, the coastal waters of Ratnagiri present a valuable case for studying phytoplankton dynamics and their light absorption properties. We integrated parameters like phytoplankton taxonomy, pigment composition, Coloured Dissolved Organic Matter (CDOM), and phytoplankton absorption characteristics to give a comprehensive view of the phytoplankton community and its optical environment. Phytoplankton samples from Ratnagiri were analyzed with microscopy and high-performance liquid chromatography (HPLC) for taxonomy and pigment profiling, while CDOM and absorption properties were measured using spectrophotometry. Our findings reveal more or less spatial uniformity in phytoplankton composition (Pielou's Evenness Index (J’) ranged between 0.84 and 0.9), with significant diversity (Shannon Diversity Index (H’) ranged from 3.7 to 4.19) within the phytoplankton community. Pigment analysis revealed key marker pigments associated with various phytoplankton classes including bacillariophytes (diatoms), chlorophytes, cyanophytes, haptophytes and cryptophytes, providing insights into community structure. Concurrently, CDOM absorption coefficient (aCDOM (440)) that ranged between 0.08 and 0.3 m−1 indicated varying levels of dissolved organic matter, influencing light absorption and availability within the water column. The phytoplankton absorption data highlighted the specific wavelengths most affected by phytoplankton presence, notably those contributed by diatoms and cyanophytes, providing insights into the optical properties of Ratnagiri's coastal waters. This integrative approach underscores the importance of considering multiple parameters to accurately assess phytoplankton dynamics and their impact on the aquatic environment. The results contribute to a better understanding of the ecological processes governing coastal waters in Ratnagiri and offer valuable information for future monitoring and management efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phytoplankton, the microscopic photosynthetic organisms in aquatic environments, play a crucial role in global biogeochemical cycles and form the foundation of the marine food web. Their diversity and abundance significantly influence primary productivity, nutrient cycling, and the overall health of marine ecosystems. Understanding phytoplankton dynamics is crucial for comprehending marine ecosystems and their responses to environmental changes. One effective approach to studying these dynamics involves examining bio-optical properties, which provide valuable insights into the composition and behaviour of phytoplankton populations. Key parameters include phytoplankton absorption, which reveals the amount of light absorbed by pigments within phytoplankton cells, offering clues about their concentration and health. Additionally, coloured dissolved organic matter (CDOM) can influence light absorption and scattering, indirectly affecting phytoplankton dynamics by altering light availability. Analyzing phytoplankton pigments, such as chlorophyll-a and carotenoids, allows for the identification and quantification of different phytoplankton groups, facilitating assessments of community structure and primary production. Furthermore, integrating phytoplankton taxonomy enhances our ability to link specific species or functional groups with observed optical signatures. By adopting such a multidisciplinary approach that integrates various biological and optical parameters, we can develop a more comprehensive understanding of phytoplankton dynamics, ultimately contributing to better management and prediction of marine environments.
The coastal region of Ratnagiri, located along the western coast of India, is characterized by its rich biodiversity and productive waters [21, 24]. The waters of Ratnagiri are also economically important, sustaining local fisheries and providing livelihoods to coastal communities. Additionally, the region has become a focus for marine research due to its unique ecological characteristics and the increasing pressures from human activities such as fishing, tourism, and industrial development. Rapid urbanization, industrialization and developmental activities are causing damage to the marine ecosystem and diversity of the coastal areas along the Maharashtra coast [21]. Ratnagiri coastal waters was reported to be polluted from diffuse and direct sources of agricultural, urban and industrial pollutants by Sankpal et al. [29]. Such activities increase their vulnerability to anthropogenic pressures and climate change thereby decreasing the resilience of coastal ecosystems [3].
Despite the ecological significance of the coastal waters of Ratnagiri, very few studies exist on the phytoplankton communities in this region [14, 21, 25]. The present study, thus, focuses on addressing this knowledge gap by combining phytoplankton taxonomy that provides insights into the composition and diversity of the community with pigment analysis using high-performance liquid chromatography (HPLC). Further, understanding the optical properties of CDOM and phytoplankton absorption is essential for elucidating their impacts on underwater light fields and primary production. By integrating these parameters, this study aims to provide a comprehensive understanding of the phytoplankton community structure and its optical environment in Ratnagiri’s coastal waters. The findings will contribute to a deeper knowledge of the ecological processes governing these coastal waters and offer valuable insights for future monitoring and management efforts in the region.
2 Materials and methods
2.1 Study area
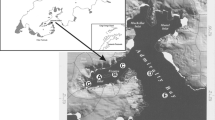
The coastal waters of Ratnagiri, located along the Konkan coastline in the state of Maharashtra, India, are renowned for their ecological richness and biodiversity. This region, situated between latitudes 17° 00ʹ N and 17° 30ʹ N and longitudes 73° 00ʹ E and 73° 30ʹ E, encompasses a diverse range of marine habitats, including estuaries, mangroves, sandy beaches, and rocky shores. The Arabian Sea influences the coastal dynamics of Ratnagiri, contributing to its high biological productivity and complex hydrological features. Ratnagiri's coastal waters are characterized by a tropical monsoon climate, with distinct seasonal variations affecting the oceanographic conditions. The sampling was conducted during the pre-monsoon period, i.e., March 2018, when the region experiences increasing temperatures and reduced freshwater input. Surface water samples for various biological and bio-optical parameters were collected using Niskin sampler from 21 locations namely S1 to S21 as shown in Fig. 1.
2.2 Methodology
2.2.1 Phytoplankton identification and enumeration
For phytoplankton identification and enumeration, 1 L of water was fixed with 1% of Lugol’s Iodine and preserved with 4% formaldehyde solution and further stored under dark and cool (4 °C) conditions until microscopic analysis. Samples were concentrated approximately up to 10–15 mL by siphoning the top layer of the sample carefully with a tube. 1 mL of concentrated sample was transferred to the Sedgwick- Rafter slide for identification and counting using Zeiss® Observer A1 AX10 inverted microscope. Phytoplankton samples were enumerated and identified to the lowest possible taxonomic level using standard taxonomic key [33].
2.2.1.1 Diversity and Evenness analysis
A diversity index is a measure of species diversity in a community that consists of a simultaneous population of several different species whereas evenness index shows the relative abundance of species within a selected region. Species diversity was calculated using Shanon’s diversity index [30] and species evenness was calculated using Pielou’s evenness index [28] for the purpose of understanding the phytoplankton community within the sampled area.
Shannon’s index
where H’: diversity index, ln: natural log, S: number of species in community, pi: the proportion of the individuals found in ith species.
Pielou’s Evenness Index
J’: Pielou evenness index, H’: The observed value of Shannon index, H’max: lnS, S: Total number of species.
2.2.2 Spectrophotometric chlorophyll-a
Surface water samples for chlorophyll-a measurement were collected using Niskin bottles and filtered (0.4 L) onto 47 mm diameter Whatman® GF/F filters (0.7 μm pore size) under low vacuum (~ 125 mmHg) to preserve the integrity of chlorophyll-containing cells. Filters were immediately stored in dark conditions to prevent degradation, placed in histoprep tissue capsules, and frozen in liquid nitrogen before being transferred to a − 80 °C freezer until analysis. For spectrophotometric analysis, filters were removed from the freezer and placed in 10 mL 90% acetone to ensure complete extraction of chlorophyll-a. The samples were incubated in the dark at 4 °C for 24 h, then sonicated using probe sonicator to facilitate proper extraction. This was followed by centrifugation at 4 °C for 10 min at 10,000 rpm (rotations per minute). Absorbance of the acetone extract was measured using a spectrophotometer at wavelengths of 750 nm (turbidity correction), 664 nm (chlorophyll-a maximum absorbance), 647 nm (chlorophyll-b), and 630 nm (pheophytin), with 90% acetone as the blank reference. Absorbance readings were corrected for turbidity by subtracting the absorbance at 750 nm from those at 664 nm, 647 nm, and 630 nm. Chlorophyll-a concentration was calculated using the equation: Chlorophyll-a (mg m−3) = [(11.85 * A664) – (1.54 * A647) – (0.08 * A630)]*v/V, where v is volume of acetone in millilitres and V is volume of sea water filtered in litres [15]. Results were expressed in milligrams per cubic metre (mg m−3) of water, ensuring accurate measurement of chlorophyll-a concentrations in marine samples.
2.2.3 Quantification of phytoplankton pigments using HPLC
For the analysis of phytoplankton pigments, 0.4 L of water samples were collected and filtered through 25 mm Whatman GF/F (pore size 0.7 μm) filter paper in dark and stored in liquid nitrogen. Samples were transferred to − 80 °C deep freezer upon reaching the laboratory and stored there until analysis. Pigment extraction and analysis were performed using a modified method of Zapata et al. [35]. The frozen filters were soaked in 2 mL extraction solution (90% acetone containing internal standard) for 45 min at 0 °C. Further, it was subjected to sonication for 30 s and allowed to stand for 15 min at 0 °C. This was followed by centrifugation at 4 °C for 10 min at 10,000 rpm. The extracts were then filtered through a 25 mm nylon syringe filter with 0.2 μm pore size to remove cellular debris. 100 μL of the final extract was injected into the reverse-phase HPLC (Agilent Technologies 1260 Infinity) equipped with a C8 column. The column was maintained at 25 °C, and the detection wavelengths of the diode array detector were set at 440 nm and 660 nm. The method involved a two solvent system, eluent A composed of methanol:acetonitrile:aqueous pyridine solution (50:25:25, v/v/v), and eluent B composed of methanol:acetonitrile:acetone (20:60:20, v/v/v), for the separation of individual pigments. The flow rate was set at 0.2 mL/min. Pigments were identified and quantified using commercially available pure pigment standards obtained from DHI Inc. (Denmark). Identification was done based on retention time and spectral matching with the standard pigment library. The library comprised of spectra of the following 30 pigments: chlorophyll-a, chlorophyll-b, chlorophyll-c2, chlorophyll-c3, divinyl chlorophyll-a, pheophorbide-a, pheophytin-a, chlorophyllide-a, chlorophyllide-b, chlorophyll C2-MGDG, alpha carotene, beta carotene, fucoxanthin, 19ʹbut-fucoxanthin, 19ʹhex-fucoxanthin, diadinoxanthin, diatoxanthin, dinoxanthin, alloxanthin, astaxanthin, prasinoxanthin, peridnin, lutein, violaxanthin, antheraxanthin, gyroxanthin diesterase, canthaxanthin, zeaxanthin, neoxanthin and myxoxanthophyll.
2.2.4 Phytoplankton absorption
Water samples were collected using Niskin bottles and filtered (0.4 L) onto 25 mm diameter Whatman® GF/F filters with 0.7 μm pore size. Filtration was carried out in dim light and cool conditions under low vacuum pressure (~ 125 mmHg) to preserve the integrity of phytoplankton cells, with the volume of water filtered adjusted to achieve optical densities of 0.05 < ODf (675) ≤ 0.25 and ODf (440) ≤ 0.4 (ODf is the optical density of filtered samples). Wet filters were stored in histoprep tissue capsules, frozen in liquid nitrogen, and then transferred to a − 80 °C deep freezer until analysis using Shimadzu UV-Spectrophotometer with integrated sphere attachment (Model no. UV-2450). Baseline scans without filters removed instrument signals, with air baselines or filter pad baselines used for corrections. Optical density (OD) of particulate matter on sample filters was measured against a clean wet reference filter, and total particulate absorption (ap(λ)) was calculated using the formula: ap(λ) = 2.303*ODs*A/V, with pathlength amplification corrected by ODs = 0.679*(ODf)1.2804, where ODs is the absorbance corrected for pathlength amplification [31], A is the effective area of the filter (m2) and V is the volume filtered (m3). This was followed by de-pigmentation that involved treating sample and blank filters with 100% methanol and rinsing with filtered seawater, repeating if necessary until peaks disappeared. The OD spectra of de-pigmented samples must be measured in the spectrophotometer in the same way as that performed for total particulate matter samples. Phytoplankton pigment absorption coefficient (aph) is indirectly measured from the total particulate absorption (ap(λ)) by subtracting the de-pigmented particulate absorption (ad) from the total particulate absorption (ap(λ)). This protocol ensures accurate measurement of phytoplankton absorption by mitigating degradation and interference during sample processing and analysis [12].
2.2.5 Estimation of CDOM absorption coefficient and spectral slope
Surface water samples for measuring CDOM concentration were collected using Niskin bottles and stored in acid-washed, pre-combusted amber borosilicate glass bottles, which were rinsed thrice with the sample before final collection and stored at 4 °C. In the laboratory, samples were filtered through 47 mm cellulose acetate filters with a pore size of 0.2 μm under low vacuum using Millipore filtration units and vacuum pumps, after thoroughly cleaning the filtration unit with distilled water, rinsing with 10% HCL (hydrochloric acid), and then with distilled water. The filtered samples were equilibrated to room temperature to avoid temperature differences with the blank. Absorbance of the filtrate sample was measured in 10 cm cuvettes on a Shimadzu UV–Vis 1800 spectrophotometer against Milli-Q water from 400 to 750 nm, with cuvettes rinsed with 10% HCL, Milli-Q water, and the samples. The CDOM absorption coefficient aCDOM(λ) was calculated as aCDOM(λ) = 2.303*[(ODs(λ) − ODbs(λ)) − ODnull]/l, where l is the cuvette path length, ODs(λ) is the optical density of the sample relative to purified water, ODbs(λ) is the optical density of the purified water blank treated like a sample, and ODnull is the residual optical density at a wavelength where absorption by dissolved materials is assumed to be zero [8, 11]. This method ensures accurate measurement by correcting for any residual optical density and using consistent null wavelength regions for both sample and blank.
CDOM absorption varies exponentially with wavelength according to the equation.
a(λ) = a(443) exp[−S(λ−443)] where, a(λ) is theCDOM absorption at wavelength λ; a(443) is the CDOM absorption at 443 nm; and the CDOM spectral slope (S) was estimated by fitting the above equation to the in-situ CDOM absorption data in the wavelength range 400–550 nm [11].
3 Results
3.1 Phytoplankton taxonomy
Analysis of phytoplankton samples collected from various sites in Ratnagiri revealed significant diversity in community composition. The phytoplankton community structure in this study area comprised of 252 species of phytoplankton from 68 different genera. This includes 148 species of bacillariophytes (diatoms), 96 species of dinoflagellates, and 7 species from other algal groups. In terms of the percent contribution of different phytoplankton groups, the community was predominantly dominated by diatoms (61–94%), followed by dinoflagellates (5–35%), and other algae (1–8%) (Fig. 2). Regarding phytoplankton cell density, diatoms were the most abundant group, with cell densities ranging from 0.161 * 105 to 3.96 * 105 cells l−1. Dinoflagellates had cell densities ranging from 0.024 * 105 to 1.98 * 105 cells l−1, and other algal groups contributed cell densities from 0.0086 * 105 to 0.3 * 105 cells l−1 at the surface during the pre-monsoon season. Among the diatoms, the genera Chaetoceros and Coscinodiscus were notably diverse, with 27 and 23 species identified, respectively. In the dinoflagellate group, the genera Ceratium and Protoperidinium were major contributors, with 45 and 16 species, respectively. Commonly observed diatom species included Coscinodiscus argus, Coscinodiscus radiatus, Bellerochea horologicalis, Chaetoceros curvisetus, Thalassionema nitzschioides, Odontella aurita, Chaetoceros lorenzianus, Chaetoceros compressus, Odontella sinensis, Fragilariopsis oceanica, Thalassionema frauenfeldii, Pseudo-nitzschia liniola, Pseudo-nitzschia delicatissima, Coscinodiscus albornii, Bacteriastrum delicatulum, Dactyliosolen fragilissimus, and Bacillaria paxillifera. Among other algal groups, silicoflagellates (Dictyochophyceae) such as Dictyocha fibula, Dictyocha octonaria, Octatis octonaria, and cyanophyceae (blue-green algae) such as non-heterocystous cyanobacteria like Trichodesmium erythraeum and Trichodesmium thiebautii, as well as heterocystous cyanobacteria like Richelia intracellularis and Calothrix sp., were observed in the study area.
Table 1 shows the diversity and evenness index observed at the sampled locations. The Shannon diversity index ranged from 3.7 to 4.19 across the study area. These values indicate relatively high diversity, with S14 showing the highest diversity (H’ = 4.19) and S18 showing the lowest (H’ = 3.7). Pielou's Evenness values ranged between 0.84 and 0.9, indicating that most stations had a relatively even distribution of species. Among the sampled sites, highest evenness (J’ = 0.9) was represented by S14, S16 and S20.
3.2 Spectrophotometric chlorophyll-a
The chlorophyll concentrations in the coastal waters of Ratnagiri varied between 1.8 and 5.12 mg m−3 across different locations (Fig. 3), reflecting diverse environmental conditions and nutrient availability. This also shows varying levels of primary productivity across the sampled sites. At S4, situated farthest from the coast, the chlorophyll concentration peaked at 5.12 mg m−3. In contrast, S1, located closest to the land (approx. 1 km from the shoreline and 5 km from the bar-mouth), showed a markedly lower concentration of 1.8 mg m−3.
3.3 Pigment composition
HPLC analysis identified a range of pigments, including chlorophyll-a, chlorophyll-b, zeaxanthin, violaxanthin, fucoxanthin, 19’hex-fucoxanthin, lutein, diadinoxanthin, diatoxanthin, alloxanthin and beta-carotene (Fig. 4). Among the identified pigments, chlorophyll-a, fucoxanthin and zeaxanthin were present in major concentrations. Fucoxanthin, a marker for diatoms, was the most dominant accessory pigment, corroborating the abundance of diatoms observed in the taxonomic analysis. At S4, where highest chlorophyll-a concentration was observed, the concentration of fucoxanthin (0.62 mg m−3) was also the highest, indicating the dominance of microphytoplankton especially the diatoms. The invariable presence of chlorophyll-b across the study area indicates green algae to have a consistent occurrence. The constant presence of 19’hex-fucoxanthin and alloxanthin, point out to the presence of haptophytes as well as cryptophytes in these waters. The presence of more or less similar pigment composition revealed the existence of almost comparable phytoplankton communities in the coastal waters of Ratnagiri.
3.4 CDOM characteristics
The absorption coefficient of coloured dissolved organic matter (aCDOM) in the study area exhibited an exponential decrease with increasing wavelength. Specifically, aCDOM at 440 nm ranged from 0.08 to 0.3 m−1, reflecting variations in CDOM concentration and composition across different sampling sites. Notably, S12 had the highest aCDOM(440), while S2 had the lowest (Fig. 5). For comparison, during the spring inter-monsoon season of 2015, Vishnu et al. [34] reported that aCDOM(443) in the coastal waters off Cochin varied between 0.009 and 0.143 m−1.
The spectral slope of CDOM (S400–550) explains the spectral dependence of aCDOM with wavelength and can provide information on the source of CDOM [22]. In the study area, the values of CDOM slope (S400–550) ranged from 0.0076 to 0.0126 nm−1, with approximately 70% of the values falling between 0.0076 and 0.0097 nm−1 (Fig. 6). This suggests that CDOM in the region predominantly originates from similar sources. Additionally, an inverse relationship was observed between aCDOM(440) and S400–550 (R2 = 0.51, n = 18) (Fig. 7).
3.5 Phytoplankton absorption
The phytoplankton absorption measurements exhibited notable variability at S8 relative to other coastal locations (Fig. 8). The absorption spectra of phytoplankton revealed distinct peaks corresponding to chlorophyll-a (at approximately 437 nm and 676 nm) and accessory pigments. The chlorophyll-a specific absorption coefficient at 440 nm (a*ph(440)) varied between 0.09 and 0.21 m2 mg−1, with highest value observed at S8. Secondary peaks in the blue and green regions of the spectrum correspond to accessory pigments like fucoxanthin and phycocyanin. These variations in absorption characteristics are indicative of differences in phytoplankton community structure and cell size.
The absorption characteristics majorly observed in our study were typical of diatoms, with the exception noted at S8. The work by Bidigare et al. [2] provides similar absorption features for chlorophyll-a and fucoxanthin in marine diatoms. The highest a*ph and chlorophyll-b concentration observed at S8, shows the dominance of green algae. These results once again prove the reliability of multi-parameter approach in understanding and establishing the bio-optical signatures of respective aquatic ecosystem.
4 Discussion
This study undertook a detailed analysis of phytoplankton taxonomy, pigment composition, and CDOM and phytoplankton absorption properties to elucidate the complex interactions and ecological significance of phytoplankton in the coastal ecosystem of Ratnagiri. By combining taxonomic analysis with HPLC-derived phytoplankton pigment analysis, we revealed a diverse phytoplankton community dominated by diatoms and cyanobacteria. This composition is typical for nutrient-rich coastal environments [4, 23].
The high abundance of diatoms, evidenced by both taxonomic analysis and fucoxanthin concentrations [16], suggests favourable conditions for their growth, likely driven by nutrient inputs from riverine and estuarine discharges. This prevalence aligns with previous studies highlighting diatoms' roles in carbon fixation and nutrient cycling [17]. Similarly, the presence of cyanobacteria, indicated by the pigment zeaxanthin [16], underscores their contribution to nitrogen fixation, which supports the overall phytoplankton community and maintains ecological balance [18, 19]. Also, the consistent occurrence of green algae across the study area indicates the persistence of calm and stable environment that promotes their growth.
Phytoplankton diversity plays a crucial role in sustaining marine ecosystems, including fisheries [6, 20, 27]. Gajbhiye et al. [7] reported similar trends between plankton, benthos, and fish catch rates along the coastal waters of Ratnagiri. The diversity of pigments observed suggests a well-adapted phytoplankton community capable of exploiting different light niches within the water column. Accessory pigments like fucoxanthin enhance photosynthetic efficiency under varying light conditions, which is vital for maintaining high primary production in regions like Ratnagiri that experience fluctuating light environments.
The absorption spectra of phytoplankton displayed peaks corresponding to chlorophyll-a and accessory pigments, indicating efficient light absorption across various wavelengths. This efficiency facilitates photosynthesis under diverse light conditions. Our study found that phytoplankton absorption characteristics varied across the study area, with higher specific absorption coefficients in regions with smaller phytoplankton cells. Notably, S8 exhibited the highest concentration of chlorophyll-b, a marker pigment for chlorophytes, aligning with the idea that smaller cells have higher surface-area-to-volume ratios, enhancing their light absorption efficiency [1, 5, 10].
The high diversity and efficient absorption properties of phytoplankton suggest a robust ecosystem capable of sustaining primary production. Additionally, significant CDOM absorption highlights the need to consider terrestrial influences on aquatic ecosystems, particularly regarding coastal water quality. Terrestrial influences can affect light availability and quality in the water column [9], impacting phytoplankton photosynthesis and community dynamics [13, 32]. Understanding these dynamics is crucial for predicting responses to environmental changes, such as climate change and nutrient loading.
The observed invariability in CDOM absorption coefficients suggests spatial homogeneity in organic matter sources and composition, with exceptions at stations S12 and S5. These notable variations of CDOM absorption at S12 and S5 reflects distinct sources and processes affecting organic matter dynamics relative to other sampled locations. Higher grazing activity by zooplankton could probably be the reason for greater CDOM absorption at S12 (Fig. 9 shows the microscopic picture of zooplankton captured during taxonomic analysis of phytoplankton at S12; higher abundance of zooplankton was observed at S12). Nelson and Siegel [26] reported such deviations in CDOM absorption as a result of localized biological processes, including phytoplankton release, zooplankton grazing, bacterial release and uptake, and viral interactions.
While our study provides valuable insights, it is limited by its temporal scope. Future research should consider seasonal variations and broader geographic coverage to further elucidate the interactions between phytoplankton, pigments, and CDOM. Overall, this study underscores the complex interplay between phytoplankton diversity, pigment composition, CDOM, and optical properties in the coastal waters of Ratnagiri. The integrative approach adopted in this research offers a comprehensive understanding of the factors influencing phytoplankton dynamics and their ecological significance. Understanding phytoplankton absorption properties is crucial for modeling primary productivity and carbon cycling in aquatic ecosystems. The efficient light absorption by diverse phytoplankton groups suggests a robust capacity for primary production, supporting higher trophic levels and contributing to the overall functioning of the ecosystem.
5 Conclusion
Our study reveals significant interactions between phytoplankton taxonomy, pigment composition, and optical properties in Ratnagiri's coastal waters. The strong correlation between HPLC-derived pigment concentrations and absorption peaks emphasizes the critical role of pigments, such as fucoxanthin, in shaping the optical characteristics of the phytoplankton community. High fucoxanthin levels indicate diatom dominance and explain the substantial absorption in the blue-green spectral region. The observed phytoplankton diversity points to a stable, nutrient-rich environment, supporting efficient light utilization and enhancing photosynthetic performance, particularly in variable light conditions.
This research underscores the importance of multidisciplinary approaches in marine ecological studies and provides a foundation for future monitoring and management of coastal ecosystems in Ratnagiri. Future studies could explore the temporal dynamics of these interactions and their implications for local fisheries and coastal health.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Code availability
Not applicable.
References
Aknes DL, Egge JK. A theoretical model for nutrient uptake in phytoplankton. Mar Ecol Prog Ser. 1991;70:65–72.
Bidigare RR, Ondrusek ME, Morrow JH, Kiefer DA. In-vivo absorption properties of algal pigments. Ocean Optics X. 1990;1302:290–302.
Bijlsma L, Ehler CN, Klein RJT, Kulshrestha SM, McLean RF, Mimura N, Nicholls RJ, Nurse LA, Perez Nieto H, Stakhiv EZ, Turner RK, Warrick RA. Coastal zones and small Islands. In: Watson RT, Zinyowera MC, Moss RH, Editors. Climate change 1995: Impacts, adaptations and mitigation of climate change—scientific-technical analyses. Contribution of working group II to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, UK, 1995; p. 289–324.
Caroppo C. Ecology and biodiversity of picoplanktonic cyanobacteria in coastal and brackish environments. Biodivers Conserv. 2015;24:949–71. https://doi.org/10.1007/s10531-015-0891-y.
Eppley RW, Thomas WH. Comparison of half-saturation constants for growth and nitrate uptake of marine phytoplankton. J Phycol. 1969;5:375–9.
Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–6.
Gajbhiye SN, Mehta P, Mustafa S, Nair R. Fishery potential along the Indian Coastal Waters Between Porbandar and Ratnagiri. J Indian Fisher Assoc (Proc Nat Symp Aquacorps). 1994;24:63–72.
Green SA, Blough NV. Optical absorption and fluorescence properties of chromophoric dissolved organic matter in natural waters. Limnol Oceanogr. 1994;39:1903–16.
Guallar C, Flos J. Linking phytoplankton primary production and chromophoric dissolved organic matter in the sea. Progr Oceanogr. 2019;176:102116. https://doi.org/10.1016/j.pocean.2019.05.008.
Hein M, Pedersen MF, Sand-Jensen K. Size-dependent nitrogen uptake in micro- and macroalgae. Mar Ecol Prog Ser. 1995;118:247–53.
Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr. 2008;53:955–69. https://doi.org/10.4319/lo.2008.53.3.0955.
IOCCG Protocol Series. Inherent Optical Property Measurements and Protocols: Absorption Coefficient. In: Neeley AR, Mannino A, Editors. IOCCG Ocean Optics and Biogeochemistry Protocols for Satellite Ocean Colour Sensor Validation, Volume 1.0, IOCCG, Dartmouth, NS, Canada. 2018.
Isada T, Iida T, Liu H, Saitoh S-I, Nishioka J, Nakatsuka T, et al. Influence of Amur River discharge on phytoplankton photophysiology in the Sea of Okhotsk during late summer. J Geophys Res. 2013;118:1995–2013. https://doi.org/10.1002/jgrc.20159.
Jadhav SB, Chavan NS. Study of phytoplankton population from water resources of Jotiba (Wadi Ratnagiri), Maharashtra. Nat Environ Pollut Technol. 2009;8(1):37–42.
Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophyll a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz. 1975;167:191–4.
Jeffrey SW, Mantoura RFC, Wright SW. Phytoplankton pigments in oceanography. Paris, France: UNESCO Publishing; 1997. p. 661.
Jin X, Gruber N, Dunne JP, Sarmiento JL, Armstrong RA. Diagnosing the contribution of phytoplankton functional groups to the production and export of particulate organic carbon, CaCO3, and opal from global nutrient and alkalinity distributions. Global Biogeochem Cycles. 2006. https://doi.org/10.1029/2005gb002532.
Karlson AML, Duberg J, Motwani NH, Hogfors H, Klawonn I, Ploug H, Sveden JB, Garbaras A, Sundelin B, Hajdu S, Larsson U, Elmgren R, Gorokhova E. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio. 2015;44(Suppl 3):413–26. https://doi.org/10.1007/s13280-015-0660-x.
Li H, Miller T, Lu J, Goel R. Nitrogen fixation contribution to nitrogen cycling during cyanobacterial blooms in Utah Lake. Chemosphere. 2022;302: 134784. https://doi.org/10.1016/j.chemosphere.2022.134784.
Mathivanan V, Vijayan P, Sabhanayakam S, Jeyachitra O. An assessment of plankton population of Cauvery River with reference to pollution. J Environ Biol. 2007;28(2):523.
Mhatre VD, Khandagale PA, Thomas S. Spatio-temporal variations of phytoplankton assemblage in coastal waters of Maharashtra, north-eastern Arabian Sea. Indian J Fisher. 2022;69(1):61–74. https://doi.org/10.21077/ijf.2022.69.1.115317-07.
Mohd-Shazali SM, Madihah J, Ali N, Cheng-Ann C, Brewin RJW, Idris MS, Noir PP. Dynamics of absorption properties of CDOM and its composition in Likas estuary, North Borneo, Malaysia. Oceanologia. 2022;64:583–94. https://doi.org/10.1016/j.oceano.2022.04.005.
Muñiz O, Rodríguez JG, Revilla M, Laza-Martínez A, Seoane S, Franco J. Seasonal variations of phytoplankton community in relation to environmental factors in an oligotrophic area of the European Atlantic coast (southeastern Bay of Biscay). Reg Stud Mar Sci. 2018;17:59–72. https://doi.org/10.1016/j.rsma.2017.11.011.
Naikwade PV, Bansode RB, Sankpal ST, Jadhav BB. Biodiversity of mangroves in estuarine ecosystems of Ratnagiri district. J Aquatic Biol Fisher. 2014;2:472–5.
Nair SR, Achuthankutty CT, Nair VR, Devassy VP. Plankton composition in the coastal waters between Jaigarh and Rajapur along west coast of India. Mahasagar-Bull Nat Inst Oceanogr. 1980;13(4):343–52.
Nelson NB, Siegel DA. Chromophoric DOM in the open ocean. In: Hansell DA, Carlson CA, editors. Biogeochemistry of marine dissolved organic matter. San Diego: Academic Press; 2002. p. 547–78.
Otero J, Álvarez-Salgado XA, Bode A. Phytoplankton diversity effect on ecosystem functioning in a coastal upwelling system. Front Mar Sci. 2020;7: 592255. https://doi.org/10.3389/fmars.2020.592255.
Pielou EC. The measurement of diversity in different types of biological collections. J Theor Biol. 1966;13:131–44.
Sankpal ST, Patil RM, Naikwade PV. Studies on seasonal trend in sea water quality of Ratnagiri coast, Maharashtra, India. Biosci Discov. 2015;6(1):85–92.
Shannon CE, Wiener W. The Mathematical Theory of Communication. Urbana, IL: University of Illinois Press; 1949.
Stramski D, Reynolds RA, Kaczmarek S, Uitz J, Zheng G. Correction of pathlength amplification in the filter-pad technique for measurements of particulate absorption coefficient in the visible spectral region. Appl Opt. 2015;54:6763–82.
Tanaka Y, Nakaoka M. Interspecific variation in photosynthesis and respiration balance of three seagrasses in relation to light availability. Mar Ecol Prog Ser. 2007;350:63–70. https://doi.org/10.3354/meps07103.
Tomas CR. Identifying marine phytoplankton. New York, NY, USA: Academic Press; 1997. p. 858.
Vishnu PS, Shaju SS, Tiwari SP, Menon N, Nashad M, Joseph AA, Raman M, Hatha M, Prabhakaran MP, Mohandas A. Seasonal variability in bio-optical properties along the coastal waters off Cochin. Int J Appl Earth Observ Geoinf. 2017;66:184–95. https://doi.org/10.1016/j.jag.2017.12.002.
Zapata M, Rodríguez F, Garrido JL. Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar Ecol Prog Ser. 2000;19:59–45.
Acknowledgements
The authors would like to express their gratitude to the Director, SAC (ISRO) and the Deputy Director, EPSA for the facilities and encouragement. The authors would like to thank the AVIRIS-NG team for their generous financial support in conducting field-work for in-situ data collection. Special thanks to Dr. Mangesh Shirdhankar, College of Fisheries, Ratnagiri, for the support extended in conducting field and laboratory analysis. We would like to acknowledge Mr. Srijith. B for the contribution to this research paper, specially for plotting the station map.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Aswathy Vijaya Krishna—Conceptualisation, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualisation, Writing- original draft; Anima Tirkey—Data curation, Formal analysis, Investigation, Methodology, Resources, Visualisation, Writing—original draft; Mini Raman- Project administration, Resources, Funding acquisition, Supervision; Syed Moosa Ali—Data curation, Project administration; Anurag Gupta—Data curation, Project administration; Sanjib Kumar Deb—Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Krishna, A.V., Tirkey, A., Raman, M. et al. Exploring the interplay between phytoplankton taxonomy, pigments, and optical properties in Ratnagiri coastal waters using a multi-parameter approach. Discov Oceans 1, 23 (2024). https://doi.org/10.1007/s44289-024-00025-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44289-024-00025-6