Abstract
We conducted an integrated study of phytoplankton taxonomic composition, biomass and physicochemical properties of the water in Admiralty Bay, South Shetlands. The aim of the study was to provide detailed information on phytoplankton composition and diversity related to relevant environmental conditions. High-performance liquid chromatography pigment analysis and microscopy were performed in the summers of 2007 and 2009–2010. Results for 2007 showed a typical high-nutrient, low-chlorophyll system. Total carbon biomass and cell numbers were dominated by nanoflagellates, while diatoms made up 1.2–4.5 % of the total algal numbers and contributed a maximum of 23.4 % to the total cell carbon. A small algal bloom occurred in the center of the bay, with chlorophyll a values of ~1.0 µg l−1. The prevalent pigment was 19′-hexanoyloxyfucoxanthin, characteristic of the Prymnesiophyceae. In January 2010, the values of chlorophyll a (8–24 µg l−1) and cell carbon (150 µg l−1) were the highest ever recorded for Admiralty Bay. In general the algal populations were dominated numerically by nanoflagellates, but a bloom of diatoms (maximum 30 % of total cells and 50 % of total cell carbon) was observed. Diatoms were dominated by those of micro-size: Thalassiosira ritscheri and T. antarctica. The dominant pigment was fucoxanthin, mainly found in diatoms. The diatom bloom could be related to the southeast wind direction, stable water column conditions and an inflow of diatom-rich waters from the Bransfield Strait, while the input of high-turbidity, low-salinity water from melting glaciers and strong katabatic northwest winds could favor nanoflagellates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous investigations of phytoplankton community structure and composition in Admiralty Bay, carried out in various seasons and summarized by Kopczyńska (2008), have revealed year-round dominance (in number and biomass) of nano-size cells, mainly nanoflagellates, over micro-size cells such as diatoms. Recently, the contribution of diatoms to the total cell numbers has been declining, from approximately 44 % in 1996–1998 to 5 % in 2003–2005. This finding was based on water samples from both the central bay and shore assemblages, the latter in the vicinity of the Henryk Arctowski Polish Antarctic Station.
Examination of surface samples (0–5 m) has shown that diatom blooms usually start near the shore and rarely merge with open water diatom assemblages (Kopczyńska 2008). Near the shore, the composition of diatom blooms is influenced by benthic species, while the composition in the bay as a whole is mainly influenced by diatoms, which are transported in water masses coming from the Brainsfield Strait.
Chlorophyll a concentrations in the bay are generally low (~0.5 µg l−1) and have not previously been found to exceed 2.0 µg l−1 (Tokarczyk 1986; Lipski 1987; Rakusa-Suszczewski 1993; Lange et al. 2007). Randomly performed primary productivity measurements in Admiralty Bay also showed low values. For example, the total annual productivity in surface waters in the center of the bay was estimated at 6.6 g C m−3 year−1; in summer 1988, the mean primary productivity was only 10 mg C m−3 year−1 (Domanov and Lipski 1990).
The present research initiated phytoplankton pigment monitoring in Admiralty Bay, enabling the investigation of large areas of water in the bay for the first time. We attempted to ascertain whether the seasonal and spatial distribution of major algal groups, calculated from the concentration of diagnostic carotenoids using the CHEMTAX program (Mackey et al. 1996), reflected the distribution of the same groups as determined by light microscopy analysis. By introducing pigment studies we hoped to find a suitable and relatively time-effective tool for estimating the composition of phytoplankton assemblages in this dynamic environment characterized by phytoplankton blooms that vary in both space and time (Kopczyńska 2008). The study included analysis of the physicochemical properties of water including temperature, salinity, turbidity, Secchi disc depth, photosynthetic active radiation (PAR) measurements, meteorological observations and concentrations of nutrients (N-NO3, N-NO2, N-NH4, P-PO4, SiO2). The main aim of our study was to analyze the variation in phytoplankton biomass and composition in relation to physical-chemical properties of the water column, to better understand these variations and to identify the causes of the typically low phytoplankton biomass and of the incidental phytoplankton blooms in coastal Antarctic waters.
Materials and methods
Study area
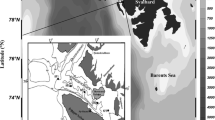
Investigations were conducted in Admiralty Bay (62°03′–12′S; 58°18′–38′W) (Fig. 1). It is the largest embayment on King George Island (South Shetlands), with a surface area of 119 km2, water volume of ca. 21 km3 (Lipski 1987) and maximum depth of 530 m. It has a number of features typical of a fjord: characteristic water circulation and a current system dependent on tides, wind, the morphology of the bottom and local upwelling. Exchange of waters between Admiralty Bay and the open ocean is one of the most important factors affecting the hydrography of the fjord. In summer (January, February) surface water movements in Admiralty Bay have been observed to be mostly determined by the strength and direction of katabatic winds (Nowosielski 1980; Pruszak 1980), which cause the outflow of surface water into the Bransfield Strait. In contrast, southeastern (SE) winds bring inflows of Bransfield Strait water into Admiralty Bay and Ezcurra Inlet (Nowosielski 1980; Pruszak 1980; Szafrański and Lipski 1982; Tokarczyk 1986; Domanov and Lipski 1990; Brandini and Rebello 1994). The winds induce water turbulence and resuspension of sediments in shallow parts of the bay. The input of fresh water from melting glaciers bearing mineral suspensions causes an increase in turbidity, decrease in light intensity and decrease in surface salinity (Lipski 1987; Schwarz and Schodlock 2009; Vernet et al. 2011). These phenomena contribute to the complex hydrology of the area, particularly near retreating glaciers and close to freshwater streams (Dierssen et al. 2002).
Location of sampling stations in Admiralty Bay for summer 2007 and 2009–2010: A Ezcurra Inlet (close to Dufayel Island); B center of Ezcurra Inlet; C exit of Ezcurra Inlet; D central Admiralty Bay; E Admiralty Bay exit to the Bransfield Strait., G Goulden Cove. White areas indicate retreat of the ice front between 1956 and 1995. The delineation of glacier drainage areas (black line) was taken from Simões et al. (1999)
Sampling
In summer 2007 we collected samples from four sites: Goulden Cove (site G, maximal depth 83 m) situated at the end of the bay; Ezcurra Inlet (site C, depth 225 m), the central part of Admiralty Bay (site D, depth 504 m); the entrance to the Bransfield Strait (site E, depth 522 m (Fig. 1). Collections were made on 9 (site C), 22 (sites D) and 26 January (site E) and 10 February (sites C and G) (Table 1). Sites D and E were sampled only once in the season, because bad weather conditions prevented us from sailing to the center and outlet of the bay.
In 2009–2010 most of the sampling sites in the bay were the same as in 2007 (C, D, E, G), and two other sites were included at Dufayel Island (site A, depth 66 m) and in the center of Ezcurra Inlet (site B, depth 154 m) (Fig. 1). Samples were taken three times in the season: at the end of December (29th), the beginning of January (2nd); mid-January (22nd, 26th); and mid-February (11th, 15th). Detailed sampling dates are presented in Table 1.
In both summer seasons, samples for chemical, pigment, and microscopic analysis were taken from the surface to a depth of 100 m, or to the sea floor at shallower stations, at 2.5-m intervals in the euphotic zone and at 5.0–10.0-m intervals below the euphotic zone. Samples were collected from a fishing boat using Niskin bottles and were transferred into 2.5-l opaque Nalgene plastic bottles.
Water samples for nutrient analysis (N-NO3, N-NO2, N-NH4, P-PO4, SiO2) were filtered through 45-mm Whatman GF/F filters and quickly deep-frozen (−70 °C) in the Arctowski laboratory.
Water samples for pigment analysis were filtered immediately after collection through Whatman GF/F glass-fiber filters under a gentle vacuum in dim light. Filters were stored in a freezer at −70 °C until analysis, which was carried out within 1 week of collection.
For phytoplankton estimation, 100-ml aliquots were preserved with glutaraldehyde-lugol solution at a final concentration of 1 and 2 %, respectively, then stored in the dark at 4 °C until analysis. In 2007 cell counting and identification were done for samples from 5-m and in 2009–2010 from 5-, 20- and 40-m depths from all the sampling sites.
In 2007, the physical characteristics of the water column (temperature, turbidity, conductivity and transparency) were determined in situ simultaneously with sample collection using a dissolved oxygen meter (WTW Oxi197i), a conductivity meter (WTW LF 197i) and a Secchi disc. In 2009–2010, the physicochemical properties of water were determined using a Midas Conductivity-Temperature-Depth (CTD) multiparameter profiler additionally equipped with a series of automatic sensors, an electromagnetic current meter (ECM), Chelsea Mini Tracka IIC Chlorophyll fluorometer, a PAR irradiance meter and Seapoint turbidity sensor.
The euphotic zone in 2007 was estimated as 2× Secchi depth. Although the euphotic depth is not a fixed multiple of Secchi depth, the 2× Secchi depth value is often used to determine the euphotic zone. In different studies estimates of euphotic depth have been reported from 1.16 to 2.3 times the Secchi depth (e.g., Davies-Colley and Vant 1988). We estimated the euphotic zone depth using PAR values from the water profiles for the 2009/2010 season. Euphotic zone depth reflects the depth where PAR is 1 % of its surface value (e.g., Lee et al. 2007).
Mixed layer depth (MLD) was calculated using the potential density anomaly, σ 0, where σ 0 = ρ − 1000 and ρ is the density in kgm−3. MLD is the depth at which σ 0 = σ 0 (surface) + 0.05 (Barth et al. 2001; Clarke et al. 2008).
Meteorological data were collected using automatic meteorological station type DAVIS Vantage pro 2.
Nano- and microplankton
Prior to counting and species identification, phytoplankton samples were placed for 24 h in 100 ml settling chambers (Utermöhl 1958). Algal cells were counted at 400× magnification with a Nikon inverted microscope, according to the procedures described by Utermöhl (1958). In every sample at least 300 cells were counted along randomly chosen transects made across the counting chamber. We considered the number of cells counted (ca. 300) to give a good representation of the algae contained in a sample. The method is the same as the one used in our previous work from Admiralty Bay (e.g., Kopczyńska 2008). Diatoms were identified to the species or genus level. Flagellates of the nano- (<20 µm) and micro-size (>20 µm) fraction were counted and identified to the class level. Nanoflagellates in Admiralty Bay include the classes of Prymnesiophyceae, Prasinophyceae (both autotrophic) and Cryptophyceae, which contain auto- and heterotrophic species. We did not distinguish heterotrophic from autotrophic flagellates. Dinophyceae were not included within the group of flagellates. We did identify the obvious heterotrophic dinoflagellates (e.g., micro-size species >20 µm of Gyrodinium) to the genus level. The following works were used for identification of the algae: Hasle (1965), Sournia et al. (1979), Priddle and Fryxell (1985), Medlin and Priddle (1990) and Steidinger and Tangen (1997).
Cell carbon was estimated from cell volumes and cell abundances. Cell volumes were calculated by comparing cell shapes to appropriate geometric figures (Eppley et al. 1970). The following cell volume-to-carbon relationships were used (Eppley et al. 1970; Smayda 1978): for diatoms, log C = 0.76 (logV) − 0.352; for other phytoplankton, log C = 0.94 (logV) − 0.60, with V representing total cell volume (µm3) and C representing cell carbon (pg). The carbon biomass of dinoflagellates (>20 µm) was calculated using the formula for other phytoplankton as shown above. Picoplankton cells were not counted.
Pigment analysis
The pigments were extracted in 3 ml of methanol in 6-ml centrifuge tubes by ultrasonification (2 min at 10 W; Omni-Ruptor 250) while kept on ice. Analyses were carried out in the laboratory at the Henryk Arctowski Station using a Shimadzu HPLC system equipped with a UV-Vis and fluorescence detector on a Waters Spherisorb C18 ODS2 column. The gradient method recommended by SCAR (Wright et al. 1991) was used to separate pigments. The pigments were identified by comparing their retention times and absorption spectra with standards (DHI Lab Products) and also with data in the literature (Jeffrey et al. 1997; Roy et al. 2011). Calibration curves were developed using external standards.
The chlorophyll a contribution of the different algal classes to the measured total chlorophyll a was calculated based on the fact that taxonomic classes differ in the composition and content of characteristic pigments. Some pigments can be used as biomarkers, e.g., fucoxanthin for Bacillariophyceae, 19′-hexanoyloxyfucoxanthin for Prymnesiophyceae, peridinin for Dinophyceae, prasinoxanthin for Prasinophyceae, alloxanthin for Cryptophyceae and chlorophyll b for Chlorophyceae (e.g., Wright et al. 1991; Peeken 1997). The ratio of chlorophyll a concentration in a given taxonomic group of phytoplankton to the total chlorophyll a concentration in the entire sample was used to calculate the chlorophyll a biomass (%) of the algal group using the CHEMTAX program.
Nutrient analysis
Analyses were carried out in our home laboratory in Poland 60–90 days later, following standard analytical procedures (Grasshoff et al. 1999) using a Shimadzu UV-160A spectrophotometer.
Statistics
Principal component analysis (PCA) was used to relate the CHEMTAX results showing the chlorophyll a of individual phytoplankton groups, as well as total chlorophyll a, to abiotic environmental factors (nutrients, water temperature, salinity and turbidity). Only the complete data sets from all sampled stations and all depths in the 2009–2010 season were included. The NIR test, Kolmogorow-Smirnow test and Mann-Whitney test (Statistica version 8) were used to assess the statistical significance of differences between mean values of the studied parameters.
Results
Physicochemical data
In 2007, the average water column salinities varied from 33.8 ± 0.3 practical salinity units (PSU) at a cove in the vicinity of melting glaciers (site G) to 34.1 ± 0.09 PSU in Ezcurra Inlet (site C). Generally, the salinity of surface waters (0–5 m) at all study sites was lower than in the deeper water (Table 1). Surface water temperature in January ranged from 0.6 °C at site G to 2.2 °C at site D. The MLD was the shallowest (5 m) at station G; at site D it reached 12.5 m and was 15 m at sites C and E. Meteorology data for the 2007 season showed dominating katabatic winds from the northwest (NW) with a mean velocity of 1.9–6.4 m s−1, which caused a flow of surface water toward the outlet of the bay. The bay was ice-free.
Weather records for the summer of 2009–2010 show low atmospheric pressure (976.7 hPa for January) and prevailing winds from the SE during the sampling period. Thick ice cover was present in December and at the beginning of January in small coves, while later in January only thin ice partly covered the coves. During sampling, transect sites were located in recently ice-free water, while site G was covered with pack ice. Average values of surface (0–5 m) salinity ranged from 34.12 ± 0.12 PSU in December and 34.10 ± 0.30 PSU in February to 33.84 ± 0.45 PSU in January. Surface water temperature increased from a minimum of 0.63 °C in December to 1.31 °C in January and 1.11 °C in February (Table 1).
In December 2010, when the small coves were covered with ice, the highest turbidity—10.98–11.66 nephelometric turbidity units (NTU)—was observed at sites A and B in Ezcurra Inlet. Following ice melting in January, turbidity values varied between 10.75 NTU in Admiralty Bay and 60.0 NTU in the cove (site G). The relatively great depth of the euphotic zone in December (60 m at site A; 92 m at site B) had decreased by half in the 3rd week of January and ranged between 24 m (site G) and 49 m (site D). In February, the euphotic depth increased to values similar to those in December (52 at site G; 99 m at site D). A very shallow MLD (from 1.7 m at site G to 8.2 at site D) was observed on 22–26 January and a slightly deeper MDL (from 10.3 m at site G to 15.8 at site E) on the other sampling dates in the 2009–2010 season. Nutrient data recorded for both seasons were typically high for Admiralty Bay (Table 1).
Phytoplankton variability
Nano- and micro-plankton
The average value of phytoplankton biomass measured as cell carbon for all study stations (at 5 m depth) in the 2007 season (January, February) was 29 ± 14 µg C l−1. There were no statistically significant differences (Mann-Whitney test, p = 0.35) between mean biomass values in January (25 ± 16 µg C l−1) and February (36 ± 16 µg C l−1). The highest biomass (49 µg C l−1) was recorded on 26 January, at the exit of Admiralty Bay to the Bransfield Strait (site E) (Fig. 2a). This site was also characterized by high total phytoplankton abundance (4.3 × 106 cells l−1) (Fig. 2b), while the average abundance for all sites and dates was 3.5 ± 0.8 × 106 cells l−1. Total carbon biomass was dominated by prymnesiophytes at all stations, except in site C at the beginning of January (Fig. 2a). Their contribution ranged from 27 % (site C, January 9th) to 80 % (site G, 10 February 10). The dominance of prymnesiophytes was also reflected in their high cell numbers (average value 3.0 ± 0.8 × 106 cells l−1). Cryptophytes constituted more than 50 % of total carbon biomass only in Ezcurra Inlet (site C) on 9 January (Fig. 2a). However, at the other stations and dates, their contribution did not exceed 17 %, similar to prasinophytes, whose contribution to the total carbon biomass was less than 20 %. The contribution to the total cell number of these two phytoplankton groups was also low, at 0.3–18 %. Diatoms contributed a maximum of 23.4 % (11.5 µg C l−1) to the total carbon biomass at the exit of Admiralty Bay (site E) on 26 January (Fig. 2a). At the same station the highest number of diatoms (1.9 × 105 cells l−1) was noted (Fig. 2b). Diatoms made up 1.2–4.5 % of the total algal cells, while dinoflagellates accounted for only 1 % of the cells wherever they were detected. Diatoms included both nano- and micro-size species. On 9 January, only small (10–15 µm) Thalassiosira spp. (total carbon biomass—0.5 µg C l−1) and Pseudo-nitzschia spp., mainly P. lineola (total carbon biomass—0.1 µg C l−1), were very common in Ezcurra Inlet. Two weeks later, small Thalassiosira spp. prevailed (maximum = 2.6 µg C l−1) at all sampling sites. They were accompanied by lower biomass (maximum = 0.2 µg C l−1) of Pseudo-nitzschia spp., Corethron pennatum, Rhizosolenia spp. and Navicula sp.
In the 2009/2010 season, the average value of phytoplankton biomass for the whole sampling period (31 ± 16 µg C l−1) was similar to the value in 2007, but there was much greater variation between study sites and dates, which is reflected in the high standard deviation. The mean value of 57 ± 25 µg C l−1 for January was statistically significantly higher than in December (21 ± 6 µg C l−1) and February (17 ± 16 µg C l−1) (Mann-Whitney test, p = 0.016 and p = 0.006, respectively). Generally prymnesiophytes contributed most to the carbon biomass (37 ± 8 %), and dinoflagellates provided 29 ± 12 %, diatoms 18 ± 9 %, prasinophytes 11 ± 12 % and cryptophytes 5 ± 3 %. There were no statistically important differences between mean biomass at the three sampling depths (5, 20, 40) (Fig. 3). The highest total carbon biomass (144 µg C l−1) was recorded on 22 January at site A, at a depth of 20 m (Fig. 3b). Diatom biomass changed from 3.9 ± 1.9 µg C l−1 in December through 15.9 ± 12.1 µg C l−1 in January to 1.5 ± 1.7 µg C l−1 in February. The highest diatom biomass was detected at site A on 22 January (47 µg C l−1), at a depth of 20 m, and the highest contribution of diatoms to the total biomass (51 %) was given at site B, also at a depth of 20 m (Fig. 3b). The January populations along the transect were composed almost exclusively of two chain-forming micro-size species: T. ritscheri (Hust.) Hasle (40–92 µm) and T. antarctica Comber (>20 µm). Their contribution in total carbon biomass of diatoms exceeded 93 % at all study sites except for the small cove (site G). A few other species, including T. tumida (Jan.) Hasle (>20 µm), the small (<10 µm) Thalassiosira spp. Fragilariopsis pseudonana, Fragilariopsis cylindrus, Navicula glacialis, Ps. lineola, Porosira sp., Navicula sp., Chaetoceros socialis and Biddulphia sp., were present in low biomass in all samples. It is interesting to note that in January and February the Goulden Cove (site G) samples were entirely devoid of live, intact diatoms; the samples collected in January contained very thick detritus and were dominated by small flagellates. The biomass of prymnesiophytes was a little higher in January than in other months at all study stations (Fig. 3), but the differences were not statistically significant. The contribution of cryptophytes to the total carbon biomass was significantly higher in February (10 ± 6 %) than in other sampling months.
The variations in phytoplankton cell numbers are less visible than in cell carbon biomass (Figs. 3, 4). There were no statistically significant differences in total cell number between sampling dates. Only prasinophytes and especially diatoms were found in greater abundance in January than in December and February (Mann-Whitney test for diatoms, p = 0.006 and p = 0.003, respectively). The maximum diatom numbers (3.8 × 105) were noted at the same station where the greatest diatom biomass was found (site A, 20 m, 22 January) (Fig. 4b). An unexpectedly high density (2.7 × 106 cells l−1) of Chaetoceros socialis, a relatively small (3–4 µm) chain-forming species, senescent in appearance, was found only at 5 m in February at site A (Fig. 4a). In most of the December and February samples, diatoms contributed 1.2–6 % to the total cell density, while in January they contributed 10–49 %. Nanoflagellates were always the most abundant (76 ± 6.7 %). Prymnesiophytes were dominated by motile and colonial forms (broken colonies) of Phaeocystis antarctica. Total cells comprised prasinophytes (9.5 ± 4.8 %), cryptophytes (3.5 ± 1.2) and dinoflagellates (3.9 ± 4.9 %). The senescent dinoflagellate cells were small and shrunken, with partly emptied contents and drab colors. Small (<12.5 µm) gymnodinioid forms and Gyrodinium spp. were most abundant at all times. Prorocentrum spp. and Amphidinium spp. were found less frequently.
Distribution of chlorophyll a
In 2007, chlorophyll a concentrations differed between January and February, as well as between the sampling stations. In January, the highest values were found in the central part of the bay (site D) and in the entrance to Bransfield Strait (site E). In surface waters (0–5 m), the concentrations were low (maximum values 0.1 and 0.7 µg l−1, respectively), but they increased with depth, reaching peaks (>1 µg l−1) at 7.5, 17.5 m and below the euphotic zone at 30–70 m (Fig. 5a). The January water profile average chlorophyll a concentration of 0.6 ± 0.3 µg l−1 in the central part and exit of Admiralty Bay (sites D and E) was significantly higher (NIR test, p < 0.05) than in Ezcurra Inlet (site C) and in the cove (site G). In the latter two sites, the average concentration of chlorophyll a was 0.3 ± 0.3 µg l−1 and 0.4 ± 0.1 µg l−1, and the highest value was 0.6 µg l−1 (Fig. 5a). Generally, chlorophyll a concentrations at all study sites were higher in February than in January (Kolmogorow-Smirnow test p < 0.05).
In 2010, chlorophyll a showed a distinct seasonal variability, increasing from December to a maximum in January and returning to low concentrations in February. The differences between January and other months are statistically significant (test NIR, p < 0.05). In December, the highest values of chlorophyll a at sites A, B and D occurred at depths of 0–25 m (site B; 0.78 µg l−1), but values exceeding 0.34 µg l−1 were still found at 70 m (Fig. 5b). A significant increase in chlorophyll a concentration (>1.13 µg l−1) was recorded on 2 January in the surface waters in the center of Admiralty Bay (site D) and in the Bransfield Strait (site E). Very high chlorophyll a values (11.5–13.7 µg l−1) were observed 20 days later in the inner part of the bay (Ezcurra Inlet; sites A and B), with the maximum reaching 24.0 µg l−1 in Goulden Cove (site G) at 10–25 m. Generally, in January (22nd and 26th) values exceeded 3 µg l−1 up to 8 µg l−1 at 50 m up to a depth of 90 m (e.g., site C; Ezcurra Inlet) (Fig. 5b). A return to much lower chlorophyll a concentrations (<2.0 µg l−1) was noted in February at all study sites (Fig. 5b).
Distribution of carotenoid pigments
Nineteen photosynthetic pigments were identified in the samples during both the 2007 and 2009–2010 seasons. These were chlorophyll and its derivatives (chlorophyll a, b and c; pheophytin a; pyropheophytin a; pheophorbide a; chlorophyllide a; achlorophyll a allomer) and carotenoids (fucoxanthin, 19′-butanoyloxyfucoxanthin, 19′-hexanoyloxyfucoxanthin, diadinoxanthin, peridinin, prasinoxanthin, diatoxanthin, alloxanthin, monadoxanthin and ββ-carotene).
In 2007, 19′-hexanoyloxyfucoxanthin was the dominant carotenoid during the entire season in Ezcurra Inlet (site C) and in Goulden Cove (site G), with an average value of 0.5 ± 0.07 µg l−1 and a maximum of 0.3 µg l−1. The concentration of fucoxanthin at these stations was much lower than 19′-hexanoyloxyfucoxanthin (average value 0.05 ± 0.05 µg l−1; maximum 0.13 µg l−1). 19′-Hexanoyloxyfucoxanthin is the pigment characteristic of the nano-size (<10 µm) prymnesiophytes. CHEMTAX results showed that prymnesiophytes made up 100 % of the phytoplankton biomass, measured as total chlorophyll a, at some depths. Prymnesiophytes were also dominant in the open waters of Admiralty Bay (site D); however, diatoms (characteristic pigment, fucoxanthin), cryptophytes (alloxanthin), prasinophytes (prasinoxanthin) and dinoflagellates (peridinin) also contributed significantly to the phytoplankton assemblages (Fig. 6). At site E (Admiralty Bay exit), all phytoplankton groups mentioned above were present with domination of prymnesiophytes, but a high concentration of fucoxanthin (average value 0.13 ± 0.2 µg l−1, maximum 0.6 µg l−1) indicated the increasing importance of diatoms.
In 2010, the dominant carotenoid pigment throughout the season was fucoxanthin (average value 0.9 µg l−1). Maximum concentrations (7 µg l−1 to >14 µg l−1) occurred at 10–20 m at sites A, B, C, D and G, indicating the occurrence of a diatom bloom in the middle of January (Fig. 7). The average value of 19′-hexanoyloxyfucoxanthin concentrations for the entire season was 0.04 µg l−1, and the maximum value of 0.18 µg l−1 was noted in January at station B. In December and February, high Spearman’s correlation coefficients (R = 0.932, R = 0.882, respectively, p < 0.05) between the concentrations of fucoxanthin and 19′-hexanoyloxyfucoxanthin at all sites indicated that a significant portion of fucoxanthin originated from prymnesiophytes. However, in January, the correlation between these pigments was much lower (R = 0.477, p < 0.05), indicating a diatom bloom. CHEMTAX results showed that the diatom contribution to the total chlorophyll a reached nearly 90 % at all study sites (Fig. 7). Other diagnostic pigments identified at all sites included prasinoxanthin, chlorophyll b, alloxanthin and monadoxanthin. Concentrations of prasinoxanthin were very low in December (average value 0.02 µg l−1), but increased significantly in January (average value 0.24 µg l−1) at all sites (Fig. 7). The lowest prasinoxanthin values were recorded in February (average value 0.004 µg l−1). A very high correlation (R = 0.851, p < 0.05) between prasinoxanthin and chlorophyll b indicated that chlorophyll b originated from prasinophytes rather than from chlorophytes. Alloxanthin and monadoxanthin (characteristic of cryptophytes) were not detected in December and January, but were found in February in the center of Admiralty Bay, when the contribution of cryptophytes to the total algal biomass was <10 % (Fig. 7).
Statistics
PCA showed that chlorophyll a in December 2009 could be mainly attributed to prymnesiophytes, followed by prasinophytes and diatoms. In January 2010, diatoms and prasinophytes were the major contributors to chlorophyll a. The diatom biomass was positively correlated with salinity and negatively with turbidity. In February 2010, all algal groups contributed equally to chlorophyll a concentrations (Fig. 8).
Phytoplankton composition versus carbon and pigment biomass during 2007 and 2009–2010
In 2007, the highest concentrations of chlorophyll a coincided with the sites of maximum carbon biomass, dominated by prymnesiophytes. The two dominant pigments, 19′-hexanoyloxyfucoxanthin and fucoxanthin, reflected this. The maximum carbon biomass in January 2010, attributed to diatoms and nanoflagellates, coincided with the sites of the highest chlorophyll a concentrations (Fig. 9). These results and the significant correlations of total phytoplankton carbon (heterotrophic dinoflagellates were excluded) with chlorophyll a (Fig. 9a), and also of diatom carbon with fucoxanthin (Fig. 9b), highlight the important contribution of both diatoms and nanoflagellates to the phytoplankton biomass in the summer of 2010. The important contribution of nanoflagellates to the phytoplankton biomass was also shown by significant positive correlations of the combined pigments (19′-hexanoyloxyfucoxanthin, prasinoxanthin and alloxanthin, characteristic of the major nanoflagellate groups) with the cellular carbon of nanoflagellates (Fig. 9c).
Discussion
Except for January 2010, when a large algal bloom occurred in Admiralty Bay, the present results from the summers of 2007 and 2009–2010 are very similar to the results of previously reported studies (Kopczyńska 1980, 1992, 2008). Nanoflagellates were numerically the most abundant group, and the carbon biomass of phytoplankton was low. Other studies have also reported the typical year-round numerical dominance of nanoflagellates in the seawater around the Antarctic Peninsula (Kang et al. 1997; Rodriguez et al. 2002; Annett et al. 2010). Also the chlorophyll a concentrations, which were generally observed in the lower part of the euphotic zone, did not exceed 1 µg l−1. They were comparable to those reported in previous summers at various locations including Admiralty Bay (Lange et al. 2007, 0.22 µg l−1); Potter Cove (Schloss et al. 2012, < 2 µg l−1); Marion Cove, King George Island (Kang et al. 1997, 0.17–0.63 µg l−1); the polar front zone and the Weddell-Scotia Confluence (Buma et al. 1990, 0.6 µg l−1); and the Gerlache and Bransfield Straits (Rodriguez et al. 2002, 0.18 µg l−1).
However, the sampling period in January 2010 showed a bloom of micro-size diatoms, predominantly the two chain-forming species T. ritscheri and T. antarctica. The values of chlorophyll a and cell carbon in January 2010 were the highest concentrations ever recorded in Admiralty Bay. The same phenomenon was observed at the same time in nearby Potter Cove, with a maximum chlorophyll a concentration of 14.7 µg l−1 (Schloss et al. 2014). Chlorophyll a values were of the order of those in Ryder Bay (9.04–20.0 µg l−1) during the summer, as reported by Clarke et al. (2008) and Annett et al. (2010), and typical for Marguerite Bay (2–5 µg l−1), based on SeaWiFS data (Smith et al. 2008). Conversely, the maximum summer carbon biomass in Ryder Bay (710 µg C l−1) and in Marguerite Bay (603 µg C l−1), which typically exhibit high algal standing stocks (Garibotti et al. 2003a; Annett et al. 2010), far exceeded the concentrations found in Admiralty Bay.
Pigment analysis, carotenoid concentrations, their proportions and correlations, and CHEMTAX results indicate that in many cases a large amount of the chlorophyll a in Admiralty Bay was contributed by prymnesiophytes. However, in the last 10 days of January, more than 70–90 % was contributed by diatoms. The maximum chlorophyll value was recorded on 22 January in Goulden Cove (G). The high chlorophyll a level must have been a result of both the great abundance of nanoflagellates at this site and the very thick detritus present in the water, evidently freshly formed and still containing pigments. HPLC results indicate that the main source of detritus might be the senescent diatoms because of the high concentration of fucoxanthin detected. Another probable additional source of some chlorophyll could be the picoplankton (>2 μm), which was not included in our microscopic counts.
The relative distribution of major algal groups estimated from concentrations of diagnostic carotenoids and chlorophyll a using the CHEMTAX program shows a similar pattern of phytoplankton composition and distribution as carbon biomass, but the calculation of cell carbon biomass using microscopic results in comparison with chlorophyll a values seems to be an underestimate. This may be because of the counting method used. Because of the numerical dominance of small cells, the counting of 300 cells at one (high) magnification might lead to an underestimation of the contribution of larger cells that, although less abundant, are often the major contributors to biomass. Other studies comparing phytoplankton characterization by microscopy and chemotaxonomy in the region of WAP showed high qualitative agreement (Garibotti et al. 2003a; Kozlowski et al. 2011), but the correlation coefficient was usually higher for the intermediate and high biomass communities (diatoms) than for low biomass communities (prasinophytes and mixed flagellates). The differences may result from variations and modifications in the cell carbon calculation methods, which are still under debate. Therefore, carbon calculations should only be treated as approximate estimates (Kopczyńska et al. 1995). However, considering variations in the physiological state of algal cells and the influence of the ambient environment on the pigment contents, as well as the occurrence of the same marker pigments in several algal groups, the HPLC results should also be interpreted with caution (Buma et al. 1990; Mackey et al. 1996; Peeken 1997).
It remains unclear why there are so few diatoms in Admiralty Bay. It is also unclear why diatom blooms occur so rarely, in spite of the presence of high silica concentrations and high concentrations of macro- and micronutrients, and also why phytoflagellates usually prevail numerically. Phytoplankton growth in the bay is unlikely affected by nutrient availability given the high macronutrient concentrations (Brandini 1993; Dick et al. 2007; Ardelan et al. 2010) and the influence of the Antarctic Peninsula ensuring high iron supply (De Jong et al. 2012). Additionally, during summer 2010, sea-ice melt should have further contributed to iron supply in the bay, promoting the diatom bloom. Sea ice acts as a medium for significant temporal iron storage, and sea-ice-entrained iron is one of the most bioavailable forms because of an abundant organic complexation coupled with photo-oxidation, either in situ or upon release into strongly stratified meltwater (van der Merwe et al. 2011). van der Merwe et al. (2011) concluded that if an increase of 1 nM of iron is sufficient for Antarctic diatoms to bloom, melting ice could fertilize 419 m3 of iron-limited surface water with total dissolved iron per m2 of fast ice.
In January 2010, fresh water inflow from melting ice and increased water temperatures caused low surface salinities (<34 PSU) in sites located in Ezcurra Inlet, which created suitable conditions for a shallow mixed layer of <2 m and increased water column stability. Throughout the region of the Western Antarctic Peninsula, many studies have found significant correlations between water column stability (largely resulting from freshwater lenses produced by sea-ice melt and glacial runoff) and the diatom concentration in this region (Kang and Lee 1995; Smith and Stammerjohn 2001; Garibotti et al. 2003b, 2005). The shallow mixed layer may allow algal cells to remain in the upper portion of the water column and to grow under a propitious light regime (Garibotti et al. 2005). The euphotic zone depth of 24–98 m, observed in 2010 in Admiralty Bay, also suggests good light conditions for phytoplankton bloom. In contrast, low transparency and high turbidity seem to be the major factors hindering growth of diatoms and promoting nanoflagellates, which are better adapted to dim light conditions. The results of PCA analysis show the negative relationship between high turbidity and diatom biomass. An earlier study in Admiralty Bay, concentrating on the small-scale vertical distribution of phytoplankton (every 1 m) (Kopczyńska 1980), revealed that diatoms were mainly found within the surface water layer of 0–30 m, while peaks of algal cells, formed in most cases by nanoflagellates, were often found below the euphotic zone. Subsequent studies in large areas of West Antarctica including the Bransfield Strait, Drake Passage and Admiralty Bay have documented that flagellates usually dominate numerically in areas of attenuated light conditions (Kopczyńska 1992). Evidence for light limitation of diatoms and of an apparently better adaptation to dim light conditions of nanoflagellates in the Antarctic was first reported by Holm-Hansen et al. (1977) and El-Sayed (1978). Experiments with cultured strains of Phaeocystis and the diatom Fragilariopsis cylindrus from the Ross Sea under several irradiance regimes have shown that Phaeocystis is physiologically well adapted to dim light in a deeply mixed water column, while F. cylindrus thrives in shallow mixed layers because of its ability to minimize photoinhibition (Kropuenske et al. 2009; Mills et al. 2010).
Another factor affecting phytoplankton biomass and composition in Admiralty Bay is the strength and direction of winds. Usually, in early summer, strong katabatic NW winds induce deep vertical mixing of shallow waters and generate strong currents responsible for the outflow of nutrient-rich surface water toward the open ocean. This results in the period of stability in the water column being too short to ensure the development of phytoplankton. In January 2010, the situation was different, and the diatom bloom was concomitant with long-lasting, prevailing weak SE winds, which might have caused an inflow of diatom-rich waters from the Bransfield Strait.
The maximum carbon biomass in January 2010 was made up of nanoflagellates, diatoms and dinoflagellates in almost equal proportions. This community structure suggests complex trophic relationships within the plankton at the peak of the bloom because of the large contribution of heterotrophic dinofalgellates. Sherr and Sherr (2007) noted that non-pigmented, phagotrophic dinoflagellates are potentially significant herbivores and likely to be more quantitatively significant consumers of bloom-forming diatoms than copepods and other mesozooplankton. The phytoplankton community in Admiralty Bay in January 2010 contained heterotrophic dinoflagellates, which may suggest a high grazing pressure at the peak of the bloom; however, the cold water (~0.6 °C) could reduce this pressure. There is some evidence that low temperature is one of the key factors limiting microzooplankton activity and grazing efficiency, leading to a decoupling of the trophic interaction between phytoplankton and herbivore grazing (Rose and Caron 2007; Chen et al. 2012). The control of nanoflagellate communities by protozoan grazers might explain the fact that nanoflagellates rarely contribute to bloom biomass, with the exception of Ph. antarctica, which forms large colonies. On the other hand, some iron enrichment experiments (Air-Sea Gas Exchange SAGE or Southern Ocean iron fertilization experiment LOHAFEX) showed the significant increase in the biomass of small flagellates. Their grow rate distinctly exceeded the grazing rate; as a result, their contribution to the phytoplankton bloom was much greater than that of diatoms, which were limited by the low concentration of silicic adic (Harvey et al. 2011; Peloquin et al. 2011; Martin et al. 2013).
Grazing of macrozooplankton is another factor controlling the phytoplankton biomass and community structure, but the role of these three groups of zooplankton in reducing the phytoplankton cell abundance is still under discussion.
The occurrence of the diatom bloom in 2010 might be the effect of various environmental factors operating in concert (Prézelin et al. 2004; Garibotti et al. 2005; Vernet et al. 2008; Montes-Hugo et al. 2008; Ducklow et al. 2012). However, the differences in wind direction between 2007 and 2009/2010 are probably the decisive factor. In 2007, katabatic winds led to an export of turbid, low-salinity waters out of Admiralty Bay, while in 2009/2010, SW winds led to advection of oceanic waters into Admiralty Bay. This might explain the different phytoplankton patterns observed in the two summers. The former scenario seems to be more common in Admiralty Bay and might explain the low chlorophyll biomass usually observed. The advection of high-turbidity, low-salinity surface waters from melting glaciers facilitated by the katabatic winds will favor nanoflagellates over diatoms. However, advection of oceanic diatoms into Admiralty Bay might explain the unusual bloom observed in January 2010. Once these diatoms enter the bay, they are likely to be exposed to elevated iron levels, which may trigger the bloom.
Conclusions
The results of the present study for January 2010 show that the development of diatom blooms in Admiralty Bay is occasionally possible with favorable weather conditions (prevailing weak SE winds), initially high levels of macro- and micronutrients (especially iron), increased stability of the water column and/or an inflow of phytoplankton-rich oceanic waters into the bay. In contrast, strong NW katabatic winds (observed in 2007), deeper mixed layers, turbulence and high turbidity leading to limited light availability are the likely factors favoring the growth of nanoflagellates in Admiralty Bay and consequently hindering the development of diatom blooms during most summer seasons.
The plankton community structure in January 2010 suggested complex trophic relationships within the plankton and an important contribution of the entire assemblage to carbon transport at all depths. Analysis of the phytoplankton pigments, chlorophyll a and carotenoids in Admiralty Bay and the results of the CHEMTAX program based on pigment analysis have mirrored the general trends in the spatial and temporal distribution of major algal groups observed by light microscopy.
References
Annett AL, Carson DS, Crosta X, Clarke A, Ganeshram RS (2010) Seasonal progression of diatom assemblages in surface waters of Ryder Bay, Antarctica. Polar Biol 33:13–29
Ardelan MV, Holm-Hansen O, Hewes CD, Reiss CS, Silva NS, Dulaiova H, Steinnes E, Sakshaug E (2010) Natural iron enrichment around the Antarctic Peninsula in the Southern Ocean. Biogeosciences 7:11–25
Barth JA, Cowles TJ, Pierce SD (2001) Mesoscale physical and bio-optical structure of the Antarctic Polar Front near 170°W during austral spring. Geophys Res 106:13879–13902
Brandini FP (1993) Phytoplankton biomass in an Antarctic coastal environment during stable water conditions - implications for the iron limitation theory. Mar Ecol Prog Ser 93:267–275
Brandini FP, Rebello J (1994) Wind field effect on hydrography and chlorophyll dynamics in the coastal pelagial of Admiralty Bay, King George Island, Antarctica. Antarct Sci 6:433–442
Buma AGJ, Treguer P, Kraay GW, Morvan J (1990) Algal pigment patterns in different water masses of the Atlantic sector of the Southern Ocean during fall 1987. Polar Biol 11:55–62
Chen B, Landry MR, Huang B, Liu H (2012) Does warming enhance the effect of microzooplankton grazing on marine phytoplankton in the ocean? Limnol Oceanogr 57:519–526
Clarke A, Meredith MP, Wallace MI, Brandon MA, Thomas DN (2008) Seasonal and interannual variability in temperature, chlorophyll and macronutrients in northern Marguerite Bay, Antarctica. Deep-Sea Res II 55:1998–2006
Davies-Colley RJ, Vant WN (1988) Estimation of optical properties of water from Secchi disk depths. Water Res Bull 24:1329–1335
De Jong J, Schoemann V, Lannuzel D, Croot P, de Baar H, Tison JL (2012) Natural iron fertilization of the Atlantic sector of the Southern Ocean by continental shelf sources of the Antarctic Peninsula. J Geophys Res. doi:10.1029/2011JG001679
Dick D, Philipp E, Kriews M, Abele D (2007) Is the umbo matrix of bivalve shells (Laternula elliptica) a climate archive? Aquat Toxicol 84:450–456
Dierssen HM, Smith RC, Vernet M (2002) Glacial meltwater dynamics in coastal waters west of the Antarctic peninsula. Natl Acad Sci USA 99:1790–1795
Domanov MM, Lipski M (1990) Annual cycle of chlorophyll a and primary production in Admiralty Bay (Antarctica). Pol Arch Hydrobiol 37:471–478
Ducklow HW, Clarke A, Dickhut R, Doney SC, Geisz H, Huang K, Martinson DG, Meredith MP, Moeller HV, Montes-Hugo M, Schofield O, Stammerjohn SE, Steinberg D, Fraser W (2012) The marine system of the Western Antarctic Peninsula. In: Rogers AD, Johnston NM, Murphy EJ, Clarke A (eds) Antarctic ecosystems: an extreme environment in a changing world. Blackwell, London, pp 121–159
El-Sayed SZ (1978) Primary productivity and estimates of potential yields of the Southern Ocean. In: McWhinnie MA (ed) Polar research, to the present and the future. AAAS Selected Symposium 7. Westview Press, Boulder, pp 141–159
Eppley RW, Reid FMH, Strickland JDH (1970) The ecology of the plankton off La Jolla, California, in the period April through September 1967. Bull Scripps Inst Oceanogr 17:33–42
Garibotti IA, Vernet M, Kozlowski WA, Ferrario ME (2003a) Composition and biomass of phytoplankton assemblages in coastal Antarctic waters: a comparison of chemotaxonomic and microscopic analyses. Mar Ecol Prog Ser 247:27–42
Garibotti IA, Vernet M, Ferrario ME, Smith RC, Ross RM, Quetin LB (2003b) Phytoplankton spatial distribution patterns along the western Antarctic Peninsula (Southern Ocean). Mar Ecol Prog Ser 261:21–39
Garibotti IA, Vernet M, Smith RC, Ferrario ME (2005) Interannual variability in the distribution of the phytoplankton standing stock across the seasonal sea-ice zone west of the Antarctic Peninsula. J Plankton Res 27:825–843
Grasshoff K, Ehrhardt M, Kremling K, Anderson LG (1999) Methods of seawater analysis. Wiley, New York
Harvey M, Law C, Smith M, Hall J, Abraham E, Stevens C, Hadfield M, Ho D, Ward B, Archer S, Cainey J, Currie K, Devri D, Ellwood M, Hill P, Jones G, Katz D, Kuparinen J, Macaskill B, Main W, Marriner A, McGregor J, McNeil C, Minnett P, Nodder S, Peloquin J, Pickmere S, Pinkerton M, Safi K, Thompson R, Walkington M, Wright S, Ziolkowski L (2011) The SOLAS Air-Sea Gas Exchange Experiment (SAGE). Deep Sea Res II 58:753–763
Hasle GR (1965) Nitzschia and Fragilariopsis species studied in the light and electron microscope. II The group Pseudonitzschia. Skrifter Litgitt Av Det Norske Videnskaps-Akademi 18:1–45
Holm-Hansen O, El-Sayed SZ, Franceschini GA, Cuhel K (1977) Primary production and the factors controlling phytoplankton growth in the Southern Ocean In: Adaptations within Antarctic ecosystems. Proceedings of the 3rd SCAR Symposium on Antarct Biol, Houston, Texas, pp 11–50
Jeffrey SW, Mantoura RFC, Wright SW (1997) Phytoplankton pigments in oceanography: guidelines to modern oceanography. UNESCO Publishing, Paris
Kang SH, Lee SH (1995) Antarctic phytoplankton assemblage in the western Bransfield Strait region, February 1993: composition, biomass, and mesoscale distributions. Mar Ecol Prog Ser 129:253–267
Kang SH, Kang JS, Chung KH, Lee MY, Lee BY, Chung H, Kim Y, Kim DY (1997) Seasonal variation of near shore Antarctic microalgae and environmental factors in Marian Cove, King George Island, 1996. Korean J Polar Res 8:9–27
Kopczyńska EE (1980) Small scale vertical distribution of phytoplankton in Ezcurra Inlet (Admiralty Bay, South Shetland Islands). Pol Polar Res 4:77–96
Kopczyńska EE (1992) Dominance of microflagellates over diatoms in the Antarctic areas of deep vertical mixing and krill concentrations. J Plankton Res 14:1031–1054
Kopczyńska EE (2008) Phytoplankton variability in Admiralty Bay, King George Island, South Shetland Islands: six years of monitoring. Pol Polar Res 29:117–139
Kopczyńska EE, Goeyens L, Semeneh M, Dehairs F (1995) Phytoplankton composition and cell carbon distribution in Prydz Bay, Antarctica: relation to organic particulate matter and its delta 13 C values. J Plankton Res 17:685–707
Kozlowski WA, Deutschmann D, Garibotti I, Trees C, Vernet M (2011) An evaluation of the application of CHEMTAX to Antarctic coastal pigment data Deep-Sea Res I 58:350–364
Kropuenske LR, Mills MM, van Dijken GL, Bailey S, Robinson DH, Welschmeyer NN, Arrigo KR (2009) Photophysiology in two major Southern Ocean phytoplankton taxa: photoprotection in Phaeocystis antarctica and Fragilariopsis cylindrus. Limnol Oceanogr 54:1176–1196
Lange PK, Tenenbaum DR, de Santis BragaE, Campos LS (2007) Microphytoplankton assemblages in shallow waters of Admiralty Bay (King George Island, Antarctica) during the summer 2002–2003. Polar Biol 30:1483–1492
Lee ZP, Weidemann A, Kindle J, Arnone R, Carder KL, Curtiss D (2007) Euphotic zone depth: its derivation and implication to ocean-color remote sensing. J Geophys Res. doi:10.1029/2006JC003802
Lipski M (1987) Variation of physical conditions, nutrients and chlorophyll a contents in Admiralty Bay (King George Island, South Shetland Islands, 1979). Pol Polar Res 8:307–322
Mackey MD, Mackey DJ, Higgins HW, Wright SW (1996) CHEMTAX – a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Mar Ecol Prog Ser 144:265–283
Martin P, Rutgers van der Loeff M, Caspar N, Vandromme P, d’Ovidio F, Stemmann L, Rengarajan R, Soares M, González HE, Ebersbach F, Richard S, Lampie RS, Sanders R, Burnett BA, Smetacek V, Wajch S, Naqvi A (2013) Iron fertilization enhanced net community production but not downward particle flux during the Southern Ocean iron fertilization experiment LOHAFEX, Global Biogeochem Cycles 27:871–881. doi:10.1002/gbc.20077
Medlin LK, Priddle J (1990) Polar marine diatoms. British Antarctic Survey, Cambridge, UK
Mills MM, Kropuenske LR, van Dijken GL, Alderkamp AC, Berg MG (2010) Photophysiology in two southern ocean phytoplankton taxa: photosynthesis of Phaeocystis antarctica (Prymnesiophyceae) and Fragilariopsis cylindrus (Bacillariophyceae) under simulated mixed-layer. J Phycol 46:1114–1127
Montes-Hugo MA, Vernet M, Martinson D, Smith R, Iannuzzi R (2008) Variability on phytoplankton size structure in the western Antarctic Peninsula (1997–2006). Deep-Sea Res II 55:2106–2117
Nowosielski L (1980) Meteorological conditions at Arctowski Station in 1978 (South Shetland Islands). Pol Polar Res 1:83–93
Peeken I (1997) Photosynthetic pigments fingerprints as indicators of phytoplankton biomass and development in different water masses of the Southern Ocean during austral spring. Deep-Sea Res II 44:261–282
Peloquin J, Hall J, Safi K, Ellwood M, Law CS, Thompson K, Kuparinen J, Harvey M, Pickmere S (2011) Control of the phytoplankton response during the SAGE experiment: a synthesis. Deep-Sea Res II 58:824–838
Prézelin BB, Hofmann EE, Moline M, Klinck JM (2004) Physical forcing of phytoplankton community structure and primary production in continental shelf waters of the Western Antarctic Peninsula. J Mar Res 62:419–460
Priddle J, Fryxell G (1985) Handbook of the common plankton diatoms of the Southern Ocean: Centrales except the genus Thalassiosira. British Antarctic Survey, Cambridge, UK
Pruszak Z (1980) Current circulation in Admiralty Bay (region of Arctowski Station on King George Island). Pol Polar Res 1:55–74
Rakusa-Suszczewski S (1993) Hydrography and hydrochemistry. In: Rakusa-Suszczewski S (ed) The maritime Antarctic coastal ecosystem of Admiralty Bay. PAS, Warsaw, pp 32–34
Rodriguez F, Ravela M, Zapata M (2002) Phytoplankton assemblages in the Gerlache and Bransfield Straits (Antarctic Peninsula) determined by light microscopy and CHEMTAX analysis of HPLC pigment data. Deep Sea Res II 49:723–747
Rose JM, Caron DA (2007) Does low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnol Oceanogr 52:886–895
Roy S, Llewellyn C, Egeland ES, Johnsen G (2011) Phytoplankton pigments: characterization, chemotaxonomy and applications in oceanography. Cambridge University Press, Cambridge, UK
Schloss IR, Abele D, Ferreyra GA, González O, Moreau S, Bers V, Demers S (2012) Response of Potter Cove phytoplankton dynamics to long term climate trends. J Mar Sys 92:53–66
Schloss IR, Wasilowska A, Dumont D, Almandoz GO, Hernando MP, Michaud-Tremblay CA, Saravia L, Rzepecki M, Monien P, Monien D, Kopczyńska EE, Bers V, Ferreyra GA (2014) On the phytoplankton bloom in coastal waters of southern King George Island (Antarctica) in January 2010: an exceptional feature? Limnol Oceanogr 59:195–210
Schwarz JN, Schodlock MP (2009) Impact of drifting icebergs on surface phytoplankton biomass in the Southern Ocean: ocean colour remote sensing and in situ iceberg tracking. Deep-Sea Res Part Oceanographic Res Papers 56:1727–1741
Sherr E, Sherr B (2007) Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar Ecol Prog Ser 352:187–197
Simões JC, Bremer UF, Aquino FE, Ferron FA (1999) Morphology and variations of glacial basins in the King George Island ice field, Antarctica. Ann Glaciol 29:220–223
Smayda TJ (1978) From phytoplankters to biomass. In: Sournia A (ed) Monographs on oceanographic methodology, phytoplankton manual. UNESCO, Paris, pp 273–279
Smith RC, Stammerjohn SE (2001) Variations of surface air temperature and sea-ice extent in the western Antarctic Peninsula region. Ann Glaciol 33:493–500
Smith RC, Martinson DG, Stammerjohn SE, Iannuzii RA, Ireson K (2008) Bellingshausen and western Antarctic Peninsula region: pigment biomass and sea ice spatial/temporal distributions and interannual variability. Deep-Sea Res II 55:1949–1963
Sournia A, Grall JR, Jacques G (1979) Plankton diatoms and dinoflagellates along a meridian transect in the Southern Indian Ocean (campagne “Antiprod I” du Marion-dufresne, mars 1977). Mar Bot 22:183–198
Steidinger KA, Tangen K (1997) Dinoflagellates. In: Tomas CR (ed) Identifying marine phytoplankton. Academic Press, California, pp 387–584
Szafrański Z, Lipski M (1982) Characteristics of water temperature and salinity at Arctowski Bay (King George Island, South Shetland Islands, Antarctic) during the austral summer 1978/1979. Pol Polar Res 3:7–24
Tokarczyk R (1986) Annual cycle of chlorophyll a in Admiralty Bay 1981–1982 (King George, South Shetlands). Pol Arch Hydrobiol 33:177–188
Utermöhl H (1958) Zur Vervollkommung der quantitativen Phytoplankton Mathodik Mitteilungen Internationale Vereinigung Theoretische und Angewandte. Limnology 9:1–38
van der Merwe P, Lannuzel D, Mancuso Nichols CA, Meiners K, Bowie AR (2011) Iron fractionation in pack and fast ice in East Antarctica: temporal decoupling between the release of dissolved and particulate iron during spring melt. Deep-Sea Res II 58:1222–1236
Vernet M, Martinson D, Iannuzzi R, Stammerjohn S, Kozlowski W, Sines K, Smith R, Garibotti I (2008) Primary production within the sea-ice zone west of the Antarctic Peninsula: I—Sea ice, summer mixed layer, and irradiance. Deep-Sea Res II 55:2068–2085
Vernet M, Sines K, Chakos AO, Ekern L (2011) Impacts on phytoplankton dynamics by free-drifting icebergs in the NW Weddell Sea. Deep-Sea Res II 58:1422–1435
Wright SWS, Jeffrey RFC, Mantoura CA, Liewellyn T, Bjornland D, Repeta D, Welschmeyer N (1991) Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar Ecol Prog Ser 77:183–196
Acknowledgments
We thank our friends who helped us with the field and laboratory work: Tadeusz Cieśluk, Elżbieta Krystyna Gromadka, Joanna Zubek and Krzysztof Zubek. The work was carried out as part of the IMCOAST Project (PolarCLIMATE-PP-001) European Partnership in Polar Climate Science. We would like to thank Doris Abele, the coordinator of this project, and Valeria Bers for valuable discussions. We would also like to sincerely thank the anonymous reviewers for their constructive comments with respect to our manuscript. The paper was supported by the project UMO—2012/05/B/ST10/01130 financed by the National Science Centre Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wasiłowska, A., Kopczyńska, E.E. & Rzepecki, M. Temporal and spatial variation of phytoplankton in Admiralty Bay, South Shetlands: the dynamics of summer blooms shown by pigment and light microscopy analysis. Polar Biol 38, 1249–1265 (2015). https://doi.org/10.1007/s00300-015-1691-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1691-2