Abstract
Hydrogen fuel is recognized as a promising energy carrier for the sustainable development of global energy system and the green hydrogen production via water electrolysis attracts great interest. The development of cost-effective electrocatalysts for water electrolysis is important for enhancing green hydrogen production efficiency. Recently, tungsten pnictides (phosphides and nitrides) have emerged as promising catalysts for water electrolysis, and efficient tungsten pnictide-based catalysts with different nanostructures, compositions, and surface chemical properties have been developed. In this review, recent progress in the design of tungsten pnictide-based electrocatalysts for water electrolysis is comprehensively analyzed. The synthesis of tungsten pnictide-based electrocatalysts are discussed briefly. Then, current achievements in developing efficient tungsten pnictide electrocatalysts for water electrolysis are detailed, and four key catalyst design strategies (i.e., nanostructure control, heteroatom doping, defect engineering, and heterostructure design) are outlined. The physicochemical properties-catalytic performance relationship of tungsten pnictide-based electrocatalysts is also discussed. At last, perspectives in this field are put forward for guiding further research on the design and application of high-performance tungsten pnictide-based electrocatalysts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
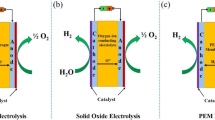
The global climate change and energy crisis evoke the development of sustainable energy systems. Hydrogen, with zero carbon footprint, has gained growing interest as a favorable energy carrier [1,2,3,4]. To meet the high demand of green hydrogen fuels, water electrolysis becomes a critical process in the sustainable hydrogen energy supply chain [5, 6]. Water electrolysis consists of oxygen evolution reaction (OER, anode part) and hydrogen evolution reaction (HER, cathode part). The hydrogen generation efficiency is highly dependent on the applied catalysts which are used to reduce the energy barrier of reactions [7,8,9]. Thus, great efforts have been made to develop high-performance electrocatalysts for water electrolysis. Although noble metal (e.g., Ru, Ir, Pt, Os)-based materials exhibit high intrinsic activities, their high costs hinder their large-scale applications [10, 11]. To address this issue, researchers have turned to low-cost transition metal-based materials.
Among diverse transition metal-based catalysts, tungsten pnictides (phosphides and nitrides) gain growing interest due to their high electronical conductivity, flexible phase/electronic structure, good catalytic activity, high earth abundance, and excellent chemical stability [12, 13]. Recent work has focused on enhancing the catalytic performance of tungsten pnictides with diverse catalyst design strategies [14, 15]. For example, Kavinkumar et al. developed a W2N3/Fe2N composite electrocatalyst for overall water splitting (OWS) [16]. Compared with single W2N3 or Fe2N, the optimal W2N3/Fe2N composite shows enhanced activities. For W2N3/Fe2N, the overpotentials at the current density of 50 mA·cm–2 (η50) for OER and HER are 268.5 and 187.2 mV respectively, and the cell voltage at 50 mA·cm–2 for OWS is just 1.68 V. Further density functional theory (DFT) calculations indicate that the coupling of W2N3 with Fe2N leads to an upshift of the d band center, which helps to optimize the adsorption/desorption of reaction intermediates and ultimately improve the catalytic performance. Aside from constructing composite catalysts, nanostructure control [17], heteroatom doping [18], and defect engineering [19] are also powerful methods to enhance the catalytic performance of tungsten pnictides (Scheme 1). To date, diverse efficient tungsten pnictide-based catalysts with different nanostructures, compositions, and surface chemical properties have been designed for water electrolysis, especially for HER. Hence, it is urgent to provide a comprehensive review that focuses on recent achievements of tungsten pnictide-based catalysts, which remains unavailable.
In this review, we thoroughly analyze recent advances in the design of tungsten pnictide-based electrocatalysts for water electrolysis. The water electrolysis mechanism is not included here, which has been extensively reviewed [20,21,22,23]. The synthesis of tungsten pnictide-based electrocatalysts are first discussed briefly. Then, current efforts in developing efficient tungsten pnictide electrocatalysts for water electrolysis are detailed, and four key catalyst design strategies are outlined. Additionally, catalysts’ physicochemical properties-catalytic performance relationship is emphasized. Finally, several perspectives in this field are pointed out for guiding further research.
2 Synthesis of tungsten pnictide-based electrocatalysts
The synthesis of tungsten pnictide-based materials generally involves a high-temperature process, namely phosphization or nitridation. To date, various tungsten pnictide-based catalysts have been fabricated from different chemical precursors. In this section, the one-step direct phosphization/nitridation and multi-step synthesis methods for constructing different types of tungsten pnictide-based electrocatalysts are discussed.
2.1 Electronic properties of representative tungsten pnictides
Both tungsten phosphides and nitrides have many different crystal structures with various stoichiometric ratios. Currently, only several tungsten phosphides and nitrides have been explored as electrocatalysts for water electrolysis. As shown in Fig. 1, orthorhombic WP, monoclinic WP2 (α-WP2), and orthorhombic WP2 (β-WP2) are the widely used tungsten phosphides for HER [24]. The electronic structures of the three tungsten phosphides suggest that these materials have a metallic feature, indicating ultrahigh electronical conductivity. Since the electrons near Fermi level have a major influence on the catalytic properties of materials [25, 26], it can be concluded that W sites in these tungsten phosphides possess high activity for catalysis. Compared with β-WP2, α-WP2 has a higher proportion of electrons located near the Fevel level, which could lead to its better catalytic performance [27].

Reproduced with permission from copyright Materials Project® and Ref. [24], Open Accessed 2013
Crystal structures and electronic structures (P: Purple; W: Blue) of representative tungsten phosphides.
Compared with tungsten phosphides, tungsten nitrides have more flexible compositions (e.g., WN, W2N3, WN2, W7N6, W3N4, WN6). However, only several of them have been developed for electrocatalytic applications. Figure 2 shows the crystal structures and electronic structures of cubic WN, trigonal W2N, and hexagonal W2N3, which have shown good HER performances [17, 28, 29]. Similar to tungsten phosphides, these tungsten nitrides possess a metallic feature, making them highly conductive materials for electrocatalysis. From the density of states (DOS) figures, it can be seen that W sites in these three tungsten nitrides have high activity. In addition, N sites in hexagonal W2N3 also hold promising activity for electrocatalysis due to the significantly high density of the N electron near the Fermi level. Overall, tungsten pnictides have high electronical conductivity which is highly important for electrochemical reactions [30, 31]. Also, the DOS of tungsten pnictides suggest rich active sites in these materials.

Reproduced with permission from copyright Materials Project® and Ref. [24], Open Accessed 2013
Crystal structures and electronic structures (N: Light grey; W: Blue) of representative tungsten nitrides
2.2 One-step phosphization/nitridation
One-step phosphization/nitridation methods are generally used to synthesize tungsten pnictide nanoparticles under a high temperature and oxygen-free atmosphere [32]. The phosphization/nitridation conditions significantly influence the properties of obtained materials. For example, Pi et al. found that α-WP2 and β-WP2 could be obtained from WO3 and red phosphorus precursors under different phosphization conditions [27]. The former one can be synthesized at 700 °C for 3 h, while the latter takes 950 °C for 5 h (Fig. 3a, b). The different phases of WP2 lead to various HER performance, and α-WP2 are more active than β-WP2. It is interesting to find that the precursor ratio also governs the phase structure of WP2. Nkabinde and coauthors successfully synthesized WP and α-WP2 with the W (WCl6):P (trioctylphosphine) precursor ratios of 1:1 and 1:20 respectively, at 340 °C for 6 h [33]. Compared with the WP catalyst, the phosphorus rich α-WP2 displayed better HER activity owing to the lower hydrogen adsorption energy, lower H–H formation energy barrier, longer M–P bond length and lower d-band center. The effect of phosphization temperature on the crystallinity of tungsten pnictides also has been investigated. Starting from Na2WO4·2H2O and NaH2PO2·H2O precursors, Zhang et al. synthesized a series of WP catalysts under 300–800 °C [34]. The crystallinity of WP increases with the growth of reaction temperature, and the optimal one for HER is determined to be obtained at a low temperature of 300 °C, due to the formation of rich W vacancies and relatively high conductivity.

Reproduced with permission from Ref. [27]. Copyright 2016, Elsevier. c Illustration of the synthesis of VN/WN@NC; d TEM and e HRTEM images of VN/WN@NC; f schematic of VN/WN@NC during electrocatalytic process. Reproduced with permission from Ref. [37]. Copyright 2022, Elsevier
a Scheme of the synthesis of α-WP2 and β-WP2; b X-ray powder diffraction patterns of WO3, α-WP2 and β-WP2.
Composite materials also can be synthesized by one-step phosphization/nitridation methods, especially carbon/tungsten pnictide heterostructures. During the high-temperature process, carbon precursors (e.g., melamine) can be pyrolyzed and form carbon materials [35, 36]. Heating melamine together with W and N/P precursors can thus generate carbon supported/covered tungsten pnictides. As shown in Fig. 3c, the one-step pyrolysis treatment at 900 °C generates a composite material which is composed of heterogeneous VN/WN particles isolated in N-doped carbon [37]. The transmission electron microscopy (TEM) and high-resolution TEM images suggest that active VN/WN nanoparticles are well dispersed on the N-doped carbon (Fig. 3d, e). The conductive N-doped carbon substrate not only populates the active sites by limiting the aggregation of VN/WN particles but also provides rich channels for efficient electron transfer during the electrochemical reaction (Fig. 3f).
2.3 Multi-step synthesis
To enlarge the active sites of tungsten pnictides, many studies have focused on designing nanostructures (e.g., nanoarrays) for water electrolysis. Nanoarrays with an open architecture can provide rich active sites, facilitate mass/charge transfer, and accelerate gaseous product transport. The fabrication of tungsten pnictide-based nanoarrays generally requires a tungsten-based precursor (e.g., WO3) that has a nanoarray structure, and thus the final tungsten pnictides inherit the nanostructure of the precursor after the phosphization/nitridation process. For instance, Wang and coauthors fabricated a porous Mo-W-P nanosheet catalyst with a hydrothermal treatment-phosphization method (Fig. 4a) [38]. In the first step, the Mo-W-O with an interconnected nanowire structure was obtained by the hydrothermal process. As indicated by the scanning electron microscopy (SEM) images, the original Mo-W-O nanowires evolved into porous Mo-W-P nanosheets with a thickness of ~ 100 nm, after the phosphization treatment at 700 °C (Fig. 4b, c). In the phosphization process, the skeleton of Mo-W-O is maintained, and the P/O exchange process would trigger the coarsening growth of nanowires and leads to the formation of nanosheets. Similar tungsten oxides to tungsten pnictides conversion strategies are widely reported for the construction of nanoarray catalysts [39,40,41,42].
The synthesis process of tungsten pnictide-based composites involves multiple steps, especially for the design of tungsten pnictide/oxides, tungsten pnictide/sulfides, and tungsten pnictide/hydroxides. In these composites, the formation conditions of oxides, sulfides, and hydroxides differ significantly from those of tungsten pnictides, necessitating the use of various methods in combination to obtain the final composites. Recently, Chen et al. designed a ternary nanosheet heterostructure (CeP5O14/WP/WS2) with a four-step hydrothermal treatment-electrodeposition-sulfurization-phosphization method (Fig. 4d) [43]. In the final step, both CeO2 and WS2 react with NaH2PO2 H2O and leads to the formation of tri-component CeP5O14/WP/WS2. Despite the lengthy synthesis process involved, the flexibility of active components (e.g., alloys, metal (hydr)oxides, sulfides, selenides, borides, carbides, fluorides) and synthesis methods (e.g., pyrolysis, electrodeposition, solvothermal/hydrothermal process, sulfurization, selenization, phosphization, chemical vapor deposition, nitridation, boronation, and ball milling) make it possible to construct high-performance tungsten pnictide-based electrocatalysts.
3 Advanced tungsten pnictides for water electrolysis
To date, tungsten pnictides with different nanostructures, compositions, and surface chemical properties have exhibited high catalytic performance towards HER, OER, and OWS, especially HER. In this section, current efforts in developing efficient tungsten pnictide-based electrocatalysts for water electrolysis are discussed, and key catalyst design strategies are outlined. In addition, catalysts’ physicochemical properties-catalytic performance relationship is emphasized.
3.1 Nanostructure control
The nanostructure of electrocatalysts directly influences the active surface area, exposure of active sites, catalyst/electrolyte contact, and gaseous product transport during the electrocatalytic process [44,45,46]. Therefore, nanostructure design has attracted great interest. Catalysts with smaller sizes exhibit a larger specific surface area (SSA) and therefore possess more electroactive sites for catalytic reactions [47]. Many studies have thus fabricated tungsten pnictide submicroparticles and nanoparticles for water electrolysis [32, 48, 49]. With a temperature-programmed hydrogen reduction method, Xing and coauthors prepared WP2 submicroparticles with diameters ranging from 150 to 400 nm and a Brunauer–Emmett–Teller (BET) SSA of 37.4 m2·g−1 [32]. The HER performance of the WP2 submicroparticles is much higher than the bulk WP2 counterpart with a low SSA of 7.8 m2·g−1. McEnaney et al. prepared an amorphous tungsten phosphide nanoparticle catalyst (WP NP) with an average diameter of ~ 3 nm [48]. Compared with the bulk crystalline WP, WP NP shows significant improved HER activities (η10 = 120 vs. 200 mV) in 0.5 mol·L−1 H2SO4. While the authors attribute the enhanced performance to the reduced size, it is important to note that the amorphous phase also contributes to the improved HER activity due to its flexible electronic structure, abundant defects, and presence of rich active sites [50,51,52].
Open architectures like nanoarrays are favorable for electrochemical reactions, which can facilitate the mass/charge transfer, improve electrolyte transport and penetration, and accelerate gaseous product release. WP2 nanosheets [39], flower-like W2N3 [17], WP nanorod arrays [53], network-like WP [54], three-dimensional (3D) flower-like WP2 nanowire arrays [55], 3D WP nanorod arrays [56], and WN nanorod arrays [42] are representative open structures for water electrolysis. In general, these arrays are supported on a large surface area substrate and synthesized from tungsten-based precursors with an open architecture, like hydrothermally synthesized WO3. In 2020, Liu et al. reported self-supported 3D WP2 and WP nanosheet arrays with a solvothermal process-phosphatization method (Fig. 5a) [41]. In Fig. 5b–d, WP2 nanosheet arrays retain the vertical growth morphology of the solvothermally synthesized WO3 precursor. WP nanosheet arrays show a similar open structure, and the thickness of WP nanosheets is slightly higher than that of WP2 nanosheets (Fig. 5e–g). Further computational studies suggest that the calculated adsorption free energy of hydrogen (ΔGH ∗) value of WP2 is closer to zero than WP ((− 0.56) eV vs. (− 0.66) eV), implying WP2 possesses better HER activities than WP (Fig. 5h–j). Compared with binder-involved electrodes, two advantages of these self-supported electrodes are the enhanced structural stability and the reduced fabrication cost [57].

Reproduced with permission from Ref. [41]. Copyright 2020, Elsevier
a Scheme of the synthesis of WP2 and WP nanosheet (NS) array; b-d SEM images of WP2 NS arrays; e–g SEM images of WP NS arrays; crystal models of h WP2 and i WP; j calculated ΔGH ∗ for Pt, WP, and WP2.
Porous materials with abundant voids and channels are particularly advantageous for electrocatalytic reactions, as they can provide a high SSA that promotes better catalytic performance [58, 59]. The high surface area can offer plentiful catalytically active sites, and ample channels can improve reactant transport [60, 61]. Additionally, the abundant nanoscale voids deliver defective sites and nano-confinement effects, thereby enhancing catalysts’ activity and durability [62]. In the binder-free 3D mesoporous WP2 nanowire arrays, the integrated 3D nanostructures and rich mesopores not only expose rich active sites but also facilitate electrolyte penetration for HER [63]. As a result, the mesoporous WP2 nanowire arrays exhibit good catalytic performance (η10 = 109 mV, Tafel slope = 56 mV·dec−1). Similarly, the porous WP2 nanosheet arrays (P-WP2 NSs) synthesized by a pulsed laser deposition-hydrothermal process-vacuum phosphization method show high activities towards HER (Fig. 6a) [64]. The P-WP2 NSs exhibit a 3D configuration with a rough surface, as numerous pores are formed during the phosphization process (Fig. 6b, c). Compared with WP2 nanosheet arrays (WP2 NSs), the porous catalyst shows obviously improved HER activities in 0.5 mol·L−1 H2SO4 (Fig. 6d, e), which should be due to the increased catalyst loading (4.8 mg·cm−2 vs. 3.5 mg·cm−2) and the reinforced charge/mass transport.

Reproduced with permission from Ref. [64]. Copyright 2017, Elsevier
a Schematic of the synthesis of P-WP2 NSs/GP (GP: graphite paper); b SEM and c TEM images of P-WP2 NSs/GP; d linear sweep voltammetry (LSV) curves and e Tafel plots of P-WP2 NSs/GP, WP2 NSs/GP, and Pt/GP.
3.2 Heteroatom doping
Although tungsten pnictides exhibit promising performance towards water electrolysis, their intrinsic activities need further improvements. Heteroatom doping is a powerful strategy to enhance catalytic performance by regulating the electronic properties of electrocatalysts [65]. Depending on the property of dopants (anion doping, cation doping, and anion-cation dual doping), a series of heteroatom doped tungsten pnictides have been developed. For cation doping, transition metals like Co [66], Mo [67], Mn [18], Cu [18], and Ni [68] are widely used as dopants. Wang et al. have investigated the HER performance of WP, Co-doped WP, and Mo-doped WP [69]. Compared with WP (η10 = 261 mV, Tafel slope = 112 mV·dec−1) and Mo-doped WP (η10 = 175 mV, Tafel slope = 75 mV·dec−1), Co-doped WP shows a lower η10 of 119 mV and a smaller Tafel slope of 55 mV·dec−1 in 1 mol·L−1 KOH (Fig. 7a, b). This activity trend is also witnessed in both acidic and medium electrolytes. Thus, Co is more favorable as a dopant than Mo. DFT calculations unveil that both Mo and Co can help to increase the ΔGH ∗ and reduce the energy barrier in acidic HER (Fig. 7c). In the case of neutral/alkaline HER, which begins with the dissociation of water molecules, the addition of Co dopants significantly reduces the energy barrier for water dissociation. However, the addition of Mo shows limited effects on water dissociation (Fig. 7d). The Co dopant can accelerate water dissociation and molecular hydrogen formation during HER, therefore leading to better performance. Kim et al. also compared the effect of four cation dopants (Co, Cu, Cr, Mn) on the alkaline HER performance of WP/WOx nanowires [18]. These dopants are suggested to promote the water dissociation step, and Co shows an optimum oxophilicity and leads to the best HER performance.

Reproduced with permission from Ref. [69]. Copyright 2019, Elsevier
a HER LSV curves and b corresponding Tafel plots of catalysts in 1 mol·L−1 KOH; DFT calculations of HER energy profiles of Co-WP, Mo-WP, and WP in c acid electrolyte and d neutral/alkaline electrolytes.
Following these comparative studies, many studies have employed Co dopant to enhance the HER performance of tungsten pnictides [66, 70]. Huo and coworkers fabricated a Co-doped WP nanoparticle catalyst with a η10 of 111 mV [71], while the reason of the enhanced catalytic activity was not given. With experimental and computational tools, Liu et al. have uncovered the beneficial role of Co dopant in improving the performance of WN [66]. The Co-doped WN (Co/WN-20) shows a nanowire array nanostructure with a low diameter (Fig. 8a–c). Compared with bare WN, the optimal Co/WN-20 exhibits better HER performance in acidic, neutral, and alkaline solutions (Fig. 8d–f). Further poisoning tests indicate that the Co dopant itself works as HER active sites (Fig. 8g), in addition to improving the intrinsic activity of W sites. DFT calculations verify that the Co dopant is highly active for HER, and the activity of W sites in Co-doped WN is higher than the analogue in pristine WN (Fig. 8h). Further DOS results indicate that Co doping leads to a lower d band center (Fig. 8i), which optimizes the interaction between catalyst surface and the reaction adsorbate and ultimately improves the HER activity. Differently, in the Co-doped WP2, Yang et al. suggested that the Co dopant site is inert for HER [70]. The Co dopant improves the HER performance of WP2 by reducing the water dissociation energy barrier and ΔGH* and enhancing the conductivity due to the strong electron-donor ability of Co.

Reproduced with permission from Ref. [66]. Copyright 2020, WILEY‐VCH
a and b SEM images, and c TEM image of Co/WN-20; HER LSV curves of WN, Co/WN-20 and Pt/C catalyst in d alkaline, e acidic, and f neutral solutions; g HER LSV curves of WN and Co/WN-20 in the absence and presence of SCN−; h ΔGH* over W(WN), Co(Co/WN), and W(Co/WN) sites; i DOS of d orbitals of W(WN), Co(Co/WN), and W(Co/WN) and scheme of bond formation between adsorbates (Ads.) and surface.
Besides cation dopants, several studies also employ anions to improve the catalytic performance of tungsten pnictides by regulating the electronic properties. Yan and coauthors developed a P-modified WN nanoparticle catalyst for HER, which only takes an overpotential of 85 mV at 10 mA·cm–2 [72]. The presence of a second anion P holds the promise to enhance the activity of WN, while the underlying mechanism for the upgraded performance is unclear. In 2022, Liu et al. investigated the role of S dopant in regulating the catalytic properties of WP2 [73]. By manipulating the electronic configuration of WP2, S dopant can help to reduce the interaction between reaction intermediates (e.g., H*) and catalyst surface, thereby enhancing the intrinsic activity. Harnessing the beneficial roles of both cation and anion dopants, cation–anion dual-doping has become an efficient method to boost the catalytic performance of tungsten pnictides. Zhou and coworkers recently developed Fe, C co-doped WN and Fe, N co-doped WC catalysts from a WO3 nanoarray precursor (Fig. 9a) [40]. Compared with bare WN and WC, the dual-doped catalysts exhibit better performance in both alkaline and acidic electrolytes. In acidic solution, Fe, N co-doped WC outperforms Fe, C co-doped WN, while Fe, C co-doped WN shows the best performance in alkaline electrolyte (Fig. 9b, c). Computational results imply that the presence of dopants can help to regulate the adsorption of key reaction intermediates (i.e., H*, *H2O) (Fig. 9d, e). Especially, Fe, C co-doped WN has a strong adsorption towards H2O, leading to facilitated water dissociation step in alkaline HER. In addition, the free energy profiles of HER are in line with the experimental results (Fig. 9f, g).

Reproduced with permission from Ref. [40]. Copyright 2023, Springer Nature
a Illustration of the fabrication of Fe-N-WC and Fe-C-WN catalysts (FW-GSR: ferrocene induced WO3-based in situ gas–solid reaction); HER LSV curves of catalysts in b acidic and c alkaline solutions; calculated adsorption free energy of d H atom and e H2O molecule on catalysts (vertical axis unit: eV). Free energy profiles of HER process in f acidic and g alkaline solutions.
3.3 Defect engineering
Introducing defects/vacancies into the crystal structure of tungsten pnictides can activate the catalytic properties via engineering the electronic structure. In 2018, Ma et al. performed a comprehensive computational study on the defective WP-based HER catalysts [74]. Figure 10a shows the perfect WP (101) plane and monovacancy (W or P vacancy)-modified WP (101) planes. Based on the ΔGH* values, it can be seen that high HER activity can be maintained on the defective (101) planes in the presence of W/P monovacancy (Fig. 10b). With the introduction of defects, new catalytically active sites could be generated near the defective site because of the decreased coordination number of related atoms. Of note, introducing W monovacancy on the WP (101) plane is effective for enhancing HER activity over a wide range of hydrogen coverage. The key effect of the W vacancy was also investigated by Li et al., who found that W vacancies in an ordered mesoporous WP catalyst helped to lower the activation barrier for HER [75]. With a relatively low crystallinity and rich W vacancies, the WP nanoparticles developed by Zhang and coauthors exhibit good performance for HER in acidic electrolyte (η10 = 170 mV, Tafel slope = 52 mV·dec−1) [34]. Although W vacancies could activate the adjacent P atoms and the bridge sites as electroactive sites, they would deteriorate the conductivity of WP. As such, controlling the amount of W vacancies and attaining a balance between good conductivity and rich active sites is necessary.

Reproduced with permission from Ref. [74]. Copyright 2018, Royal Society of Chemistry. c Schematic of the preparation of Co(OH)2/c-WN1−x/CNTs; d ΔGH* on c-WN, c-WN1−x, Pt (111), and the O site and Co site of Co(OH)2/c-WN1−x; e illustration of HER process over Co(OH)2/c-WN1−x/CNTs. Reproduced with permission from Ref. [19]. Copyright 2021, Royal Society of Chemistry
a Schemes of perfect WP (101) plane and four defective (101) planes, and the defective sites are noted with green color; b ΔGH* values as a function of hydrogen coverage for the five planes (ML: monolayer).
Nitrogen vacancies in WN catalysts have been intentionally created for enhancing the catalytic performance. In a cobalt hydroxide/defective cubic phase tungsten nitride composite (Co(OH)2/c-WN1−x) catalyst developed via a three-step nitridation-annealing-precipitation method (Fig. 10c) [19]. The N vacancies were introduced in the second annealing step. Compared with perfect c-WN (111) surface which shows low adsorption of water molecules, the N vacancies in c-WN1−x promotes water molecules adsorption/dissociation. In addition, N vacancies on c-WN1−x tunes the ΔGH* value to near zero and provide electroactive sites for H* adsorption (Fig. 10d). Overall, alkaline HER kinetics can be profoundly improved via the Co(OH)2/c-WN1−x composite, in which Co(OH)2 and N vacancies helps to promote H2O adsorption and dissociation, and the N vacancies-rich c-WN1−x offers rich highly active sites for H* adsorption and enhances H2 formation (Fig. 10e).
3.4 Heterostructure design
To meet the multiple requirements for high conductivity, rich active sites, high intrinsic activity, and good durability, many studies have focused on designing heterostructures that combine two or more favorable components into one catalyst [52, 76, 77]. The as-formed composite catalysts can integrate the advantages of different parent materials (e.g., rich active sites, high activity and good stability) and thus leads to high performance [78, 79]. In addition, the interfacial electronic interaction between different components can promote the intrinsic activity of catalysts [80]. To date, conductive carbon materials and active metal materials have been used to alleviate the catalytic performance of tungsten pnictides.
Carbon materials like N-doped carbon [81, 82], carbon black [83, 84], and graphene [85] with large SSA and high conductivity are employed to promote the electronical conductivity of tungsten pnictides-based electrocatalysts and isolate electroactive sites [86, 87]. Interestingly, some carbon materials can not only work as conductive supports but also improve the intrinsic activity by providing active sites and/or tuning the electronic properties of tungsten pnictides [88]. For example, Zhu et al. found that the N-rich (9.5 at.%) porous graphene-like carbon (NRPGC) in the WNx-NRPGC catalyst is active for HER due to the high percentage of active pyridinic-N (54.3 at.%) [82]. In addition, the highly porous structure of NRPGC populates electroactive sites and promotes charge/mass transfer during HER process. In the NC-covered WP nanowire arrays (WP@NC NA) synthesized by a hydrothermal process-pyrolysis-phosphatization method (Fig. 11a, b), the presence of NC can improve the HER activities and stability of WP [89]. In 0.5 mol·L−1 H2SO4, WP@NC NA has a low η10 of 110 mV, with a Tafel slope of 50 mV·dec−1 (Fig. 11c, d). Also, WP@NC NA shows a high faradaic efficiency of 95% for hydrogen production, with good stability for 90 h (Fig. 11e, f). In-depth DFT calculations imply that the presence of N-doped carbon can regulate electronic properties of WP and optimize the ΔGH ∗ in acidic electrolyte. For alkaline HER, the N-doped carbon can improve the water adsorption/decomposition processes, and consequently accelerates the Volmer step (Fig. 11g, h). For tungsten pnictide@carbon composites, the highly stable carbon shells can preserve the initial active site structure during electrocatalysis by limiting the exposure of the active sites to the harsh (electro)chemical environment.

Reproduced with permission from Ref. [89]. Copyright 2022, Royal Society of Chemistry
a Illustration of the fabrication process of WP@NC NA (CFP: carbon fiber paper); b SEM image of WP@NC NA; c HER LSV curves and d Tafel plots of electrocatalysts (electrolyte: 0.5 mol·L−1 H2SO4); e faradaic efficiency and f durability tests of WP@NC NA in acid electrolyte; HER energy profiles for WP@NC NA and WP NA in g acidic and h alkaline solutions.
Coupling tungsten pnictides with catalytically active metal-based materials is a powerful strategy to boost the catalytic performance by providing additional active sites and electronic synergistic effects [14, 90, 91]. For example, the synergy between WN and Co in a porous Co/WN composite adjusts the electronic structure of WN, thereby optimizing the adsorption/desorption of reaction intermediates (e.g., H*, OH*, OOH*) [92]. The combination of metals with tungsten pnictides generally leads to the formation of Mott-Schottky heterojunctions [93], such as Ni/W5N4, in which the spontaneous electron transfer from Ni to W5N4 results in the creation of a built-in electric field [94]. The established built-in electric field can accelerate electron transfer and improve electronical conductivity, which further helps to tune the adsorption/desorption behavior of reaction intermediates and thus enhance the catalytic performance. Considering the high activity of noble metals, several studies hybridizing tungsten pnictides with noble metals (i.e., Pt [95] and Ru [28, 96]) for water electrolysis. Ru is an active material for OER [97], and thus the Ru-NiWNx is a promising bifunctional electrocatalyst for OWS [96]. In the alkaline electrolyte, the bifunctional Ru-NiWNx driven electrolyzer attains 50 mA·cm–2 at 1.54 V, surpassing the NiWNx counterpart (1.75 V). Another merit of constructing such noble metal-based composites is the high utilization efficiency of noble metals, which largely cuts the catalyst fabrication cost [98,99,100].
Aside from metals, alloys [101], metal carbides [29, 102, 103], oxides [43], hydroxides [104, 105], pnictides [16, 106], and sulfides [107] also have been used to construct composite catalysts with tungsten pnictides. By performing a facile phase-controlled phosphization process, Pi et al. developed a polymorphic tungsten diphosphide (p-WP2) that contains α- and β- phase WP2 [106]. Compared with the single phase α-WP2 and β-WP2, the mixed phase p-WP2 shows better HER performance. The reason should be the electronic interaction between α-WP2 and β-WP2, which generally leads to a highly active interface for catalysis. Guided by DFT calculations, Diao et al. developed a bifunctional W2N/WC heterostructure for OWS [29]. At the interface of W2N/WC composite, C atoms can accept more electrons from W atoms than N atoms, leading to electron transfer from W2N to WC (Fig. 12a, b). In addition, the W2N/WC heterojunction exhibits metallic behavior, suggesting high electronical conductivity and thereby would benefit the electrochemical process (Fig. 12c). Also, it can be seen that the localized distribution of electrons at the interface is different from that at the bulk structure of W2N and WC. More ionic bond is suggested at the interface of the heterojunction, while the bulk structure of W2N and WC shows high covalent bonds (Fig. 12d). Based on these computational results, it is reasonable to consider fabricating a W2N/WC composite for water splitting applications. Electrochemical results suggest that the W2N/WC heterostructure outperforms the single phase W2N and WC for OER and HER, implying that the synergistic effect between W2N and WC leads to high catalytic activities (Fig. 12e–g). Further applied in an electrolyzer, the bifunctional W2N/WC shows a comparable activity to noble metal-based Pt/C||RuO2 electrodes, while the stability of W2N/WC is significantly better than the Pt/C||RuO2 pair (Fig. 12h, i). Therefore, the W2N/WC catalyst is a promising cost-effective bifunctional catalyst for practical water electrolysis applications.

Reproduced with permission from Ref. [29]. Copyright 2020, WILEY–VCH
a Models of WC (100) and W2N (111) surfaces; b distribution of charge density difference at WC (100) and W2N (111) interface; c DOS of WC (100)/W2N (111) heterojunction; d electronic local function of (010) plane of WC (100)/W2N (111) heterojunction; e OER LSV curves and f Tafel plots of electrocatalysts; g HER LSV curves of electrocatalysts; h photograph of a water electrolyzer; polarization curves for OWS with bifunctional W2N/WC and Pt/C||RuO2 pair as the electrodes; i durability tests for Pt/C||RuO2 and W2N/WC for OWS.
In addition to the common two-component composites, several studies have focused on designing tri-component heterostructures based on tungsten pnictides, such as W3P/WP/W [108], CeP5O14/WP/WS2 [43], and MoxW1-xO2/MoxW1-xS2/MoxW1-xP [107]. Considering the rich interfaces in these ternary heterostructures, the synergistic effect between different active phases is suggested to enhance the catalytic performance. Chen et al. found that the CeP5O14/WP/WS2 had a lower d band center than WP, WS2, and WP/WS2, thereby facilitating the H desorption in HER process [43].
Overall, these strategies are efficient for the development of active tungsten pnictides for HER, OER, and OWS, as shown in Table 1. However, it remains highly challenging to reveal the catalytic mechanism over such complex composites due to the uncertainty of the crystal structure, as well as the multi-factors (e.g., nanostructure, size, and surface chemistry) that govern the catalytic performance.
4 Summary and prospects
Developing high-performance tungsten pnictide-based electrocatalysts for water electrolysis is of great importance. In this review, current advances in the design of tungsten pnictides for water electrolysis (especially HER) applications are comprehensively analyzed. Tungsten pnictide-based catalysts can be directly synthesized by one-step phosphization/nitridation and multi-step synthesis, the latter one is widely used for fabricating nanoarray catalysts and composites. For the design of advanced tungsten pnictide catalysts, strategies like nanostructure control, heteroatom doping, defect engineering, and heterostructure design show high potentials. Notwithstanding current progress, there are some key challenges that need further consideration.
-
(1)
Design cost-effective tungsten pnictide-based catalysts with advanced strategies. Although tungsten pnictides exhibit promising performance towards water electrolysis, the intrinsic activity of tungsten pnictides need further enhancements. Nanostructure control, heteroatom doping, defect engineering, and heterostructure design discussed in this review are efficient for boosting the performance of tungsten pnictides, while applying one of these strategies often fails to meet the multiple requirements of electrolysis, such as high conductivity, large electroactive area, high intrinsic activity, as well as high stability. Therefore, further studies are recommended to integrate different strategies into the design of a single catalyst in order to enhance the overall performance of tungsten pnictides. DFT calculations are also suggested to uncover the reason for enhanced activities and illustrate the structure-performance relationship. In addition, it is sensible to construct single-atom catalysts and high entropy catalysts based on tungsten pnictides, which would lead to significantly improved catalytic performance.
-
(2)
Unlock bifunctional catalytic activity of tungsten pnictides by modular design. Currently, most tungsten pnictide-based catalysts are active towards HER, and only several studies have investigated their OER and OWS properties. The reason could be the relatively low OER activity of tungsten-based materials. Considering the huge benefit of developing bifunctional electrocatalysts, it is suggested to integrate HER-active tungsten pnictides with OER-active materials, typically earth abundant Ni, Co, and Fe-based nanomaterials (e.g., sulphides, phosphides, (hydr)oxides) into composite catalysts. With such a modular design route, the as-obtained composite catalysts will be convinced of good performance for both HER and OER.
-
(3)
Apply tungsten pnictide-based catalysts in large-scale water electrolysis applications. To achieve large-scale hydrogen production through water electrolysis, it is crucial to evaluate the activity and durability of tungsten pnictide-based catalysts under industrial conditions in real electrolyzers, which involve high temperatures and high current densities. Besides (acidized or alkalized) pure water electrolysis, using tungsten pnictide-based catalysts for seawater and wastewater (e.g., urine) electrolysis is highly suggested [109, 110]. The impure water electrolysis would largely reduce the running cost and help the water management simultaneously. Especially, the urine electrolysis can realize energy-saving hydrogen production compared to pure water electrolysis due to the lower energy barrier of urea oxidation reaction than OER.
Data availability
The data generated during and/or analysed in this article are available from the corresponding author on reasonable request.
References
Zhou P, Navid IA, Ma YJ, Xiao YX, Wang P, Ye ZW, Zhou BW, Sun K, Mi ZT. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature. 2023;613(7942):66. https://doi.org/10.1038/s41586-022-05399-1.
Li S, Jiang JX, Zhai NN, Liu JY, Feng K, Chen YF, Wen Z, Sun XH, Zhong J. A half-wave rectifying triboelectric nanogenerator for self-powered water splitting towards hydrogen production. Nano Energy. 2022;93:106870. https://doi.org/10.1016/j.nanoen.2021.106870.
Dai W, Wang RH, Chen ZJ, Deng SM, Huang CZ, Luo WJ, Chen H. Highly-efficient photocatalytic hydrogen evolution triggered by spatial confinement effects over co-crystal templated boron-doped carbon nitride hollow nanotubes. J Mater Chem A. 2023;11(14):7584. https://doi.org/10.1039/D3TA00199G.
Zeng J, Xu L, Luo X, Chen T, Tang SH, Huang X, Wang LL. Z-scheme systems of ASi2N4 (A= Mo or W) for photocatalytic water splitting and nanogenerators. Tungsten. 2022;4:52. https://doi.org/10.1007/s42864-021-00116-3.
Ma MY, Yu HZ, Deng LM, Wang LQ, Liu SY, Pan H, Ren JW, Maximov YM, Hu F, Peng SJ. Interfacial engineering of heterostructured carbon-supported molybdenum cobalt sulfides for efficient overall water splitting. Tungsten. 2023;5:589. https://doi.org/10.1007/s42864-023-00212-6.
Chen ZJ, Wei WF, Zou WS, Li J, Zheng RJ, Wei W, Ni BJ, Chen H. Integrating electrodeposition with electrolysis for closing loop resource utilization of battery industrial wastewater. Green Chem. 2022;24(8):3208. https://doi.org/10.1039/D1GC04891K.
Chen ZJ, Zheng RJ, Bao T, Ma TY, Wei W, Shen YS, Ni BJ. Dual-doped nickel sulfide for electro-upgrading polyethylene terephthalate into valuable chemicals and hydrogen fuel. Nano-Micro Lett. 2023;15:210. https://doi.org/10.1007/s40820-023-01181-8.
Li J, Guo M, Yang X, Wang JL, Wang KX, Wang AR, Lei FC, Hao P, Xie JF, Tang B. Dual elemental modulation in cationic and anionic sites of the multi-metal Prussian blue analogue pre-catalysts for promoted oxygen evolution reaction. Prog Nat Sci: Mater Int. 2022;32(6):705. https://doi.org/10.1016/j.pnsc.2022.12.001.
Chen ZJ, Zheng RJ, Zou WS, Wei WF, Li J, Wei W, Ni BJ, Chen H. Integrating high-efficiency oxygen evolution catalysts featuring accelerated surface reconstruction from waste printed circuit boards via a boriding recycling strategy. Appl Catal B: Environ. 2021;298:120583. https://doi.org/10.1016/j.apcatb.2021.120583.
Sanati S, Morsali A, Garcia H. First-row transition metal-based materials derived from bimetallic metal–organic frameworks as highly efficient electrocatalysts for electrochemical water splitting. Energy Environ Sci. 2022;15(8):3119. https://doi.org/10.1039/D1EE03614A.
Sahoo DP, Das KK, Mansingh S, Sultana S, Parida K. Recent progress in first row transition metal layered double hydroxide (LDH) based electrocatalysts towards water splitting: A review with insights on synthesis. Coord Chem Rev. 2022;469:214666. https://doi.org/10.1016/j.ccr.2022.214666.
Bhunia K, Chandra M, Sharma SK, Pradhan D, Kim SJ. A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coord Chem Rev. 2023;478:214956. https://doi.org/10.1016/j.ccr.2022.214956.
Han N, Liu PY, Jiang J, Ai LH, Shao ZP, Liu SM. Recent advances in nanostructured metal nitrides for water splitting. J Mater Chem A. 2018;6(41):19912. https://doi.org/10.1039/C8TA06529B.
Men LL, Shi TC, Li J, Li X, Sun B, Pan QQ, Su ZM. Bimetallic polyoxometalate derived Co/WN composite as electrocatalyst for high-efficiency hydrogen evolution. Int J Hydrogen Energy. 2022;47(64):27452. https://doi.org/10.1016/j.ijhydene.2022.06.061.
Zhang J, Yu AM, Sun CH. Theoretical insights into heteronuclear dual metals on non-metal doped graphene for nitrogen reduction reaction. Chin J Catal. 2023;52:263. https://doi.org/10.1016/S1872-2067(23)64500-0.
Kavinkumar T, Yang H, Sivagurunathan AT, Jeong H, Han JW, Kim DH. Regulating electronic structure of iron nitride by tungsten nitride nanosheets for accelerated overall water splitting. Small. 2023;19:2300963. https://doi.org/10.1002/smll.202300963.
Tan S, Tackett BM, He Q, Lee JH, Chen JG, Wong SS. Synthesis and electrocatalytic applications of flower-like motifs and associated composites of nitrogen-enriched tungsten nitride (W2N3). Nano Res. 2020;13:1434. https://doi.org/10.1007/s12274-020-2687-y.
Kim D, Kim D, Oh S, Park T, Yong K. Ex situ flame vapor-doped oxophilic metals on WP/WOx nanowires for enhanced alkaline hydrogen evolution activity. Appl Surf Sci. 2023;608:155044. https://doi.org/10.1016/j.apsusc.2022.155044.
Liu H, Wu ZS, Huang L, Zhang BW, Yin LC, Xu CY, Zhen L. Mechanistic insights into interfaces and nitrogen vacancies in cobalt hydroxide/tungsten nitride catalysts to enhance alkaline hydrogen evolution. J Mater Chem A. 2021;9(18):11323. https://doi.org/10.1039/D1TA01560E.
Chen ZJ, Yun SN, Wu L, Zhang JQ, Shi XD, Wei W, Liu YW, Zheng RJ, Han N, Ni BJ. Waste-derived catalysts for water electrolysis: circular economy-driven sustainable green hydrogen energy. Nano-Micro Lett. 2023;15:4. https://doi.org/10.1007/s40820-022-00974-7.
Anantharaj S, Ede SR, Sakthikumar K, Karthick K, Mishra S, Kundu S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe Co, and Ni: A review. ACS Catal. 2016;6(12):8069. https://doi.org/10.1021/acscatal.6b02479.
Chatenet M, Pollet BG, Dekel DR, Dionigi F, Deseure J, Millet P, Braatz RD, Bazant MZ, Eikerling M, Staffell I, Balcombe P, Horn YS, Schäfer H. Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments. Chem Soc Rev. 2022;51(11):4583. https://doi.org/10.1039/D0CS01079K.
Yang HY, Driess M, Menezes PW. Self-supported electrocatalysts for practical water electrolysis. Adv Energy Mater. 2021;11(39):2102074. https://doi.org/10.1002/aenm.202102074.
Jain A, Ong SP, Hautier G, Chen W, Richards WD, Dacek S, Cholia S, Gunter D, Skinner D, Ceder G, Persson KA. Commentary: the materials project: A materials genome approach to accelerating materials innovation. APL Mater. 2013;1(1):011002. https://doi.org/10.1063/1.4812323.
Wang CX, Wang XX, Zhang TY, Qian P, Lookman T, Su YJ. A descriptor for the design of 2D MXene hydrogen evolution reaction electrocatalysts. J Mater Chem A. 2022;10(35):18195. https://doi.org/10.1039/D2TA02837A.
Chen ZJ, Duan XG, Wei W, Wang SB, Ni BJ. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J Mater Chem A. 2019;7(25):14971. https://doi.org/10.1039/C9TA03220G.
Pi MY, Wu TL, Zhang DK, Chen SJ, Wang SX. Phase-controlled synthesis and comparative study of α- and β-WP2 submicron particles as efficient electrocatalysts for hydrogen evolution. Electrochim Acta. 2016;216:304. https://doi.org/10.1016/j.electacta.2016.09.038.
Liu GC, Zhang JC, Ren HY, Tang YW, Sun HJ. Optimizing the hydrogen adsorption strength on interfacial Ru sites with WN for high-efficiency hydrogen evolution. Mater Chem Front. 2023;7(18):4100. https://doi.org/10.1039/D3QM00483J.
Diao JX, Qiu Y, Liu SQ, Wang WT, Chen K, Li HL, Yuan WY, Qu YT, Guo XH. Interfacial engineering of W2N/WC heterostructures derived from solid-state synthesis: A highly efficient trifunctional electrocatalyst for ORR, OER, and HER. Adv Mater. 2020;32(7):1905679. https://doi.org/10.1002/adma.201905679.
Guo FH, Chen Q, Liu ZH, Cheng DL, Han N, Chen ZJ. Repurposing mining and metallurgical waste as electroactive materials for advanced energy applications: Advances and perspectives. Catalysts. 2023;13:1241. https://doi.org/10.3390/catal13091241.
Zhou PY, Lv JJ, Huang XB, Lu YF, Wang G. Strategies for enhancing the catalytic activity and electronic conductivity of MOFs-based electrocatalysts. Coord Chem Rev. 2023;478:214969. https://doi.org/10.1016/j.ccr.2022.214969.
Xing ZC, Liu Q, Asiri AM, Sun XP. High-efficiency electrochemical hydrogen evolution catalyzed by tungsten phosphide submicroparticles. ACS Catal. 2015;5(1):145. https://doi.org/10.1021/cs5014943.
Nkabinde SS, Mwonga PV, Mpelane S, Ndala ZB, Kolokoto T, Shumbula NP, Nchoe O, Maphanga RR, Ozoemena KI, Mubiayi KP, Moloto N. Phase-dependent electrocatalytic activity of colloidally synthesized WP and α-WP2 electrocatalysts for hydrogen evolution reaction. New J Chem. 2021;45(34):15594. https://doi.org/10.1039/D1NJ00927C.
Zhang XY, Guo T, Liu TY, Lv KY, Wu ZY, Wang DZ. Tungsten phosphide (WP) nanoparticles with tunable crystallinity, W vacancies, and electronic structures for hydrogen production. Electrochim Acta. 2019;323:134798. https://doi.org/10.1016/j.electacta.2019.134798.
Liu D, Xu GY, Fan BM, Wang HT. In situ encapsulation of abundant WP/Ni2P heterointerfaces in N, P co-doped two-dimensional carbon frameworks for boosting hydrogen evolution electrocatalysis. Dalton Trans. 2022;51(46):17911. https://doi.org/10.1039/D2DT02606F.
Wei P, Sun XP, Wang MH, Xu JH, He ZM, Li XG, Cheng FY, Xu Y, Han JT, Yang H, Huang YH. Construction of an N-decorated carbon-encapsulated W2C/WP heterostructure as an efficient electrocatalyst for hydrogen evolution in both alkaline and acidic media. ACS Appl Mater Interfaces. 2021;13(45):53955. https://doi.org/10.1021/acsami.1c16547.
He DY, Cao LY, Huang JF, Li SN, Feng YQ, Li GD, Wang FM, Feng LL. Synergistic coupling of heterogeneous VN/WN nanoparticles embedded in N-doped carbon matrix for efficient hydrogen evolution reaction. Chem Eng J. 2022;429:131945. https://doi.org/10.1016/j.cej.2021.131945.
Wang XD, Xu YF, Rao HS, Xu WJ, Chen HY, Zhang WX, Kuang DB, Su CY. Novel porous molybdenum tungsten phosphide hybrid nanosheets on carbon cloth for efficient hydrogen evolution. Energy Environ Sci. 2016;9(4):1468. https://doi.org/10.1039/C5EE03801D.
Qin Q, Li J, Guo ZG, Jian CY, Liu W. Tungsten phosphide nanosheets seamlessly grown on tungsten foils toward efficient hydrogen evolution reaction in basic and acidic media. Int J Hydrogen Energy. 2019;44(50):27483. https://doi.org/10.1016/j.ijhydene.2019.08.235.
Zhou J, Wang FF, Wang HQ, Hu SX, Zhou WJ, Liu H. Ferrocene-induced switchable preparation of metal-nonmetal codoped tungsten nitride and carbide nanoarrays for electrocatalytic HER in alkaline and acid media. Nano Res. 2023;16:2085. https://doi.org/10.1007/s12274-022-4901-6.
Liu W, Geng P, Li SQ, Zhu R, Liu WH, Lu HD, Chandrasekaran S, Pang YY, Fan DY, Liu YP. Self-supported three-dimensional WP2 (WP) nanosheet arrays for efficient electrocatalytic hydrogen evolution. Int J Hydrogen Energy. 2020;45(53):28576. https://doi.org/10.1016/j.ijhydene.2020.07.144.
Shi JL, Pu ZH, Liu Q, Asiri AM, Hu JM, Sun XP. Tungsten nitride nanorods array grown on carbon cloth as an efficient hydrogen evolution cathode at all pH values. Electrochim Acta. 2015;154:345. https://doi.org/10.1016/j.electacta.2014.12.096.
Chen HY, Hu MH, Wang XY, Xu X, Jing P, Liu BC, Gao R, Zhang J. Constructing novel ternary heterostructure of CeP5O14/WP/WS2 to enhance catalytic activity for hydrogen evolution in a full pH range. Small Struct. 2023;4:2300026. https://doi.org/10.1002/sstr.202300026.
Li XX, Liu XC, Liu C, Zeng JM, Qi XP. Co3O4/stainless steel catalyst with synergistic effect of oxygen vacancies and phosphorus doping for overall water splitting. Tungsten. 2023;5:100. https://doi.org/10.1007/s42864-022-00144-7.
Chen ZJ, Zou WS, Zheng RJ, Wei WF, Wei W, Ni BJ, Chen H. Synergistic recycling and conversion of spent Li-ion battery leachate into highly efficient oxygen evolution catalysts. Green Chem. 2021;23(17):6538. https://doi.org/10.1039/D1GC01578H.
Du HF, Gu S, Liu RW, Li CM. Tungsten diphosphide nanorods as an efficient catalyst for electrochemical hydrogen evolution. J Power Sources. 2015;278:540. https://doi.org/10.1016/j.jpowsour.2014.12.095.
Chen ZJ, Zheng RJ, Wei WF, Wei W, Ni BJ, Chen H. Unlocking the electrocatalytic activity of natural chalcopyrite using mechanochemistry. J Energy Chem. 2022;68:275. https://doi.org/10.1016/j.jechem.2021.11.005.
McEnaney JM, Chance Crompton J, Callejas JF, Popczun EJ, Read CG, Lewis NS, Schaak RE. Electrocatalytic hydrogen evolution using amorphous tungsten phosphide nanoparticles. Chem Commun. 2014;50(75):11026. https://doi.org/10.1039/C4CC04709E.
Pu ZH, Amiinu IS, Mu SC. In situ fabrication of tungsten diphosphide nanoparticles on tungsten foil: A hydrogen-evolution cathode for a wide pH range. Energy Technol. 2016;4(9):1030. https://doi.org/10.1002/ente.201600110.
Chen ZJ, Zheng RJ, Graś M, Wei W, Lota G, Chen H, Ni BJ. Tuning electronic property and surface reconstruction of amorphous iron borides via W-P co-doping for highly efficient oxygen evolution. Appl Catal B: Environ. 2021;288:120037. https://doi.org/10.1016/j.apcatb.2021.120037.
Anantharaj S, Noda S. Amorphous catalysts and electrochemical water splitting: An untold story of harmony. Small. 2020;16(2):1905779. https://doi.org/10.1002/smll.201905779.
Chen ZJ, Han N, Zheng RJ, Ren ZJ, Wei W, Ni BJ. Design of earth-abundant amorphous transition metal-based catalysts for electrooxidation of small molecules: Advances and perspectives. SusMat. 2023;3:290. https://doi.org/10.1002/sus2.131.
Pu ZH, Liu Q, Asiri AM, Sun XP. Tungsten phosphide nanorod arrays directly grown on carbon cloth: a highly efficient and stable hydrogen evolution cathode at all pH values. ACS Appl Mater Interfaces. 2014;6(24):21874. https://doi.org/10.1021/am5060178.
Xu KK, Fu XL, Li H, Peng ZJ. A novel composite of network-like tungsten phosphide nanostructures grown on carbon fibers with enhanced electrocatalytic hydrogen evolution efficiency. Appl Surf Sci. 2018;456:230. https://doi.org/10.1016/j.apsusc.2018.06.106.
Meng FY, Yu Y, Sun DF, Li L, Lin SM, Huang L, Chu WH, Ma SF, Xu BS. Three-dimensional flower-like WP2 nanowire arrays grown on Ni foam for full water splitting. Appl Surf Sci. 2021;546:148926. https://doi.org/10.1016/j.apsusc.2021.148926.
Wu L, Pu ZH, Tu ZK, Amiinu IS, Liu SJ, Wang PY, Mu SC. Integrated design and construction of WP/W nanorod array electrodes toward efficient hydrogen evolution reaction. Chem Eng J. 2017;327:705. https://doi.org/10.1016/j.cej.2017.06.152.
Chen ZJ, Duan XG, Wei W, Wang SB, Zhang ZJ, Ni BJ. Boride-based electrocatalysts: Emerging candidates for water splitting. Nano Res. 2020;13:293. https://doi.org/10.1007/s12274-020-2618-y.
Chen ZJ, Wei W, Shen YS, Ni BJ. Defective nickel sulfide hierarchical structures for efficient electrochemical conversion of plastic waste to value-added chemicals and hydrogen fuel. Green Chem. 2023;25(15):5979. https://doi.org/10.1039/D3GC01499A.
Cai SC, Meng ZH, Li GJ, An Y, Cheng YP, Kan EJ, Ouyang B, Zhang HN, Tang HL. Nitrogen doped porous carbon-based bifunctional oxygen electrocatalyst with controllable phosphorus content for zinc-air battery. Nano Res. 2023;16:5887. https://doi.org/10.1007/s12274-022-5126-4.
Ren BW, Li DQ, Jin QY, Cui H, Wang CX. A self-supported porous WN nanowire array: an efficient 3D electrocatalyst for the hydrogen evolution reaction. J Mater Chem A. 2017;5(36):19072. https://doi.org/10.1039/C7TA04923D.
Pi MY, Wang XD, Zhang DK, Wang SX, Chen SJ. A 3D porous WP2 nanosheets@carbon cloth flexible electrode for efficient electrocatalytic hydrogen evolution. Front Chem Sci Eng. 2018;12:425. https://doi.org/10.1007/s11705-018-1726-7.
Du C, Li P, Zhuang ZH, Fang ZY, He SJ, Feng LG, Chen W. Highly porous nanostructures: Rational fabrication and promising application in energy electrocatalysis. Coord Chem Rev. 2022;466:214604. https://doi.org/10.1016/j.ccr.2022.214604.
Pi MY, Wu TL, Zhang DK, Chen SJ, Wang SX. Self-supported three-dimensional mesoporous semimetallic WP2 nanowire arrays on carbon cloth as a flexible cathode for efficient hydrogen evolution. Nanoscale. 2016;8(47):19779. https://doi.org/10.1039/C6NR05747K.
Pi MY, Guo WM, Wu TL, Wang XD, Zhang DK, Wang SX, Chen SJ. Pulsed laser deposition-assisted synthesis of porous WP2 nanosheet arrays integrated on graphite paper as a 3D flexible cathode for efficient hydrogen evolution. J Power Sources. 2017;364:253. https://doi.org/10.1016/j.jpowsour.2017.08.034.
Chen ZJ, Wei W, Shon HK, Ni BJ. Designing bifunctional catalysts for urea electrolysis: Progress and perspectives. Green Chem. 2024;26:631. https://doi.org/10.1039/D3GC03329E.
Liu ZZ, Zhang XM, Song H, Yang YX, Zheng Y, Gao B, Fu JJ, Chu PK, Huo KF. Electronic modulation between tungsten nitride and cobalt dopants for enhanced hydrogen evolution reaction at a wide range of pH. ChemCatChem. 2020;12(11):2962. https://doi.org/10.1002/cctc.202000391.
Pi MY, Zhang DK, Wang SX, Chen SJ. Enhancing electrocatalytic hydrogen evolution of WP2 three-dimensional nanowire arrays via Mo doping. Mater Lett. 2018;213:315. https://doi.org/10.1016/j.matlet.2017.11.058.
Zhang XY, Shen HJ, Moghadam BR, Liu SQ, Zhu YJ, Wang JC, Yang MH. Three-dimensional hierarchically ternary iron tungsten nitride nanosheets with slight ratio of nickel modulation for oxygen evolution reaction. NANO. 2019;14(7):1950089. https://doi.org/10.1142/S1793292019500899.
Wang JJ, Chang K, Sun ZY, Lee JH, Tackett BM, Zhang C, Chen JG, Liu CJ. A combined experimental and theoretical study of the accelerated hydrogen evolution kinetics over wide pH range on porous transition metal doped tungsten phosphide electrocatalysts. Appl Catal B: Environ. 2019;251:162. https://doi.org/10.1016/j.apcatb.2019.03.065.
Yang YX, Feng XY, Liu ZZ, Zhang XM, Song H, Pi CR, Gao B, Chu PK, Huo KF. Enhanced hydrogen evolution activity of phosphorus-rich tungsten phosphide by cobalt doping: A comprehensive study of the active sites and electronic structure. ChemElectroChem. 2021;8(9):1658. https://doi.org/10.1002/celc.202100384.
Huo DK, Sun ZC, Liu YY, Yu ZQ, Wang Y, Wang AJ. Synthesis of Co-doped tungsten phosphide nanoparticles supported on carbon supports as high-efficiency HER catalysts. ACS Sustainable Chem Eng. 2021;9(36):12311. https://doi.org/10.1021/acssuschemeng.1c03956.
Yan HJ, Tian CG, Wang L, Wu AP, Meng MC, Zhao L, Fu HG. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew Chem Int Ed. 2015;54:6325. https://doi.org/10.1002/anie.201501419.
Liu W, Xiao ZZ, Chandrasekaran S, Fan DY, Li W, Lu HD, Liu YP. Insights into the effect of sulfur incorporation into tungsten diphosphide for improved hydrogen evolution reaction. ACS Appl Mater Interfaces. 2022;14(14):16157. https://doi.org/10.1021/acsami.1c24363.
Ma YF, Yu GT, Wang T, Zhang CH, Huang XR, Chen W. Highly efficient catalytic activity for the hydrogen evolution reaction on pristine and monovacancy defected WP systems: a first-principles investigation. Phys Chem Chem Phys. 2018;20(20):13757. https://doi.org/10.1039/C8CP02038H.
Li F, Wang CR, Han XC, Feng XQ, Qu YQ, Liu J, Chen WL, Zhao LP, Song XF, Zhu H, Chen H, Zhao M, Deng Z, Wu JB, Zhang P, Gao L. Confinement effect of mesopores: in situ synthesis of cationic tungsten-vacancies for a highly ordered mesoporous tungsten phosphide electrocatalyst. ACS Appl Mater Interfaces. 2020;12(20):22741. https://doi.org/10.1021/acsami.9b22761.
Du YX, Zhou YT, Zhu MZ. Co-based MOF derived metal catalysts: from nano-level to atom-level. Tungsten. 2023;5:201. https://doi.org/10.1007/s42864-022-00197-8.
Chen ZJ, Zheng RJ, Deng SM, Wei WF, Wei W, Ni BJ, Chen H. Modular design of an efficient heterostructured FeS2/TiO2 oxygen evolution electrocatalyst via sulfidation of natural ilmenites. J Mater Chem A. 2021;9(44):25032. https://doi.org/10.1039/D1TA08168C.
Zhang YB, Xue ZM, Zhao XH, Zhang BL, Mu TC. Controllable and facile preparation of Co9S8–Ni3S2 heterostructures embedded with N, S, O-tri-doped carbon for electrocatalytic oxidation of 5-hydroxymethylfurfural. Green Chem. 2022;24(4):1721. https://doi.org/10.1039/D1GC04499K.
Zhang ZY, Song N, Wang J, Liu YQ, Dai Z, Nie GD. Polydopamine-derived carbon layer anchoring NiCo-P nanowire arrays for high-performance binder-free supercapacitor and electrocatalytic hydrogen evolution. SusMat. 2022;2(5):646. https://doi.org/10.1002/sus2.49.
Pu ZH, Ya X, Amiinu IS, Tu ZK, Liu XB, Li WQ, Mu SC. Ultrasmall tungsten phosphide nanoparticles embedded in nitrogen-doped carbon as a highly active and stable hydrogen-evolution electrocatalyst. J Mater Chem A. 2016;4(40):15327. https://doi.org/10.1039/C6TA05165K.
Li Q, Cui W, Tian JQ, Xing ZC, Liu Q, Xing W, Asiri AM, Sun XP. N-doped carbon-coated tungsten oxynitride nanowire arrays for highly efficient electrochemical hydrogen evolution. Chemsuschem. 2015;8(15):2487. https://doi.org/10.1002/cssc.201500398.
Zhu YP, Chen G, Zhong YJ, Zhou W, Shao ZP. Rationally designed hierarchically structured tungsten nitride and nitrogen-rich graphene-like carbon nanocomposite as efficient hydrogen evolution electrocatalyst. Adv Sci. 2018;5(2):1700603. https://doi.org/10.1002/advs.201700603.
Wang DZ, Lv KY, Wu ZZ. Facile synthesis of tungsten phosphide/Ketjen Black hybrid electrocatalyst for hydrogen production. Mater Res Express. 2018;5(6):065509. https://doi.org/10.1088/2053-1591/aac919.
Liu YH, Zhang DX, Zhang KW, Dong WX, Tian CG, Mao BD. Facile immobilization of polyoxometalates for low-cost molybdenum/tungsten phosphide nanoparticles on carbon black for efficient electrocatalytic hydrogen evolution. J Coord Chem. 2020;73(17–19):2590. https://doi.org/10.1080/00958972.2020.1843024.
Mtukula AC, Bo XJ, Guo LP. Highly active non-precious metal electrocatalyst for the hydrogen evolution reaction based on nitrogen-doped graphene supported MoO2/WN/Mo2N. J Alloy Compd. 2017;692:614. https://doi.org/10.1016/j.jallcom.2016.09.079.
Chen ZJ, Wei WF, Ni BJ, Chen H. Plastic wastes derived carbon materials for green energy and sustainable environmental applications. Environ Funct Mater. 2022;1(1):34. https://doi.org/10.1016/j.efmat.2022.05.005.
Graś M, Kolanowski Ł, Chen ZJ, Lota K, Jurak K, Ryl J, Ni BJ, Lota G. Partial inhibition of borohydride hydrolysis using porous activated carbon as an effective method to improve the electrocatalytic activity of the DBFC anode. Sustainable Energy Fuels. 2021;5(17):4401. https://doi.org/10.1039/D1SE00999K.
Chen ZJ, Zheng RJ, Wei WF, Wei W, Zou WS, Li J, Ni BJ, Chen H. Recycling spent water treatment adsorbents for efficient electrocatalytic water oxidation reaction. Resour, Conserv Recycl. 2022;178:106037. https://doi.org/10.1016/j.resconrec.2021.106037.
Lv CC, Liu JF, Lou PP, Wang XB, Gao LJ, Wang SF, Huang ZP. Unveiling the advantages of an ultrathin N-doped carbon shell on self-supported tungsten phosphide nanowire arrays for the hydrogen evolution reaction experimentally and theoretically. Nanoscale. 2022;14(14):5430. https://doi.org/10.1039/D2NR00423B.
Zhang CY, Du XQ, Zhang XS, Wang YH, Hu TP. In situ construction of WNiM–WNi LDH (M = Se, S, or P) with heterostructure as highly efficient electrocatalyst for overall water splitting and urea oxidation reaction. Dalton Trans. 2023;52(18):6052. https://doi.org/10.1039/D3DT00065F.
Zhu Y, Zheng HY, Liu XY, Sun CY, Dong M, Wang XL, Su ZM. Ultra-small porous WN/W2C nanoparticles for sustained hydrogen production by a polyoxometalate-intercalated pyrolysis strategy. New J Chem. 2022;46(48):23292. https://doi.org/10.1039/D2NJ04218E.
Wu AP, Gu Y, Yang BR, Wu H, Yan HJ, Jiao YQ, Wang DX, Tian CG, Fu HG. Porous cobalt/tungsten nitride polyhedra as efficient bifunctional electrocatalysts for overall water splitting. J Mater Chem A. 2020;8(43):22938. https://doi.org/10.1039/D0TA09620B.
Seenivasan S, Im H, Lim T, Han JW, Seo J. Schottky switch derived by metallic W5N4 | catalyst junction: Switch-on to enhance catalytic activity and durability in water splitting reaction. Appl Catal B: Environ. 2024;340:123233. https://doi.org/10.1016/j.apcatb.2023.123233.
Zhou YM, Chu BX, Sun ZJ, Dong LH, Wang F, Li B, Fan MG, Chen ZJ. Surface reconstruction and charge distribution enabling Ni/W5N4 Mott-Schottky heterojunction bifunctional electrocatalyst for efficient urea-assisted water electrolysis at a large current density. Appl Catal B: Environ. 2023;323:122168. https://doi.org/10.1016/j.apcatb.2022.122168.
Denny SR, Tackett BM, Tian D, Sasaki K, Chen JG. Exploring electrocatalytic stability and activity of unmodified and platinum-modified tungsten and niobium nitrides. Int J Hydrogen Energy. 2020;45(43):22883. https://doi.org/10.1016/j.ijhydene.2020.06.186.
Wang HJ, Cheng XX, Tong Y. Coupling of ruthenium with hybrid metal nitrides heterostructure as bifunctional electrocatalyst for water electrolysis. J Colloid Interface Sci. 2023;629:155. https://doi.org/10.1016/j.jcis.2022.08.147.
Chen ZJ, Duan XG, Wei W, Wang SB, Ni BJ. Electrocatalysts for acidic oxygen evolution reaction: achievements and perspectives. Nano Energy. 2020;78:105392. https://doi.org/10.1016/j.nanoen.2020.105392.
Cao XJ, Huo JJ, Li L, Qu JP, Zhao YF, Chen WH, Liu CT, Liu H, Wang GX. Recent advances in engineered Ru-based electrocatalysts for the hydrogen/oxygen conversion reactions. Adv Energy Mater. 2022;12(41):2202119. https://doi.org/10.1002/aenm.202202119.
Chen ZJ, Duan XG, Wei W, Wang SB, Ni BJ. Iridium-based nanomaterials for electrochemical water splitting. Nano Energy. 2020;78:105270. https://doi.org/10.1016/j.nanoen.2020.105270.
Wang JH, Yang SW, Ma FB, Zhao YK, Zhao SN, Xiong ZY, Cai D, Shen HD, Zhu K, Zhang QY, Cao YL, Wang TS, Zhang HP. RuCo alloy nanoparticles embedded within N-doped porous two-dimensional carbon nanosheets: a high-performance hydrogen evolution reaction catalyst. Tungsten. 2023;6(1):114. https://doi.org/10.1007/s42864-023-00223-3.
Zheng JP, Chen JP, Xiao LP, Cheng XN, Cui H. In situ integrated Co3W−WN hybrid nanostructure as an efficient bifunctional electrocatalyst by accelerating water dissociation and enhancing oxygen evolution. ChemElectroChem. 2020;7(24):4971. https://doi.org/10.1002/celc.202001454.
Chen WF, Schneider JM, Sasaki K, Wang CH, Schneider J, Iyer S, Iyer S, Zhu YM, Muckerman JT, Fujita E. Tungsten carbide–nitride on graphene nanoplatelets as a durable hydrogen evolution electrocatalyst. Chemsuschem. 2014;7(9):2414. https://doi.org/10.1002/cssc.201402454.
Shi MQ, Li W, Fang J, Jiang ZZ, Gao J, Chen ZY, Sun FF, Xu YH. Electronic structure tuning during facile construction of two-phase tungsten based electrocatalyst for hydrogen evolution reaction. Electrochim Acta. 2018;283:834. https://doi.org/10.1016/j.electacta.2018.06.190.
Kim D, Park J, Lee J, Zhang Z, Yong K. Ni(OH)2-WP hybrid nanorod arrays for highly efficient and durable hydrogen evolution reactions in alkaline media. Chemsuschem. 2018;11(20):3618. https://doi.org/10.1002/cssc.201801733.
Lv CC, Wang XB, Gao LJ, Wang AJ, Wang SF, Wang RN, Ning XK, Li YG, Boukhvalov DW, Huang ZP, Zhang C. Triple functions of Ni(OH)2 on the surface of WN nanowires remarkably promoting electrocatalytic activity in full water splitting. ACS Catal. 2020;10(22):13323. https://doi.org/10.1021/acscatal.0c02891.
Pi MY, Wu TL, Guo WM, Wang XD, Zhang DK, Wang SX, Chen SJ. Phase-controlled synthesis of polymorphic tungsten diphosphide with hybridization of monoclinic and orthorhombic phases as a novel electrocatalyst for efficient hydrogen evolution. J Power Sources. 2017;349:138. https://doi.org/10.1016/j.jpowsour.2017.03.040.
Sun BT, Yang SR, Guo YC, Xue YJ, Tian J, Cui HZ, Song XJ. Fabrication of molybdenum and tungsten oxide, sulfide, phosphide (MoxW1-xO2/MoxW1-xS2/MoxW1-xP) porous hollow nano-octahedrons from metal-organic frameworks templates as efficient hydrogen evolution reaction electrocatalysts. J Colloid Interface Sci. 2019;547:339. https://doi.org/10.1016/j.jcis.2019.04.013.
Huo DK, Sun ZC, Liu YY, Yu ZQ, Wang Y, Wang AJ. Ternary-phase nanostructure W3P/WP/W for high-performance pH-universal water/seawater electrolysis. Mater Adv. 2022;3(13):5350. https://doi.org/10.1039/D2MA00288D.
Chen ZJ, Zheng RJ, Zou HY, Wang RH, Huang CZ, Dai W, Wei W, Duan LL, Ni BJ, Chen H. Amorphous iron-doped nickel boride with facilitated structural reconstruction and dual active sites for efficient urea electrooxidation. Chem Eng J. 2023. https://doi.org/10.1016/j.cej.2023.142684.
Chen ZJ, Wei W, Ni BJ. Transition metal chalcogenides as emerging electrocatalysts for urea electrolysis. Curr Opin Electrochem. 2021;31:100888. https://doi.org/10.1016/j.coelec.2021.100888.
Acknowledgements
This work was supported by the Key Research and Development Project for the High level Technological Talent of Lvliang city (Rc2020214, 2023GXYF09, 2022RC15); Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2021L564); Research Project Supported by Shanxi Scholarship Council of China (2022-182); Scientific Research Start-up Funds of Lyuliang University; Wuhan Yingcai – Outstanding Young Talents (No. PM0218003).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, X., Tan, XH., Han, N. et al. Tungsten pnictides for water electrolysis: advances and perspectives. Tungsten (2024). https://doi.org/10.1007/s42864-024-00268-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42864-024-00268-y