Abstract
Concerns regarding the effects of heavy metals (HMs) on agricultural productivity have grown over time. Because HM stress disrupts a number of the plants' physiological-biochemical and metabolic processes, it severely limits production. Phytohormones can effectively improve plants resistance to HM stress. This work was done to examine the comparative effectiveness of salicylic acid (SA), 24–epibrassinolide (EBL) and sodium nitroprusside (SNP) on photosynthetic attributes, growth, & antioxidant enzymes activity in Linum usitatissimum cv. RLC–6 (flax) subjected to cadmium (Cd) stress during vegetative growth stages. Cd considerably decreases the length, biomass, leaf diameter, chlorophyll content, and photosynthetic traits; and further triggered ROS and MDA content in plant. Moreover, exogenous application of SA, EBL and SNP individually and in combination improved the antioxidant enzymatic machinery by increasing the levels of superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT) and decrease the superoxide, hydrogen peroxide, scavenges ROS and MDA accumulation. Furthermore, submission of phytohormones also caused proline to accumulate and the activities of carbonic anhydrase (CA) and nitrate reductase (NR) to be activated which were impaired due to Cd stress. Among the phytohormones, the most effective method for dropping the damaging impacts of Cd and promoting plant growth and development was EBL. However, combined application of all three phytohormones (SA + EBL + SNP) proved to be the best. Thus, it can be concluded that, these augmented activity of antioxidants and proline elicited by application of phytohormones, would have continued to be able to give Linum usitatissimum exposed to Cd stress resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The accretion of heavy metals (HMs) in the soil and atmosphere is primarily because of manmade actions such as mining, irrigation, sludge, fertilizer application, and business waste. These activities have led to augmented levels of HMs in the soil, which can cause toxicity in the soil and negatively affect the physiological and biochemical activities of plants [17, 19]. Among HMs, cadmium (Cd) is most dangerous for plants as it is highly mobile in soil [43]. Cd can easily be transferred from one plant part to another, resulting in impaired nutrient acceptance and disrupted ion homeostasis in plants, thus restraining the plant enlargement [74]. In plants, Cd stress include chlorosis, necrosis, wilting, leaf rolling, and root browning, as well as a decrease in photosynthesis, respiration, nitrogen metabolism, and ATPase activity [71]. The quantities of hydrogen peroxide (H2O2) and reactive oxygen species (ROS), and malondialdehyde (MDA) are all elevated in cadmium toxicity, thereby disrupts chloroplast ultrastructure and stomatal morphology, resulting in reduced transpiration, and gaseous exchange [71] and consequently restraint plants yield [28, 74, 86]. Therefore, Cd toxicity not only causes damage to agricultural land but also undermines sustainable agriculture. It is crucial to implement measures to restrict accretion of toxic metal in soil and negative effects on plants to ensure sustainable agriculture and environmental conservation.
Different methods are used to mitigate the harmful effects of Cd and exogenous supplementation of plant growth regulators (PGRs) is one such technique [65]. SA has been shown to enhance germination, photosynthesis, and the production of proline, glycine betaine, and total sugars, as well as glutathione reductase (GR), superoxide dismutase (SOD), peroxidase (POX), ascorbate peroxidase (APX), and catalase (CAT). These antioxidant molecules help to maintain the osmotic balance of the cell and scavenge ROS, which can mitigate environmental stressors such as Cd exposure [4]. Brassinosteroids (BRs) also mitigate the hazardous effect of Cd and improves plant development. BRs enhance chlorophyll content, photosynthesis and relative water content; it modulates other metabolic activity and promotes production of antioxidants during different abiotic stress [76]. Additionally, BRs application modulates ion influx in soil, enhances membrane stability, reduces ROS and diminishes accumulation of HMs. Thus, BRs application enhances stress tolerance and adaptation in plant [80]. Nitric oxide (NO) is a vital player in improving the morpho-physiological and biochemical characteristics of plants under abiotic stress. It is engaged in a number of signal transduction pathways in plants. Specifically, it promotes the antioxidant defense system by stimulating the activities of enzymes such as CAT, POX, SOD and proline. To achieve this effect, exogenous NO is often administered to plants as a donor of sodium nitroprusside (SNP). This approach helps to keep the appropriate germination, plant growth under adverse circumstances [60].
SA provides tolerance against Cd stress when supplied through various methods, such as pre-soaking, hydroponic exposure, or spraying [24]. It mitigates Cd-induced toxic responses by sustaining plant growth, Cd absorption and translocation in plants, cellular redox status, membrane stability and good performance of photosynthesis [55]. Similarly, EBL alleviate the damaging effect of Cd in plants by regulating growth, nutrient status photosynthetic pigment and antioxidative defense system [39, 69]. NO increases the activity of antioxidant enzymes, which confers resistance against cadmium exposure. It also improves seed yield and reduces Cd accumulation, and thus protects plants from Cd stress [53]. SA-mediated alleviation of Cd stress not only depends on antioxidants but by also affects other machineries of Cd reclamation [57]. SA decreases Cd assimilation and root-to-shoot translocation [24]. For instance, application of SA restricts Cd absorption and mitigated Cd-triggered growth inhibition in radish [64]. The activation of element translocators mediated by SA may be the cause of the restricted absorption and translocation of Cd into vacuoles [73] such as members of ABC transporter family. SA modifies the transcription of ABC transporters in Arabidopsis (Bovet and Martinoia). SA induces these transporters to enhance vacuolar sequestration of Cd in plants. EBL alters nutrients uptake to reduce Cd accumulation in plant tissues [39]. Similarly, EBL also limits Cd accretion by increasing absorption of calcium as well as regulating ionic homeostasis [13, 81]. Moreover, EBL promotes the uptake of Mg2+, Ca2+and K+ in roots which gets transported to leaves to minimize Cd translocation. Furthermore, EBL promotes ion homeostasis, diminishes the absorption of harmful ions, and heightens the interest in inorganic ions [79]. However, reason seems to be different in case of stress mitigation by NO. It pays to Cd2+ toxicity by augmenting Cd2+ against Ca2+ uptake [8] rather relieves Cd toxicity via alternative pathway (AP) which is not concerned in reducing the uptake of Cd [34].Therefore, plant hormones like SA, BRs and NO had the capabilities to ameliorate the environmental stress and provide plant sustenance.
Linum usitatissimum L. also called linseed oil crop or flax (common name alsi) belongs to Linaceae family. It is widely used for oil, fibber, medicine and food. Its seeds contain essential α–linolenic acid; additionally it is also a rich source of oil (41%), proteins (20%), fibber (28%), and moisture (6%), ash (4%) and trace amount of phenols and lignins. This makes it highly nutritious and helps in production of several healthy products. It also contains polysaccharides and phytochemicals which make it a valuable ingredient in health enhancing trait and nutraceutical industry [63]. Linum is also used in linen manufacturing, linoleum, cloth production and raged bags [26].
Combined application of SA, EBL, and SNP in reducing the toxicity of Cd in Linum usitatissimum has received very little attention in study so far. Determining the possible defensive interactions among these three phytohormones in the instruction of Cd lenience in Linum usitatissimum was the goal of the current study.
2 Material and methods
2.1 Plant
We got uniformly sized flaxseed or linseed (Linum usitatissimum) cv. RLC-6 seeds from IARI, New Delhi, India. Sodium hypochlorite was used for sterilization of seed.
2.2 Preparation of SA, EBL and SNP
Sigma-Aldrich Chemicals Pvt. Ltd. in India was the source of SA, EBL, and SNP. In a 100 ml volumetric flask, the appropriate amount of SA, EBL, and SNP were dissolved in 5 ml of ethanol to create the stock solution. After dilution, the stock solution was used to prepare the necessary concentrations of SNP (10–5 M), EBL (10–8 M), and SA (10–5 M).
2.3 Sources of cadmium stress
Sigma–Aldrich Chemicals Pvt. Ltd. in India provided the cadmium chloride that was employed as a Cd stress (50 mg/kg soil).
2.4 Experimental design and treatments
At the Department of Botany, Aligarh Muslim University, the experiment was carried out in November through January, during the year 2019–2020 under simple randomized complete block design. The flaxseed or linseed seeds were sown in grounded pots (diameter and length of 12 and 15 inches, respectively) containing soil and manure (3:1). Pots were setup at their natural sorroundings with 11/12 h day/night length. Overall 36 pots were used during the experiment, three pots were maintained for each treatment indicating three replicates (n = 3) for each treatment. The treatments given were (1) control (supplied with DDW only and without Cd), (2) Foliar SA treatment (10–5 M), (3) Foliar EBL treatment (10–8 M), (4) Foliar SNP treatment (10–5 M), (5) Cd treatment (50 mg/kg of soil), (6) SA + Cd, (7) EBL + Cd, (8) SNP + Cd, (9) SA + EBL + Cd, (10) SA + SNP + Cd, (11) EBL + SNP + Cd, (12) SA + EBL + SNP + Cd. As Linum is a slow growing plant and to investigate results clearly at 30 days after sowing (DAS) Cd was supplemented in soil. At 45 – 50 DAS (26th – 30th December, 2019), foliar spray of different treatments was given whereas at 60 DAS (11 January 2020), sampling was done to investigate several growth and physio–biochemical traits. Additionally, all the plants were well irrigated with tap water (300 mL) during early morning hours for healthy growth and development. Furthermore, foliar spray of different plant hormone was given in evening, one hour after sun–set with the help of the sprayer.
2.5 Growth traits
Measuring scale was used to measure SL and RL. Electronic weighing machine was used to measuring the FW. Dry weight measurement, shoot and root materials were store in oven at 80 °C for 24 h. Leaf area was assessed by drawing and evaluating the area of leaf replica on the graph paper having 1 cm X 1 cm grids.
2.6 SPAD chlorophyll
A non-invasive approach was used to estimate the amount of chlorophyll. The SPAD chlorophyll meter (SPAD–502; Konica, Minolta sensing, Inc., Japan) was used to measure the total chlorophyll in the leaf.
2.7 Photosynthetic attributes
Photosynthesis and related attributes viz; net photosynthetic rate (PN), internal carbon dioxide concentration (Ci), transpiration rate (E), and stomatal conductance (gs) of undamaged fresh leaves were measured using an infrared gas analyzer (IRGA) (LI–COR 6400, LICOR, and Lincoln, Nebraska, USA).
2.8 CA activity
We calculated the CA activity in leaves by applying the method described by Dwivedi and Randhawa [14].
2.9 NR activity
The method used to test nitrate reductase activity was Jaworski's [41].
2.10 Proline content estimation
Bates et al. [6] method was applied to evaluate the proline content.
2.11 Antioxidant enzyme activity
The Aebi [1] approach was used to measure the CAT activity. With minor adjustments, the Chance & Maehly [9] process was used to quantity the POX activity. However, the Kono [52] approach was used to measure SOD activity.
2.12 Reactive oxygen species estimation
2.12.1 Superoxide anion content
The superoxide anion content was determined using the Wu et al. [82] technique.
2.12.2 Superoxide anions localization
The location of the superoxide anions in the leaves was investigated using the Kaur et al. [44] approach.
2.12.3 Estimation of Hydrogen peroxide
The amount of hydrogen peroxide (H2O2) in the leaves was measured using Patterson et al.'s [61] method.
2.12.4 Hydrogen peroxide localization
The Kaur et al. [44] approach was utilized to study the localization of hydrogen peroxide in the leaves.
2.13 Lipid peroxidation
Using the approach of Heath and Packer [35], lipid peroxidation was related to malondialdehyde (MDA) level.
2.14 Histochemical detection of lipid peroxidation
After being submerged in Schiff's reagen for 60 min, the samples were rinsed with sulphite water (10% K2S2O3 + 1N HCl and DDW). A stero microscope was used to take the images [62].
2.15 Statistical analysis
SPSS 17.0 for Windows (SPSS, Chicago, IL, USA) was used to analyze the current data. Using standard error computations and analysis of variance (ANOVA) at a significance level of p < 0.05, the least significant difference (LSD) between treatments was determined.
3 Results
3.1 Growth characteristics
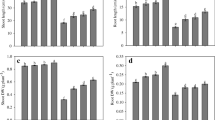
Cadmium stress in soil statistically hampered Linum growth parameters in respect of height, fresh and dry mass and leaf area. The values decreased for length of shoot by 26.95%, root by 35.89%, shoot fresh mass by 38.53%, root fresh mass by 47.46%, shoot dry mass by 29.61%, root dry mass by 44.06% and leaf area by 38.91% over their respective control (Fig. 1, D–G).However, the data depicted in the figures also shows that exogenous application of phytohormones like SA, EBL and SNP improved the above growth parameters in presence/absence of stress and EBL was superior over other two hormones in both the conditions. Furthermore, it was also noted that the stress generated by Cd is fully neutralized with combined treatment of phytohormones (Fig. 1). Amongst them SA + EBL + SNP is showed to be good and boost the length of shoot by 44.27%, root by 77.98%, fresh mass of shoot by 60.18%, root by 82.5% and leaf area by 33.33% as compared to control and even better than the Cd stressed plant.
Effect of plant hormone individually and in combination under cadmium stress and impact of cadmium on L. usitatissimum cv. RLC-6 (flax) Growth attributes that is A Shoot length, B Root length, C Leaf area, D Shoot fresh weight, E Root fresh weight, F Shoot dry weight, G Root dry weight at 60 days after sowing (DAS)
3.2 Photosynthetic performance
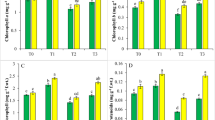
All the photosynthetic traits like PN, gs, Ci, E and SPAD level (chlorophyll content) were increased by the foliar application of SA, EBL, and SNP alone and jointly in non-stressful situations and Cd stress condition and triple combination (SA + EBL + SNP) was most active. However, the application of Cd through soil decreased the above characteristics. The respective decrease in PN value by 15.71%, gs by 50.01%, Ci by 39.44%, E by 36.53% and SPAD value by 18.95% was observed in Cd stressed plant relative to control. Furthermore application of SA + EBL + SNP improved the performance of above parameters by 62.7% (PN), 83.58% (gs), 33.33% (Ci), 7.14% (E) and 11.84 (SPAD value) respectively under Cd stress (Fig. 2 A–E).
3.3 Biochemical attributes
3.3.1 CA and NR
Plants emerged in Cd treated soil possess a significant influence and lead to reduce the content of CA (17.64%) and NR (18.42%) in respect to control. Additionally, plant applied with phytohormones SA, EBL and SNP only or in combined manner improved the activity of CA and NR. The extreme CA and NR activity was detected in combined application of SA + EBL + SNP which was about 81.23% (CA) and 22.36% (NR) more under stress. Furthermore, the adverse impact generated by Cd is mitigated by the adding of EBL, SA and SNP alone as well as in combination (Fig. 3, A–B).
Effect of plant hormone (individually and in combination) and cadmium on biochemical traits and antioxidant enzymatic machinery that is A Carbonic anhydrase activity, B Nitrate reductase activity, C Proline content, D Catalase activity, E Peroxidase activity, F Superoxide dismutase in L. usitatissimum cv. RLC-6 (flax) at 60 days after sowing (DAS)
3.3.2 Proline content
Proline is an osmoprotectant and its content was increased by 6.52% in response to Cd stress than control. However, maximum rise in proline amount during stress was observed in the presence of SA + EBL + SNP (54.34%) followed by EBL + SNP (45.65%) in comparison to control. It is observed that EBL alone gave the best results than other phytohormone (Fig. 3, C).
3.3.3 Activities of antioxidant enzyme
In contrast to the control, the data shown in figures (Fig. 3, D–E) clearly demonstrated that Cd treatment increased the CAT, POX, and SOD activity by 4.65%, 9.12%, and 6.25%, in that order. Additionally, the leaves treated with SA + EBL + SNP and receiving Cd through soil showed the greatest increases in CAT, POX, and SOD activity. The respective surge in CAT was 29.56%, POX 61.25% and SOD 36.6% over control.
3.4 Reactive oxygen species
3.4.1 Superoxide anion content and H2O2 content
The Cd stress lead to substantial increase in superoxide anion (O2·–) and accretion of H2O2 in leaves which was about 27.77% and 47.94% more, in comparison to control. After submission of SA, EBL and SNP alone or in combination production of reactive oxygen species was abridged. However, EBL was extra efficient in this regard (Fig. 4, F and F’).
Effect of plant hormone (individually and in combination) and cadmium on (F) superoxide anion content and A–E Superoxide anion localization on leaves of L. usitatissimum cv. RLC-6 (flax) images, F’ hydrogen peroxide content and A’–E’ Hydrogen peroxide localization on leaves of L. usitatissimum cv. RLC-6 (flax) images
3.4.2 Localization of superoxide anion and H2O2
Superoxide anion (O2·–) level was appeared by blue staining on leaves (Fig. 4, A–E) and H2O2 was exhibited by brownish spots on leaves (Fig. 4, A’–E’). In Cd treated plant, spots on leaves were more prominent than control. Moreover, accretion of O2·– and H2O2 in leaves was reduced after the application of phytohormone.
3.5 Lipid peroxidation (MDA content)
The application of Cd through soil caused substantial increase in MDA content (47.22%) in contrast to control. However, application of phytohormone like SA, EBL and SNP reduced the MDA amount. The foliar spray of the combination of SA + EBL + SNP was most beneficial (Fig. 5, F).
3.6 Identification of lipid peroxidation by histochemical means
MDA level in roots and leaves were appeared by pink and pinkish brown stains. Higher level of Cd stress exhibited prominent dark pink colour. However, loss of lipid peroxidation in roots and leaves was detected in the plants received phytohormone as foliar spray and the combination of SA + EBL + SNP was best (Fig. 5, A–E and A’–E’).
4 Discussion
A wide range of anthropogenic activities consequences in the accretion of HMs such as Cd in the soil. Cd is highly toxic that severely reduced the physiological and morphological attributes of the plant [16, 20, 37]. It hinders the intake of nutrients, the photosynthetic apparatus, the antioxidant apparatus, and plant growth [75]. In the current research, exposure of Linum to Cd resulted in stunted growth and reduced plant biomass along with leaf area (Fig. 1). Decrease in plant growth and biomass is one of the most common symptoms of Cd toxicity [29, 31, 33, 42]. The reduction of growth and biomass could be an after effect of decline in chlorophyll and photosynthetic values (Fig. 1A–G). Chlorosis is regarded as the main symptom which appears due to Cd toxicity [36] via exchange of Cd with Fe or Mg and affects the stability as well as biosynthesis of chlorophylls. Chlorophyll estimation is one of the important indicators of photosynthesis [11]. In the present outcome, the amendment of soil with Cd resulted in decrease of chlorophyll (Fig. 2E) which is in corroboration with the observation of Zargar et al. [88] who found a drop in SPAD values in Cd treated plants.
Plant growth and biomass directly depends on the photosynthates synthesized via photosynthesis [18]. A steep decline in gaseous exchange parameters was noted in plants raised in Cd amended soil (Fig. 2) which might be an outcome of Cd– induced stomatal deformity such as closure of stomata and reduction in stomatal density [84]. Comparable response was described by Zargar et al. [88] in mustard plants growing under Cd stress. Carbon dioxide binds with rubisco (major enzyme of Calvin cycle) in the attendance of CA to mark the commencement of C3 cycle [5]. Since, a decrease in Ci along with CA action was observed in Cd treated plants (Figs. 2B and 3A), this resulted in inducing a brake on efficient functioning of Calvin cycle, thereby reducing the photosynthate production [75]. NR is a vital enzyme linking the carbon and nitrogen assimilation in plants [58]. NR activity is closely linked to the concentration of CO2 in the environment, which is influenced by the availability of carbohydrates. Conversely, when carbon storage decreases, the uptake of nitrate decreases as well [66]. Supplementing the soil with Cd results in the inhabitation of NR activity (Fig. 3B) which suggests the reduction in protein synthesis [7] and ultimately, overall growth of the plant (Fig. 1A–G). The outcomes are lined with the study of Hayat et al. [30] where Cd mediated decrease in NR activity and growth was observed in tomato plants.
Phytohormones are important regulating molecules and hampered the development of the plants [21, 78]. In this work, the application of phytohormones (SA, SNP and/or EBL) to Linum usitatissimum resulted in enhancement of growth, SPAD value and photosynthetic attributes (Figs. 1, 2, 3) under presence/absence of Cd. The results align with previous research published by Shi et al. [73], Yuanjie et al. [85], Lu et al. [56] and Sami et al. [68]. Furthermore, the treatment of all three hormones together demonstrated the greatest efficacy in reducing the growth and photosynthetic properties' toxicity symptoms caused by Cd. (Figs. 1, 2, 3). SA + SNP, SA + EBL, SNP + EBL or SA + SNP + EBL minimized Cd–induced reduction of photosynthetic pigments (Fig. 2E), suggesting that SA– and/or NO– induced growth improvement partially relied on to their protective responses on photosynthetic pigments under Cd stress. The findings are in coherence with previous studies where combined application of SA, NO or BR enhanced resistance against abiotic stress [40, 72]). Combined and individual application of BR, NO or SA on rice under stress boosted the morphological parameters and further improved stress tolerance [22]. The stress alleviation by the application of SA and SNP might depend on their potential to control Cd–homeostasis by restraining Cd accumulation in maize and wheat plants [23, 46]. Hayat et al. [32] proposed that SA and/or BRs treatment on Brassica juncea improved CO2 assimilation inside the plant cells by enhancing CA activity. NO also participate in enhancing CA activity and mitigating abiotic stress [67]. BRs enhanced the activity of CA in several crops under stressed and non–stressed environment [3]. Our investigations are lined with the results of several researchers where treatment of SA, NO or BRs in combination or alone under stressed or non–stressed condition enhanced NR activity in several crops like mustard, tomato, mung, this further increased nutrient assimilation and membrane integrity [15, 88].
Upon encountering stressful conditions, plants tend to generate excessive levels of ROS that oxidize nucleic acids, protein and lipids, causing cellular disruptions [10, 12, 87]. Current article, outburst of O2·– and H2O2 level was triggered in plants under Cd stress which eventually resulted in higher MDA content (Figs. 4, 5) suggesting occurrence of oxidative damage. The outcomes are consistent with the earlier experiment conducted by Yan et al. [83] on Brassica napus, showing that Cd stress triggers ROS and MDA deposition in root and leaves. Against the ROS production, plants activate their defence mechanisms, including the generation of CAT, POX and SOD, and proline [45]. The documented rise in antioxidant enzymes (as shown in Fig. 3D–F) and proline levels (as illustrated in Fig. 3C) is anticipated to be a direct result of the ROS-induced response. Irfan et al. [38] reported similar response in mustard crop upon encounter with Cd stress. Current findings presented that follow–up treatment of phytohormones such as SA, NO or BRs alone or in combination to Cd–stressed reduced the ROS production and MDA accumulation (Figs. 4 and 5). SA, BRs or NO augmented antioxidant system which reduced O2·– and H2O2 content which in turn controlled the MDA level [25, 47, 54, 59, 70]. Agami [2] reported that SA and/or EBL supplementation mitigated stress by improving antioxidant machinery in maize. Similarly, application of SA and/NO on wheat unregulated the activity of CAT, POX and SOD and decreases ROS production to reduce stress [77]. Additionally, in tomato it was observed that EBL and/ or SNP treatment enhanced antioxidant enzymes. However, EBL showed best results in enhancing antioxidant enzymatic machinery [29, 33]. The osmotic adjustments done by proline help in maintaining plant turgor, membrane stability and antioxidant machinery [32]. Spray of plant hormone separately or in combination promoted proline production and its accumulation in Cd treated plants. Furthermore, combination of three plant hormones (SA + SNP + EBL) boosted up proline content followed by combination of two plant hormones. Results were supported by the investigations of Kohli et al. [50], where SA and/ or EBL application enhanced osmolyte content in mustard and reduced toxic effect of stress. Combined and individual application of SA and/or NO maintained cell turgor and osmotic balance in wheat during stress by increasing the proline content [77]. Considering the physiological aspects it could be inferred that all the three hormones altered the parameters in a similar pattern however, the difference lied amongst their efficiency in producing the response (Figs. 1, 2, 3, 4, 5).
Significant reduction of Cd toxicity in the flax plant was observed in the plants received SA, SNP, and EBL together. Kohli et al. [51] clarified that SA and EBL act combinedly to enhance crop resistance against abiotic stress moreover, BAK1 which is regarded as the receptor of BR signaling, its overexpression results in higher SA production [48]. Similarly, NO and SA collaborate to improve the accumulation of SA and jointly transmit the resistance signal by influencing the identical effectors proteins or their respective genes [49]. According to a recent finding [27], NR–reliant NO synthesis participates in the EBL–regulated mitigation of abiotic stress. Hence, simultaneous application of the three selected hormones creates a loop of signals that triggers the pronounced resistance in Linum usitatissimum against Cd stress (Fig. 6).
5 Conclusions
The present study's findings unequivocally showed that the damaging effects of Cd stress inhibited photosynthesis and the yield of Linum plant. However, by boost-up of antioxidant enzymes and detoxifying MDA, H2O2, and ROS in stressed plants, foliar administration of SA, SNP, or EBL, either alone or in grouping, to the Cd-treated Linum plants has reduced the toxicity. When applied in combination, SA, SNP, or EBL had a greater impact on reducing Cd stress in Linum plants than when applied separately. Furthermore, comprehensive research concentrating on the cellular level must be clarified in later investigations.
Data availability
The data generated and analyzed during the current study are available from the corresponding author.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agami RA (2013) Alleviating the adverse effects of NaCl stress in maize seedlings by pretreating seeds with salicylic acid and 24–epibrassinolide. S Afr J Bot 88:171–177
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ Exp Bot 62(2):153–159
Arif Y, Sami F, Siddiqui H, Bajguz A, Hayat S (2020) Salicylic acid in relation to other phytohormones in plant: a study towards physiology and signal transduction under challenging environment. Environ Exp Bot 1:104040
Badger MR, Price GD (1994) The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Biol 45(1):369–392
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water–stress studies. Plant Soil 39:205–207
Bavi K, Kholdebarin B, Moradshahi A (2011) Effect of cadmium on growth, protein content and peroxidase activity in pea plants. Pak J Bot 43(3):1467–1470
Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F et al (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149(3):1302–1315
Chance B, Maehly AC (1955) Assay of catalase and peroxidase. Methods Enzymol 2:764–775
Chen X, Honghua R, Xiaoqiao W, Youchao X, Yan Y (2022) Progresses in drought stress on the accumulation and turnover of soil organic carbon in forests. J Nanjing For Univ 46(6):195–206
Dalio RJD, Pinheiro HP, Sodek L, Haddad CRB (2011) The effect of 24-epibrassinolide and clotrimazole on the adaptation of Cajanus cajan (L.) Millsp to salinity. Acta Physiol Plant 33(5):1887–1896
di Toppi LS, Lambardi M, Pazzagli L, Cappugi G, Durante M, Gabbrielli R (1998) Response to cadmium in carrot in vitro plants and cell suspension cultures. Plant Sci 137(2):119–129
Dong C, He F, Berkowitz O, Liu J, Cao P, Tang M et al (2018) Alternative splicing plays a critical role in maintaining mineral nutrient homeostasis in rice (Oryza sativa). Plant Cell 30(10):2267–2285
Dwivedi RS, Randhawa NS (1974) Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant Soil 40(2):445–451
Faizan M, Faraz A, Hayat S (2018) Dose Dependent Response of Epibrassinolide on the Growth, Photosynthesis and Antioxidant System of Tomato Plants. Indian J Hortic 8(2 and 3): 68–76.
Faizan M, Karabulut F, Alam P, Yusuf M, Tonny SH, Adil MF, Sehar S, Ahmed SM, Hayat S (2023) Nanobionics: a sustainable agricultural approach towards understanding plant response to heavy metals, drought, and salt stress. Nanomaterials 13:974
Faizan M, Rajput VD, Al-Khuraif AA, Arshad M, Minkina T, Sushkova S, Yu F (2021) Effect of foliar fertigation of chitosan nanoparticles on cadmium accumulation and toxicity in Solanum lycopersicum. Biology 10:666
Faizan M, Sehar S, Rajput VD, Faraz A, Afzal S, Minkina T, Sushkova S, Adil MF, Yu F, Alatar AA, Akhter F, Faisal M (2021) Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 10:2254
Faizan M, Cheng SH, Tonny SH, Robab MI (2022) Specific roles of strigolactones in plant physiology and remediation of heavy metals from contaminated soil. Plant Physiol Biochem 192:186–195
Faraz A, Faizan M, Sami F, Siddiqui H, Hayat S (2020) Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Growth Regul 39(2):641–655
Fariduddin Q, Zaid A, & Mohammad F (2019) Plant Growth Regulators and Salt Stress: Mechanism of Tolerance Trade–Off. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution (pp. 91–111). Springer, Singapore.
Farooq M, Wahid A, Lee DJ, Cheema SA, Aziz T (2010) Drought stress: comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J Agron Crop Sci 196(5):336–345
Gondor OK, Pál M, Darkó É, Janda T, Szalai G (2016) Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS ONE 11(8):e0160157
Guo B, Liu C, Liang Y, Li N, Fu Q (2019) Salicylic acid signals plant defence against cadmium toxicity. Int J Mol Sci 20(12):2960
Guo J, Zhou R, Ren X, Jia H, Hua L, Xu H et al (2018) Effects of salicylic acid, Epi–brassinolide and calcium on stress alleviation and Cd accumulation in tomato plants. Ecotoxicol Environ Saf 157:491–496
Guo Y, Qiu C, Long S, Wang H, Wang Y (2020) Cadmium accumulation, translocation, and assessment of eighteen Linum usitatissimum L. cultivars growing in heavy metal contaminated soil. Int J Phytoremiat 22(5):490–496
Gupta P, Seth CS (2020) Interactive role of exogenous 24 Epibrassinolide and endogenous NO in Brassica juncea L. under salinity stress: Evidence for NR–dependent NO biosynthesis. Nitric Oxide 97:33–47
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Exogenous silicon attenuates cadmium–induced oxidative stress in Brassica napus L by modulating AsA–GSH pathway and glyoxalase system. Front Plant Sci 8:1061
Hayat S, Hasan SA, Hayat Q, Ahmad A (2010) Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma 239(1–4):3–14
Hayat S, Hasan SA, & Ahmad A (2011) Growth, nitrate reductase activity and antioxidant system in cadmium stressed tomato (Lycopersicon esculentum Mill.) cultivars. BASE.
Hayat S, Khalique G, Wani AS, Alyemeni MN, Ahmad A (2014) Protection of growth in response to 28–homobrassinolide under the stress of cadmium and salinity in wheat. Int J BiolMacro 64:130–136
Hayat S, Maheshwari P, Wani AS, Irfan M, Alyemeni MN, Ahmad A (2012) Comparative effect of 28 homobrassinolide and salicylic acid in the amelioration of NaCl stress in Brassica juncea L. Plant Physiol Bioch 53:61–68
Hayat S, Yadav S, Ali B, Ahmad A (2010) Interactive effect of nitric oxide and brassinosteroids on photosynthesis and the antioxidant system of Lycopersicon esculentum. Russ J Plant Physiol 57(2):212–221
He D, Cui J, Gao M, Wang W, Zhou J, Yang J et al (2019) Effects of soil amendments applied on cadmium availability, soil enzyme activity, and plant uptake in contaminated purple soil. Sci Total Environ 654:1364–1371
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hernández-Baranda Y, Rodríguez-Hernández P, Peña-Icart M, Meriño-Hernández Y, Cartaya-Rubio O, Postal G (2019) Toxicity of Cadmium in plants and strategies to reduce its effects. Case study: The tomato. Cult Trop 40(3):e10
Hongna M, Wei W, Lei F et al (2023) Effects of Piriformospora indica on growth and drought resistance in Osmanthus fragrans under water deficit stress. J Nanj For Univ 47(2):101–106. https://doi.org/10.12302/j.issn.1000-2006.202203014
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21(2):125–131
Jan S, Alyemeni MN, Wijaya L, Alam P, Siddique KH, Ahmad P (2018) Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol 18(1):146
Jangid KK, Dwivedi P (2017) Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol Plant 39(3):73
Jaworski EK (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279
Jinadasa N, Collins D, Holford P, Milham PJ, Conroy JP (2016) Reactions to cadmium stress in a cadmium–tolerant variety of cabbage (Brassica oleracea L): is cadmium tolerance necessarily desirable in food crops? Environmental SciPollut Res 23(6):5296–306
Jianchao W, Wenmin Q, Kangming J et al (2023) Comprehensive analysis of WRKY gene family in Sedum plumbizincicola responding to cadmium stress. J Nanj For Univ 47(2):49–60. https://doi.org/10.12302/j.issn.1000-2006.202201015
Kaur N, Sharma I, Kirat K, Pati PK (2016) Detection of reactive oxygen species in Oryza sativa L. (rice). Bio Protoc 6:1–9
Kaur R, Yadav P, Thukral AK, Walia A, Bhardwaj R (2017) Co–application of 6–ketone type brassinosteroid and metal chelator alleviates cadmium toxicity in B. juncea L. Environ Sci Pollut Res 24(1):685–700
Kaya C, Akram NA, Sürücü A, Ashraf M (2019) Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Sci Hortic 255:52–60
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant 168(2):345–360
Kim SY, Shang Y, Joo SH, Kim SK, Nam KH (2017) Overexpression of BAK1 causes salicylic acid accumulation and deregulation of cell death control genes. Biochem Biophys Res Comm 484:781–786. https://doi.org/10.1016/j.bbrc.2017.01.166
Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P, Trifa Y (2000) Nitric oxide and salicylic acid signaling in plant defense. ProcNat AcadSci 97(16):8849–8855
Kohli SK, Handa N, Bali S, Arora S, Sharma A, Kaur R, Bhardwaj R (2018) Modulation of antioxidative defense expression and osmolyte content by co–application of 24–epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol Environ Saf 147:382–393
Kohli SK, Handa N, Sharma A, Kumar V, Kaur P, Bhardwaj R (2017) Synergistic effect of 24–epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turk J Biol 41(6):943–53
Kono Y (1978) Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Kumari A, Sheokand S, Swaraj K (2010) Nitric oxide induced alleviation of toxic effects of short term and long term Cd stress on growth, oxidative metabolism and Cd accumulation in Chickpea. Braz J Plant Physio 22(4):271–284
Li Q, Wang G, Wang Y, Yang D, Guan C, Ji J (2019) Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol Environ Saf 172:317–325
Liu Z, Ding Y, Wang F, Ye Y, Zhu C (2016) Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep 35(4):719–731
Lu Q, Zhang T, Zhang W, Su C, Yang Y, Hu D, Xu Q (2018) Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. EcotoxicolEnviron Saf 147:500–508
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132(1):272–281
Mir AR, Alam P, Hayat S (2021) Effect of different levels of soil applied copper on the morpho-physiological, photochemical, and antioxidant system of Brassica juncea. J Soil Sci Plant Nutr 21:3477–3492
Mostofa MG, Rahman M, Ansary M, Uddin M, Fujita M, Tran LSP (2019) Interactive effects of salicylic acid and nitric oxide in enhancing rice tolerance to cadmium stress. Int J Mol Sci 20(22):5798
Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun BG, Yun BW (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot 161:120–133
Patterson BD, Mackae EA, Mackae I (1984) Estimation of hydrogen peroxide in plants extracts using titanium (iv). Anal Biochem 139:487–492
Pompella A, Maellaro E, Casini AF et al (1987) Histochemical detection of lipid peroxidation in the liver of bromobenzene–poisoned mice. Am J Pathol 129:295–301
Rashid F, Ahmed Z, Hussain S, Huang JY, Ahmad A (2019) Linum usitatissimum L. seeds: Flax gum extraction, physicochemical and functional characterization. Carbohydr Polym 215:29–38
Raza SH, & Shafiq F (2013) Exploring the role of salicylic acid to attenuate cadmium accumulation in radish (Raphanus sativus). Int J Agric Biol 15(3).
Rizwan M, Ali S, Adrees M, Rizvi H, Zia–ur–Rehman M, Hannan F, et al (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23(18):17859–17879
Sakr S, Wang M, Dédaldéchamp F, Perez-Garcia MD, Ogé L, Hamama L, Atanassova R (2018) The sugar–signaling hub: overview of regulators and interaction with the hormonal and metabolic network. Int JMolSci 19(9):2506
Sami F, Faizan M, Faraz A, Siddiqui H, Yusuf M, Hayat S (2018) Nitric oxide–mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO–mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 73:22–38
Sami F, Siddiqui H, Hayat S (2020) Nitric Oxide-Mediated Enhancement in Photosynthetic Efficiency, Ion Uptake and Carbohydrate Metabolism that Boosts Overall Photosynthetic Machinery in Mustard Plants. J Plant Growth Regul 11:1–23
Sanjari S, Keramat B, Nadernejad N, & Mozafari H (2019) Ameliorative effects of 24-epibrassinolide and thiamine on excess cadmium-induced oxidative stress in Canola (Brassica napus L.) plants. J Plant Interact 14(1), 359–368.
Shah AA, Ahmed S, Yasin NA (2019) 24–epibrassinolide triggers cadmium stress mitigation in Cucumis sativus through intonation of antioxidant system. S Afr J Bot 127:349–360
Shahid M, Dumat C, Khalid S, Niazi NK, & Antunes PM (2016) Cadmium bioavailability, uptake, toxicity and detoxification in soil–plant system. Rev Environ Contam T Volume 241 (pp. 73–137). Springer, Cham.
Shao RX, Xin LF, Guo JM, Zheng HF, Mao J, Han XP et al (2018) Salicylic acid–induced photosynthetic adaptability of Zea mays L. to polyethylene glycol–simulated water deficit is associated with nitric oxide signaling. Photosynthetica 56(4):1370–1377
Shi GR, Cai QS, Liu QQ, Wu L (2009) Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31(5):969–977
Shiyu QIN, Hongen LIU, Zhaojun NIE, Rengel Z, Wei GAO, Chang LI et al (2020) Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere 30(2):168–180
Siddiqui H, Ahmed KB, Sami F, Hayat S (2020) Phytoremediation of Cadmium Contaminated Soil Using Brassica juncea: Influence on PSII Activity, Leaf Gaseous Exchange, Carbohydrate Metabolism, Redox and Elemental Status. Bull Environ Contamin Toxicol 28:1–1
Siddiqui H, Hayat S, Bajguz A (2018) Regulation of photosynthesis by brassinosteroids in plants. Acta Physiol Plant 40(3):59
Siddiqui MH, Al–Whaibi, MH, Ali HM, Sakran AM, Basalah MO, & AlKhaishany MY, (2013) Mitigation of nickel stress by the exogenous application of salicylic acid and nitric oxide in wheat. Aust J Crop Sci 7(11):1780
Vardhini BV (2020) Brassinosteroids and salicylic acid as chemical agents to ameliorate diverse environmental stresses in plants. Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives 389.
Waisi HK, Petković AZ, Nikolić BR, Janković BŽ, Raičević VB, Lalević BT, Giba ZS (2017) Influence of 24-epibrassinolide on seedling growth and distribution of mineral elements in two maize hybrids. Hem Ind 71(3):201–209
Wani AS, Ahmad A, Hayat S, Tahir I (2019) Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol Bioch 135:385–394
Wani AS, Tahir I, Ahmad SS, Dar RA, Nisar S (2017) Efficacy of 24-epibrassinolide in improving the nitrogen metabolism and antioxidant system in chickpea cultivars under cadmium and/or NaCl stress. Sci Hortic 225:48–55
Wu GL, Cui J, Tao L, Yang H (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicol 19:124–132
Yan H, Filardo F, Hu X, Zhao X, Fu D (2016) Cadmium stress alters the redox reaction and hormone balance in oilseed rape (Brassica napus L.) leaves. Environ Sci Pollut Res 23(4):3758–3769
Ying RR, Qiu RL, Tang YT, Hu PJ, Qiu H, Chen HR et al (2010) Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J Plant Physiol 167(2):81–87
Yuanjie DO, Weifeng CH, Xiaoying BA, Fengzhen LI, Yongshan WA (2019) Effects of exogenous nitric oxide and 24–epibrassinolide on the physiological characteristics of peanut seedlings under cadmium stress. Pedosphere 1:45–59
Yuanyuan W, Baichao L, Bo J, Danni W, Caiqiu G (2022) Th2CysPrx gene enhanced abiotic stress tolerances of Saccharomyces cerevisiaee. J Nanj For Univ 46(4):87–94
Yuhua Y, Jingjing J, Xiaodie Q, Guijiao W, Jianwei Z (2022) Effects of combined saline-alkali stress on physiological and biochemical characteristics of OT hybrid lily. J Nanj For Univ 46(4):117–126
Zargar TB, Mir AR, Alam P, Hayat S (2022) Melatonin alleviates cadmium-induced toxicity by modulating antioxidant defence mechanisms, growth and photosynthesis in Brassica juncea. Russ J Plant Physiol 69(6):121
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
SN and YA performed the experiments and analyzed the data of the experiments. HS prepared the figures. SH Anayat Rasool Mir, MF and PA prepared the first draft of the manuscript. All authors read and approved the final manuscript. Declarations Conflict of Interest The authors declare that there is no conflict of interest between them. Ethical Approval There is no need for ethical clearance.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nazir, S., Arif, Y., Mir, A.R. et al. Comparative and interactive response of salicylic acid, 24–epibrassinolide or sodium nitroprusside against cadmium stress in Linum usitatissimum. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00145-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00145-x