Abstract
Clinically amyopathic dermatomyositis (CADM) is a rare auto-immune disease, which discriminates from classical dermatomyositis; it lacks typical muscle weakness, but has a high risk of developing rapid progressive interstitial lung disease (RP-ILD), malignancies, and other fatal complications, so it is easy to cause misdiagnosis and high mortality. To attract more attention and arouse more understanding, we cover and summarize our clinical findings. Here, we reported a male patient, who rapidly progressed to serious respiratory failure despite an aggressive treatment, a combination of methylprednisolone, cyclosporine, acetylcysteine, and intravenous immunoglobulin. He suffered from CADM with positive anti-melanoma differentiation-associated gene-5 (MDA 5) antibody. CADM, as a special phenotype of DM/PM (dermatomyositis/polymyositis), is usually rapidly progressive and fatal, which requires further investigation into specific pathogenesis and more effective diagnosis as well as treatment measures. Due to the severity and rarity, we simultaneously performed a review of recent literature to provide more theoretical references for clinicians in diagnosis and treatment and finally improve the survival rate of these patients.
Similar content being viewed by others
Introduction
Dermatomyositis (DM) is a systemic auto-immune disease, also known as the inflammatory myopathy, characterized by cutaneous lesion and progressive muscle weakness, and usually accompanied with multiple organ damages such as the joints and myocardium.
Clinically amyopathic dermatomyositis (CADM) is a rare type within the DM spectrum, which lacks muscle lesion, but has a higher risk of death and lower overall survival rate, especially when cooccurring with the fatal complication rapidly progressive interstitial lung diseases (RP-ILD).
In this article, we present a case of CADM and would summarize and review the related research and clinical findings of DM as well as CADM in recent years, to help clinicians discover the disease earlier and provide effective treatments for these patients.
Case Report
A 39-year-old Chinese male started with right orbital skin edema and dark purple erythema in October 2017. He neglected it at first, and then gradually developed red pimple on his upper chest, shoulder, and extensor of metacarpophalangeal, elbow, and knee joints. In February 2018, he went to the Department of Rheumatology and Immunology (Renji Hospital, Shanghai); the immunological tests indicated the following: anti-MDA5 antibody IgG 3 +, anti-RO-52 antibody IgG 3+. The positron emission tomography/computed tomography (PET-CT) scan showed no obvious interstitial lung lesions and no evidence of malignancies. In the light of the clinical performance and typical laboratory results, clinically amyopathic dermatomyositis was considered initially, so methylprednisolone 10 mg/day and hydroxychloroquine 0.1 g BID administered intraperitoneally were used for the skin lesions, but no significant improvement. In April 2018, the patient returned to the hospital for further consultation; the thoracic CT was performed and revealed a presence of subpleural ground-glass infiltrate in bilateral lung lobes. Based on the occurrence of interstitial pneumonia, the treatment was adjusted to methylprednisolone 40 mg QD, cyclosporine 50 mg BID, and acetylcysteine 0.6 g BID. About the end of May 2018, the patient developed a progressive dyspnea on exertion, accompanied by non-productive cough. During the course of the disease, there was frequent urination at night, but no myalgia, arthralgia, and fever. No similar history was found in his family.
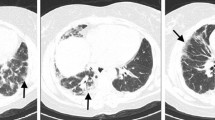
The arterial blood gas analysis on the first day of admission (he firstly entered the Department of Rheumatology and Immunology of our hospital in June 13th, 2018) showed hypoxemia (pH 7.49, PaO2 46.6 mmHg and paCO2 28.4 mmHg, SatO2 82%); the oxygenation index (OI) is 221.9. Electrocardiogram showed sinus tachycardia. Blood routine showed white blood cell 9.78 × 109/L, neutrophil% 82.6%, lymphocyte% 12.7%, and platelet 89 × 109/L. Super C active protein was 148.5 mg/mL. Erythrocyte sedimentation rate was 32 mm/h (0–15). Lactate dehydrogenase was 2150 U/L (313–618). Blood albumin was 26.6 g/L (35–50). Renal function, thyroid function, blood anti-tuberculosis antibody, mycoplasma pneumonia related antibody, and procalcitonin were normal. No positive findings were found in sputum and blood culture. The viral serologies (HIV, hepatitis B and C virus) and treponema pallidum antibody were all negative. Immunological examination Ig A, Ig G, Ig M, complement C3, complement C4, anti-cyclic citrullinated peptide antibody, antinuclear antibody, and antinuclear extract antibody (mainly including anti-ribonucleoprotein antibody, anti-dsDNA antibody, anti-Ro60 antibody, anti-Ro52 antibody, anti-La antibody, anti-nRNP antibody, anti-JO 1 antibody, anti-nucleosome antibody, anti-ScL 70 antibody, anti-histone antibody, anti-smD1 antibody, anti-PCNA antibody, anti-AMA antibody, anti-PM SCL antibody, Mi 2 antibody, KU antibody) were all negative. Creatine kinase and Creatine kinase isoenzyme were always normal. But serum ferritin was 6735μg/L, significantly higher. The serum tumor indicators are as follows: carcinoembryonic antigen was 28.11 ng/mL (0–4.3), carbohydrate antigen 199 64.82 U/mL (0–37), carbohydrate antigen 153 56.73 U/mL (0–25), cytokeratin 19 fragment 17.97 ng/mL (0–3.3), and neuron-specific enolase 38.3 mg/mL (0–16.3). For thoratic CT, apart from increased ground-glass infiltrate, there were also emerging interstitial lesions and a little pleural effusion (Fig. 1). He was treated with intravenous methylprednisolone 80 mg/day. The intravenous immunoglobulin 10 g/day was added additionally. Cyclosporine and acetylcysteine were used as before, supplemented with liver protection, prevention of osteoporosis, and appropriate fluid replenishment. Piperacillin tazobactam combined with moxifloxacin were added to cover and prevent potential infection. However, 2 days later, the patient’s respiratory failure worsens, and the transcutaneous saturation fell to 90% (under 8 L/min oxygen). So, he was admitted to the intensive care unit. Immediate monitoring of vital signs and non-invasive ventilation (mode: NSPONT, FIO2 50%, PEEP 4 cmH2O, PS 12 cmH2O) were carried out. The rest of the medication was maintained as before. Retested arterial blood gas analysis showed a progressive respiratory failure (pH 7.41, PaCO2 32.5 mmHg, paO2 53.1 mmHg, SatO2 85%, OI 87). The patient’s condition was continuously deteriorating. So, the patient and his families have a negative attitude to the latter treatment and refused the endotracheal intubation. Then, he was transferred to our department 3 days after ICU. The non-invasive ventilator assisted ventilation still continued, but the transcutaneous saturation (under 10 L/min oxygen) gradually declined. Unfortunately, 7 days later, the patient finally died of severe hypoxemia and respiratory failure. During the 12 days in our hospital, the patient had no fever, no obvious sputum, and no myalgia, but the rash had always existed.
Discussion
DM is an unusual disease, which could occur at any age, in any race, and in either males or females. The “CADM” was first proposed by Pearson in the 1960s to distinguish from “DM,” about a subset of 5–20%, which never has any evidence of muscle disease (clinical, laboratory, imaging, and histology) [1].
Cutaneous Manifestation
DM or CADM often has various cutaneous manifestations: frequently observed skin lesions are heliotrope rash (is periorbital erythema which also can be seen in other connective tissue diseases) and Gottron papules and Gotton sign (are papules and erythema at the joint extensor). The occasional observed skin lesions are as follows: facial erythema, periungual changes, inverse Gottron papules (sign), mechanic’s hands, V-neck sign, shawl sign, flagellate erythema, vesiculobullous eruptions, panniculitis, purpura, alopecia, calcinosis cutis, skin ulcers, poikiloderma, and pruritus. Most are found to be associated with autoantibodies, such as anti-Mi2 antibody, anti-TIF1 (transcriptional intermediary factor-1, anti-155/140) antibody, anti-MDA5 (melanoma differentiation-associated gene-5) antibody, anti-ARS (aminoacyl-transfer RNA synthetase) antibody, and anti-SRP (signal recognition particle) antibody. The skin biopsy pathology often revealed a cell-poor vacuolar interface dermatitis, characterized by a sparse infiltrate of inflammatory cells at the dermoepidermal junction [2]. Here, our patient also had the typical Gottron papules. Cao et al. [3] found that patients with ulcerative Gottron papules/Gottron sign had higher risk of acute or subacute interstitial pneumonia than patients with Gottron papules/Gottron sign without ulceration, as well as higher positive rate of anti-MDA5 antibody, but lower creatine kinase (CK) levels. It suggested some characteristic cutaneous change could help diagnose and judgment of patients’ condition.
Muscle Weakness
It was seen in DM patients, often starting from the symmetric proximal muscle. The pathological biopsy of muscle tissue showed typical perifascicular distribution of atrophic, degenerating, and regenerating myofibers. And laboratory inspection could find abnormal serum creatinine kinase levels [2]. However, no CADM patients have these manifestations and histopathologic feature.
Pulmonary Lesion and Anti-MDA5 Antibody
DM/CADM is often associated with interstitial lung disease (ILD),which significantly affect the prognosis.
Comparing the two patient groups with CADM-ILD or with classic DM-ILD, Mukae et al. [4] investigated that the acute ILD (not chronic/subacute) was more common in prior (the prevalence of ILD in CADM varies from 0 to 83.3%.), and their degree of respiratory failure was significantly more severe (the PaO2/FIO2 ratio was lower); the lymphocyte subset ratio in the BAL fluid was significantly higher. Few cases of CADM may only have RP-ILD singularly, without any other features including the cutaneous manifestation [5]. Ye et al. [6] demonstrated that patients with CADM-ILD showed a rapidly progressive pattern with 6-month survival rate of 40.8%, and DM-ILD manifested a progressive pattern with a 5-year survival rate of 54%, respectively, by a retrospective cohort study.
Anti-MDA5 Ab (earlier detected as an anti-140KD polypeptide antibody), closely related to the development of RP-ILD, was firstly identified in CADM patients [7]; So et al. [8] also found that Anti-MDA5 Ab is found exclusively in DM patients of the CADM subtype, similar to Sato’s [7] finding. And all these patients were predominantly male, younger, and with shorter disease duration; the Ab is associated with RP-ILD and digital ulcers. But subsequent research [9,10,11] revealed that it can also be detected among classical DM patients. The anti-MDA5 antibodies have a positive rate of 40.6% (26/64) in our Chinese patients with classic DM or clinically amyopathic DM (CADM). Thus, anti-MDA5 antibodies were not 100% sensitive nor specific for identifying CADM patients. A meta-analysis showed the pooled sensitivity and specificity of anti-MDA5 antibody for RP-ILD in patients with DM/PM were 77% (95% confidence interval [95% CI] 64–87%) and 86% (95% CI79–90%), respectively. This difference may be related to insufficient number of cases and various populations. But all these patients are frequently developing rapidly progressive interstitial lung disease (RP-ILD), especially in Asia, with an extremely low survival rate. The CADM patient we presented survived only 4 months after RP-ILD.
Newly, Kochi et al. [12] performed the first genome-wide association study, which showed the WDFY4 variant may play a critical role in the pathogenesis of CADM; the anti-MDA5 auto-antibodies were recognized by MDA5, then interacted with WDFY4, and enhanced the NF-κB signaling activation, promoting cell apoptosis and inflammation, then inducing a series of clinical symptoms.
Given the relationship between the antibody and diseases, Xu et al.’s [13] research enrolled 11 CADM patients with ILD and 29 CADM without ILD, which found that anti-MDA5 Ab+, elevated CRP, and decreased counts of lymphocyte were independent risk factors of RP-ILD by a multivariate logistic regression analysis; thus, they found out that it may consider as a precise predictive factor for the prognosis in CADM-RP-ILD patients. Furthermore, after effective treatment, the Ab titer would significantly decrease, and decrease anti-MDA5 antibody levels during the remission period, but a re-increase in anti-MDA5 antibody levels is followed by a relapse. It suggested that the examination of this antibody may be clinically useful in patients with idiopathic inflammatory myopathies and could use anti-MDA5 Ab titers to monitor the diseases activity [9, 14].
Tumor Occurrence
It came up early in 1992 that the risk of cancer is increased in patients with dermatomyositis, and there is also a higher rate of mortality from cancer than general population (the mortality ratio is 3.8; 95% confidence interval, 2.9 to 4.8). Among their 392 patients with dermatomyositis, the relative risk of cancer was higher in female patients (female 3.4; 95% confidence interval, 2.4 to 4.7, male 2.4; 95% confidence interval, 1.6 to 3.6) [15]. In contrast to this, several later studies indicated male gender was more dangerous to developing malignancies [16, 17]. And among DM patients, the most common malignancy is lymphatic/hematopoietic malignancies (more than 22-fold higher than in the general population), and lung cancer is the second one; others include ovarian, pancreatic, stomach, colorectal, colon cancer [18]. Some patients may have more than one malignancies. In addition, the risk of malignancy was the highest in the first year after diagnosis, then steadily decreases from years 2 through 5, but remains elevated after 5 years. And it is higher in those 45 years of age or older, similar to Chen’s results; the older age at onset was an independent predictive factor of malignancy [17]. But we still cannot ignore its risk and occurrence even in patients less than 45 years.
In recent years, some cases of CADM patients with tumors have been reported successively [19, 20], while CADM, compared with DM, seemed to be more susceptible to malignancy [21]. But it also has some difference with DM. The most common cancer in CADM includes breast, lung, and ovary (DM: nasopharyngeal, lung, ovary). In women, the most common is breast, ovary, cervix, or uterus cancer. In man, the most common are nasopharyngeal, bladder, colorectal, lung, and prostate cancers [22]. Interestingly, either DM or CADM patients, who developed malignancies, were less likely to have interstitial lung disease and had the complication of interstitial lung disease, with a significantly lower frequency of malignancies [16, 21].
Yet, the mechanism of high risk of malignancy in these patients is not clear, which may be related to some antibodies. Pinal-Fernandez et al. [23] proposed that TIF1-γ antibody was associated with the tumors of patients with DM. Chen et al. [24] found patients with DM with cancer had elevated sPD-L1 values, especially in those with treatment-naïve cancer (in DM). So, Labrador-Horrillo et al. [25] combined the data from the two results and concluded that the combination of TIF1-γ antibody and sPD-L1 could be a good option for cancer detection in this population, but it needs be confirmed in more clinical experiments, so that the related therapy could be conduct. Moreover, the serum ferritin level maybe correlated with the malignancy and disease activity, prior to chest radiography for monitoring clinical status [26]. Hence, patients diagnosed with DM/CAMD should be recommended to undergo malignancy screening regularly.
Other Clinical Manifestation
A rare case of sudden-onset severe pharyngeal dysphagia was reported, due to exacerbation of underlying dermatomyositis without the aggravation of other symptoms. After a multidisciplinary approach and appropriate supportive care, the patient’s condition was relieved several months later. It should arouse our attention, because the clinical symptoms of DM vary widely and may be not typical [27].
Treatment
The treatment mostly used in adult DM/CADM patients are as follows: the topical treatment mainly include sun protection, topical corticosteroids, and topical calcineurin inhibitors; the systemic treatment include dapsone, antimalarials, IVIG, non-steroidal immunosuppressants including methotrexate, azathioprine, and mycophenolate mofetil, and biologic therapies. However, there was no single treatment that was successful for all patients. Only several treatments led to improvement or remission in few cases; the IVIG is relatively the most successful one [28]. Endo et al. [29] reported an old Japanese woman diagnosed with anti-MDA5Ab-positive DM, who had a serious respiratory failure despite the combination of corticosteroids, cyclophosphamide, and calcineurin inhibitor, so PE (plasma change) was tried at 3 times/week, with fresh frozen plasma 30 units/time and repeated 1000 mg of IVCY immediately after the first PE; PE was completed in a total of 4 sessions. Finally, the patient’s condition improved and had a remission over 2 months. Ichiyasu et al. [30] reported a 35-year-old anti-MDA 5 Ab-positive woman with cervical cancer and rapidly progressive ILD associated with CADM; they successfully performed the surgery for cervical cancer after her respiratory dysfunction improved by combination therapy with corticosteroids, immunosuppressants, and direct hemoperfusion using polymyxin B immobilized fiber column (PMX-DHP), and she gradually returned to an excellent clinical condition, with complete remission of active ILD. And Li et al. [31] reported pirfenidone (an antifibrotic agent) could achieve a significantly higher survival rate in patients with subacute ILD (disease duration between 3 and 6 months), but not in acute ILD; the positivity of anti-MDA-5 Ab also had no change. Thus, these successful therapies need be confirmed in more clinical trials and help more patients.
In general, due to the severity and low survival rate of DM or CADM, it is imperative for us to pay more attention to its diagnosis, especially anti-MDA5 antibody-positive DM/CADM patients, and look for more effective treatments to save them in the future.
References
Bailey EE, Fiorentino DF. Amyopathic dermatomyositis: definitions, diagnosis, and management. Curr Rheumatol Rep. 2014;16(12):465.
Mammen AL. Dermatomyositis and polymyositis. Ann N Y Acad Sci. 2010;1184(1):134–53.
Cao H, Xia Q, Pan M, Zhao X, Li X, Shi R, et al. Gottron papules and Gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735–42.
Mukae H, Ishimoto H, Sakamoto N, Hara S, Kakugawa T, Nakayama S, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136(5):1341–7.
González-Moreno J, Raya-Cruz M, Losada-Lopez I, et al. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol Int. 2018:1–4.
Ye S, Chen X, Lu X, Wu MF, Deng Y, Huang WQ, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26(10):1647–54.
Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52(5):1571–6.
So H, IP R W K, WONG V T L, et al. Analysis of anti-melanoma differentiation-associated gene 5 antibody in Hong Kong Chinese patients with idiopathic inflammatory myopathies: diagnostic utility and clinical correlations. Int J Rheum Dis. 2018;21(5):1076–81.
Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. 2013;23(3):496–502.
Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology. 2012;51(7):1278–84.
Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, et al. Utility of anti–melanoma differentiation–associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res. 2013;65(8):1316–24.
Kochi Y, Kamatani Y, Kondo Y, Suzuki A, Kawakami E, Hiwa R, et al. Splicing variant of WDFY4 augments MDA5 signalling and the risk of clinically amyopathic dermatomyositis. Ann Rheum Dis. 2018;77(4):602–11.
Xu Y, Yang CS, Li YJ, Liu XD, Wang JN, Zhao Q, et al. Predictive factors of rapidly progressive-interstitial lung disease in patients with clinically amyopathic dermatomyositis. Clin Rheumatol. 2016;35(1):113–6.
Matsushita T, Mizumaki K, Kano M, Yagi N, Tennichi M, Takeuchi A, et al. Anti-MDA5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176:395–402.
Sigurgeirsson B, Lindelöf B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. N Engl J Med. 1992;326(6):363–7.
Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case–control study. Br J Dermatol. 2001;144(4):825–31.
Qiang JK, Kim WB, Baibergenova A, Alhusayen R. Risk of malignancy in dermatomyositis and polymyositis: a systematic review and meta-analysis. J Cutan Med Surg. 2017;21(2):131–6.
Olazagasti JM, Baez PJ, Wetter DA, Ernste FC. Cancer risk in dermatomyositis: a meta-analysis of cohort studies. Am J Clin Dermatol. 2015;16(2):89–98.
Shenoy S, Thapamagar S. Amyotrophic dermatomyositis. Can Med Assoc J. 2017;189(4):E164.
Lee SF, Lam YC, Mak SM, Wong FCS. Colon cancer presenting as amyotrophic dermatomyositis. Postgrad Med J. 2019;95(1119):49–50.
Azuma K, Yamada H, Ohkubo M, Yamasaki Y, Yamasaki M, Mizushima M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21(2):178–83.
Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5):509–18.
Pinal-Fernandez I, Ferrer-Fabregas B, Trallero-Araguas E, et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatology. 2017;57(2):388–96.
Chen H, Peng Q, Yang H, Yin L, Shi J, Zhang Y, et al. Increased levels of soluble programmed death ligand 1 associate with malignancy in patients with dermatomyositis. J Rheumatol. 2018;45(6):835–40.
Labrador-Horrillo M, Selva-O’Callaghan A. Cancer-associated dermatomyositis: does the PD-1 checkpoint pathway play a role? J Rheumatol. 2018;45(6):731–2.
Yamada K, Asai K, Okamoto A, Watanabe T, Kanazawa H, Ohata M, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11(1):34.
Kwon KM, Lee JS, Kim YH. A case report of life-threatening acute dysphagia in dermatomyositis: challenges in diagnosis and treatment. Medicine. 2018;97(17):e0508.
Callander J, Robson Y, Ingram J, Piguet V. Treatment of clinically amyopathic dermatomyositis in adults: a systematic review. Br J Dermatol. 2018;179(6):1248–55.
Endo Y, Koga T, Suzuki T, Hara K, Ishida M, Fujita Y, et al. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis: a case report. Medicine. 2018;97(15):e0436.
Ichiyasu H, Sakamoto Y, Yoshida C, Sakamoto K, Fujita R, Nakayama G, et al. Rapidly progressive interstitial lung disease due to anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis complicated with cervical cancer: successful treatment with direct hemoperfusion using polymyxin B-immobilized fiber column therapy. Respir Med Case Rep. 2017;20:51–4.
Li T, Guo L, Chen Z, Gu L, Sun F, Tan X, et al. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep. 2016;6:33226.
Acknowledgments
The authors are grateful to the editor, the associate editor, and the reviewers.
Author information
Authors and Affiliations
Contributions
Every author had an equal contribution to collect data and draft, and the manuscript is approved by all authors for publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from the patient and his families described in the report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Huang, Y., Mao, M. Rapidly Progressive Interstitial Lung Disease Originating from Clinically Amyopathic Dermatomyositis Associated with Positive Anti-MDA 5 Antibody: a Case Report and Literature Review. SN Compr. Clin. Med. 1, 915–920 (2019). https://doi.org/10.1007/s42399-019-00127-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-019-00127-9