Abstract
We report a rare case of new-onset MDA-5-positive amyopathic dermatomyositis with rapidly progressive interstitial lung disease (RP-ILD) following the second dose of the COVID-19 mRNA vaccine. Our patient was a previously healthy Asian female in her 60 s who presented with fatigue, dyspnea on exertion, and typical dermatomyositis (DM) rashes without muscle involvement two weeks after receiving the second dose of the COVID-19 mRNA BNT162b2 vaccine. Workup revealed high titer MDA-5 antibodies, abnormal pulmonary function tests, and ground-glass opacities on chest imaging. She had good response to early aggressive therapy with high-dose steroids, intravenous (IV) rituximab, mycophenolate mofetil, and intravenous immunoglobulin (IVIG). This case highlights the potential immunogenicity of COVID-19 mRNA vaccines and the possibility of new-onset systemic rheumatic syndromes after vaccination. More studies are needed to understand a definitive causal relationship and improve surveillance of adverse immunological events following COVID-19 vaccinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid and successful development of vaccines to prevent SARS-CoV-2 infection is considered the most promising approach for curbing the global COVID-19 pandemic. The mRNA vaccine is delivered in a lipid nanoparticle to express a full-length SARS-CoV-2 spike protein which is the main antigenic target for vaccination. Currently in the USA, the COVID-19 mRNA vaccines BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) are fully approved by the Food and Drug Administration (FDA) for persons aged 16 years and older and persons aged 18 years and older, respectively. The mRNA COVID-19 vaccines are highly effective in reducing infection, severity, hospitalization, and mortality [1]. Advantages of the mRNA vaccines include fast to produce, low manufacture cost, high efficacy, and exceedingly safe profile [2]. The most common reported side effects are injection site reactions and systemic events including fatigue, headache, myalgias, arthralgia, fever, and chills [3].
Vaccination against SARS-CoV-2 is crucial for patients with systemic rheumatic diseases (SRDs) who may be at increased risk of severe outcomes from COVID-19 infections. Although the mRNA vaccines have an excellent safety profile in the general population, the trials excluded patients with SRDs [4]. There are rare reports of de novo systemic rheumatologic diseases as well as flare ups of established systemic rheumatic diseases after vaccination. In a web-based survey conducted in New York City, 14.9% respondents reported systemic rheumatic disease (SRD) flare following COVID-19 vaccine [5]. Rare reports of myocarditis and pericarditis, mainly in male adolescents and young adults, have also been reported following receipt of the mRNA vaccines [6]. There are hypotheses that mRNA vaccines may trigger SRD flares or de novo SRDs through molecular mimicry activating the immune system or nonspecific adjunct effects in rare cases.
In our case report, we present a unique case of new-onset MDA-5-positive amyopathic dermatomyositis with rapidly progressive interstitial lung disease following the second dose of COVID-19 mRNA BNT162b2 vaccination.
Case Presentation
History and Physical
The patient is a previously healthy Asian woman in her early 60s who developed diffuse rash two weeks after receiving the second dose of COVID-19 mRNA vaccine. The rash was non-pruritic, painless, and covered her upper extremities, torso, palmar and dorsum of hands, chest wall, neck, bilateral eyelids, and edge of her mouth. She was initially diagnosed with Coxsackie A infection based on positive Coxsackie A IgG antibodies. Her rash continued to worsen and within two weeks, she started to have significant dyspnea on exertion with frequent coughing spells. She also reported profound fatigue but denied fevers, chills, sputum production, and history of Raynaud’s or sicca symptoms. She denied recent travels, sick contacts, or prior history of COVID infection. She has no relevant family history.
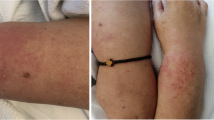
Her physical exam was notable for normal cardiovascular and neurologic exam, lungs with mild bibasilar crackles, and skin rash suspicious for dermatomyositis including Gottron’s papules (erythematous papules overlying metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints), Shawl sign on her chest wall and neck, and heliotrope rash with associated eyelid and facial swelling. She also had small ulcers on her lower lip and erythematous papules on the palmar surface of her hands (Fig. 1a–c). Interestingly, she had symmetric joint swelling in her wrists, elbows, and MCP and PIP joints without complaint of joint pain. She had a normal nailfold capillaroscopy exam. Her strength testing in all muscle groups was within normal limits.
Initial laboratory testing was notable for normal blood counts, elevated liver function tests, normal creatine kinase and aldolase, and elevated segmentation rate (76 mm/h). Antinuclear antibody (ANA), ANA comprehensive panel, and rheumatoid factor were negative, and cyclic citrullinated peptide (CCP) antibody was mildly elevated. Fungal infections ruled out with negative urine Histoplasma antigen enzyme immunoassay, Blastomyces antibodies, and serum Cryptococcus antigen. Her COVID-19 rapid antigen tests and COVID-19 IgM antibody were negative. Rocky Mountain spotted fever and syphilis screening tests were non-reactive (Table 1).
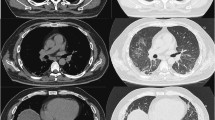
Skin biopsy revealed interface dermatitis. Pulmonary function tests (PFTs) showed forced vital capacity (FVC) 71% (normal range: 80 to 120%), forced expiratory volume during first second (FEV1) 70%, and diffuse capacity for carbon monoxide (DLCO) 31% and computed tomography (CT) of the chest had ground-glass opacities (GGOs) in the subpleural distribution (Table 2, Fig. 2a). Malignancy was ruled out with normal abdominal ultrasound, pelvis ultrasound, and CT of the abdomen and pelvis. MRI of the thighs and electromyography (EMG) were deferred given normal strength testing and muscle enzyme levels.
One week after presentation, her Myomarker® panel came back with positive anti-MDA-5 antibody 113 (normal value < 20 units) and anti-SSA 52KD antibody IgG 120 (normal value < 20 units) (Table 1).
Differential Diagnosis
The patient’s primary care physician initially diagnosed her with Coxsackie A infection given her palmar rash and positive Coxsackie A IgG antibody. She was expected to improve with time, but her rash worsened, and she developed abnormal lung findings. Sarcoidosis was considered but her skin rash was atypical for sarcoidosis, and she did not have mediastinal or hilar lymphadenopathy. Differential diagnosis also included serum sickness–like syndrome following vaccination given skin rashes and symmetric polyarthritis. However, the patient did not have fevers and her rash was more consistent with dermatomyositis than typical urticarial rash in serum sickness–like reactions. Given the diffuse GGO on CT of the chest, interstitial lung disease (ILD) with nonspecific interstitial pneumonia (NSIP) or organizing pneumonia pattern was high on the differential but infections had to be ruled out before initiation of immunosuppression.
Disseminated fungal infections can also present with acute and chronic lung diseases. Acute pneumonia from blastomycosis often presents with fever and nonproductive cough and CT of the chest may show nodules, consolidations with or without cavitation, and/or tree in bud opacities [7]. Pulmonary histoplasmosis can mimic sarcoidosis with diffuse pulmonary infiltrates and mediastinal or hilar lymphadenopathy, and cavitary lung disease in chronic cases [8]. Pulmonary cryptococcosis can be asymptomatic in immunocompetent adults and radiographic findings include noncalcified nodules, lobar infiltrates, hilar and mediastinal adenopathy, and pleural effusions [9]. Our patient did not live in an endemic area for fungal infections, and active fungal infections were ruled out with blood and urine testing (Table 1).
Given the diffuse GGOs and abnormal PFTs, COVID-19 pneumonia was also on the differential. She had multiple negative rapid antigen testing for COVID-19 and negative COVID-19 IgM antibody and nucleocapsid IgG antibody.
We diagnosed the patient with MDA-5-positive amyopathic DM with rapidly progressive interstitial lung disease (RP-ILD) based on her characteristic DM rashes with skin biopsy showing interface dermatitis, abnormal CT of the chest/PFT findings, and high titer MDA-5 antibodies.
Treatment Course
After fungal infections were ruled out, she was promptly started on methylprednisolone 1 mg/kg with slight improvement in her dyspnea on exertion and energy level. Given her extensive lung disease, she was started on combination therapy that consisted of rituximab infusions 1000 mg every 2 weeks spaced 6 months apart and mycophenolate mofetil (MMF) titrated to 1500 mg BID (Table 2). Combination therapy rituximab plus MMF was chosen over cyclophosphamide due to a more favorable side effect profile and a reported good outcome achieved in a similar case of antisynthetase syndrome with RP-ILD [10]. For her extensive skin involvement, she also received intravenous immunoglobulin (IVIG) 2 g/kg divided over 3 days every 4 weeks. Methylprednisolone was tapered slowly based on treatment response by approximately 8 mg every month.
The patient was concomitantly treated with trimethoprim/sulfamethoxazole for Pneumocystis jirovecii pneumonia (PJP) prophylaxis, calcium/vitamin D, and received one dose of IV zolendronic acid 5 mg.
Despite initial aggressive treatment, the patient had worsening of GGO on CT of the chest and slightly worse PFTs with oxygen requirement on the six-minute walk test during the first 2 months (Table 2). However, on her third month follow-up, she demonstrated mild clinical improvement with slightly less GGO on CT of the chest and stabilization of PFTs. Approximately 6 months after diagnosis, she had normalization of PFTs and significant improvement of pulmonary infiltrates on CT of the chest (Fig. 2b, c). Her dermatomyositis rash improved drastically as well with near complete resolution. Interestingly, her MDA-5 antibody titers decreased to 27 (< 20 units) at 3 months and to undetectable level by 6 months.
Discussion
We described a rare case of new-onset MDA-5-positive dermatomyositis with rapidly progressive interstitial lung disease two weeks after receiving the second dose of COVID-19 mRNA vaccination. The patient had classic dermatomyositis rashes (Gottron’s papules, shawl sign, and heliotrope rash), no evidence of muscle involvement, and RP-ILD associated with high titer MDA-5 antibodies. Her ILD and rash improved with aggressive combination therapy including high-dose steroids, rituximab, mycophenolate mofetil, and IVIG.
Interstitial lung disease (ILD) is a major cause of morbidity and mortality among patients with dermatomyositis or polymyositis. Anti-MDA-5 syndrome is a rare presentation of idiopathic inflammatory myositis associated with rapidly progressive course of ILD and poor prognosis [11]. The target antigen is the ribonucleic acid (RNA) helicase encoded by the melanoma differentiation–associated gene 5, which is an intracellular sensor of viral RNA (including coronavirus) that triggers the innate immune and subsequent production of cytokines including type I interferon, tumor necrosis factor alpha (TNFα), interleukin-1 (IL-1), IL-6, and IL-18 [11, 12]. The proposed pathogenesis involves a role of infectious and environmental factors superimposed on a genetic susceptibility [13]. RNA viruses such as Coxsackie virus and parvovirus B19 have been implicated in causation of DM in the past [13, 14].
Clinically, anti-MDA-5 DM has unique features of cutaneous vasculopathy and RP-ILD. In addition to classic rashes of DM, patients often have oral ulcers, palmar papules, and eyelid edema which were seen in our patient [15]. Arthritis typically presents in symmetric distribution similar to rheumatoid arthritis in 50–80% patients with anti-MDA-5 DM. Early and aggressive treatment with a combination of immunosuppressant is critical for survival [16, 17]. Retrospective series suggest benefit from initial combination therapy including the use of steroids, tacrolimus, cyclophosphamide, mycophenolate mofetil, and rituximab [18,19,20,21,22]. A recent retrospective study demonstrated adjunct IVIG therapy as a very effective first-line therapy for patients with MDA-5–RP-ILD, increasing survival and remission by lowering ferritin concentration, anti-MDA-5 titer, and GGO score on CT [23]. Interestingly, anti-MDA-5 concentrations correlated with RP-ILD activity as well as relapse [24]. Our patient’s anti-MDA-5 levels became undetectable after 6 months of treatment which correlated with improvement in her PFTs and radiographic imaging and resolution of her rash.
Megremis et al. identified three immunogenic linear epitopes with high sequence identity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins in patients with dermatomyositis [25]. This may suggest that exposure to Coronaviridae family might contribute to development of dermatomyositis or musculoskeletal autoimmune disease. There are reports of new-onset inflammatory myositis in COVID-19 survivors [26, 27] as well as other autoimmune and rheumatic diseases occurring after COVID-19 infection including reactive arthritis, lupus, inflammatory arthritis, cutaneous vasculitis, hemolytic anemia, and among others [27].
Development of systemic rheumatic disease after COVID-19 vaccination is rare and not well described in literature. We only found one other case of a 58-year-old man who developed probable amyopathic dermatomyositis with RP-ILD 4 days after receiving the mRNA COVID-19 vaccine. He was admitted to the intensive care unit in respiratory failure with diffuse GGO, oral sores, digital tip ischemia, and ulceration with skin biopsy consistent with dermatomyositis; his lab work showed positive ANA and elevated inflammatory markers, but MDA-5 antibodies were not reported [28]. He expired despite aggressive therapy.
Vaccine-induced inflammation may trigger or precipitate underlying autoimmunity. One proposed mechanism in development of autoimmunity to vaccines is molecular mimicry. Significant similarities between pathogenic elements contained in the vaccine and specific human proteins can potentially cause immune cross-reactivity and development of autoimmunity in predisposed individuals [29]. For instance, antibodies to SARS-COV-2 spike protein can potentially bind to human antigens such as MDA-5, extractable nuclear antigens, and nuclear antigens. Studies have also shown that SARS-COV-2 spike protein can also act as a pathogen-associated molecular pattern (PAMP) which may activate the inflammasome and TLR-mediated pathways leading to overproduction of cytokines [30, 31]. Our patient developed symptom one week after mRNA vaccination and persisted for more than four weeks. This temporal relationship suggests a distinct immunological hyperactivation rather than immediate post-vaccination immunological phenomena.
Overall, the occurrence of interstitial lung disease associated with amyopathic dermatomyositis following COVID-19 vaccination is exceedingly rare and the benefits of COVID-19 vaccination far outweigh the risks. Strength of this case report includes comprehensive patient information and good patient adherence to treatment and follow-up. The major limitation is the case report design which cannot prove direct causation and we can only postulate COVID-19 vaccination as a probable inciting trigger for ILD. Our patient also tested positive for Coxsackie A which has been associated for DM as well. However, it is important to describe this interesting case to alert clinicians the possibility of ILD associated with amyopathic dermatomyositis following COVID-19 vaccination because early aggressive therapy is critical for survival and good outcomes.
Conclusions
We described a unique case of MDA-5 syndrome with RP-ILD shortly after COVID-19 vaccination. The patient responded well to early identification and prompt aggressive immunosuppressive therapy. Larger epidemiology studies are needed to prove a causal relationship and establish surveillance for vaccine-induced immunological adverse events.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Mohammed I, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18(1):2027160.
Vitiello A, Ferrara F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacol. 2021;29(3):645–9.
Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
Singh A, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50(1):15–9.
Barbhaiya M, et al. Systemic rheumatic disease flares after SARS-CoV-2 vaccination among rheumatology outpatients in New York City. Ann Rheum Dis. 2021;80(10):1352–4.
Gargano JW, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–82.
Sheflin JR, Campbell JA, Thompson GP. Pulmonary blastomycosis: findings on chest radiographs in 63 patients. AJR Am J Roentgenol. 1990;154(6):1177–80.
Wheat LJ, et al. Pulmonary histoplasmosis syndromes: recognition, diagnosis, and management. Semin Respir Crit Care Med. 2004;25(2):129–44.
Feigin DS. Pulmonary cryptococcosis: radiologic-pathologic correlates of its three forms. AJR Am J Roentgenol. 1983;141(6):1262–72.
Ruegg, CA, et al. Jo1-antisynthetase syndrome and severe interstitial lung disease with organising pneumonia on histopathology with favourable outcome on early combined treatment with corticosteroids, mycophenolate mofetil and rituximab. BMJ Case Rep, 2019;12(9).
Mehta P, Machado PM, Gupta L. Understanding and managing anti-MDA 5 dermatomyositis, including potential COVID-19 mimicry. Rheumatol Int. 2021;41(6):1021–36.
Dias Junior AG, Sampaio NG, Rehwinkel J. A balancing act: MDA5 in antiviral immunity and autoinflammation. Trends Microbiol. 2019;27(1):75–85.
Christensen ML, et al. Prevalence of Coxsackie B virus antibodies in patients with juvenile dermatomyositis. Arthritis Rheum. 1986;29(11):1365–70.
Chevrel G, et al. Dermatomyositis associated with the presence of parvovirus B19 DNA in muscle. Rheumatol (Oxford). 2000;39(9):1037–9.
Fiorentino D, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65(1):25–34.
Chen Z, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken). 2013;65(8):1316–24.
Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78(4):776–85.
Nara M, et al. Serum interleukin 6 levels as a useful prognostic predictor of clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease. Mod Rheumatol. 2014;24(4):633–6.
Nakashima R, Hosono Y, Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus. 2016;25(8):925–33.
Matsuda KM, et al. Combined immunosuppressive therapy provides favorable prognosis and increased risk of cytomegalovirus reactivation in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. J Dermatol. 2020;47(5):483–9.
Tsuji H, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72(3):488–98.
Mao MM, et al. Ultra-low dose rituximab as add-on therapy in anti-MDA5-positive patients with polymyositis /dermatomyositis associated ILD. Respir Med. 2020;172:105983.
Wang LM, et al. Intravenous immunoglobulin for interstitial lung diseases of anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Rheumatology (Oxford). 2021.
Matsushita T, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176(2):395–402.
Megremis S, et al. Antibodies against immunogenic epitopes with high sequence identity to SARS-CoV-2 in patients with autoimmune dermatomyositis. Ann Rheum Dis. 2020;79(10):1383–6.
Beydon M, et al. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis. 2020.
Ahmed S, Zimba O, Gasparyan AY. COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol. 2021;40(7):2611–9.
Carrasco L, et al. A rapidly progressive and rare illness: autoantibodies against melanoma differentiation-associated protein 5 (anti-MDA5): amyopathic dermatomyositis with progressive interstitial lung disease that developed after COVID-19 vaccine. Chest. 2021;160(4):A680–1.
Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–94.
Khan S, et al. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-kappaB pathway. bioRxiv, 2021.
Zhao Y, et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021;31(7):818–20.
Author information
Authors and Affiliations
Contributions
Shuwei Wang: (1) substantial contributions to conception and interpretation of data; (2) drafting the article and revising it critically for important intellectual content; and (3) final approval of the version to be published.
Bassel Noumi: (1) substantial contributions to conception and interpretation of data; (2) revising it critically for important intellectual content; and (3) final approval of the version to be published.
Fardina Malik: (1) substantial contributions to conception and interpretation of data; (2) revising it critically for important intellectual content; and (3) final approval of the version to be published.
Shudan Wang: (1) substantial contributions to conception and interpretation of data; (2) revising it critically for important intellectual content; and (3) final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics Approval
This manuscript is compliant with ethical standards.
Consent to Participate
The patient provided informed consent to participate for the purpose of publication of this case study.
Written Consent for Publication
The patient has provided written consent for publication of this case report.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Covid-19
Rights and permissions
About this article
Cite this article
Wang, S., Noumi, B., Malik, F. et al. A Rare Case of MDA-5-Positive Amyopathic Dermatomyositis with Rapidly Progressive Interstitial Lung Disease Following COVID-19 mRNA Vaccination — a Case Report. SN Compr. Clin. Med. 5, 18 (2023). https://doi.org/10.1007/s42399-022-01357-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01357-0