Abstract
Anti-MDA5 (Melanoma differentiation-associated protein 5) myositis is a rare subtype of dermatomyositis (DM) characterized by distinct ulcerative, erythematous cutaneous lesions and a high risk of rapidly progressive interstitial lung disease (RP-ILD). It has been shown that SARS-CoV-2 (COVID-19) replicates rapidly in lung and skin epithelial cells, which is sensed by the cytosolic RNA-sensor MDA5. MDA5 then triggers type 1 interferon (IFN) production, and thus downstream inflammatory mediators (EMBO J 40(15):e107826, 2021); (J Virol, 2021, https://doi.org/10.1128/JVI.00862-21); (Cell Rep 34(2):108628, 2021); (Sci Rep 11(1):13638, 2021); (Trends Microbiol 27(1):75–85, 2019). It has also been shown that MDA5 is triggered by the mRNA COVID-19 vaccine with resultant activated dendritic cells (Nat Rev Immunol 21(4):195–197, 2021). Our literature review identified one reported case of MDA5-DM from the COVID-19 vaccine (Chest J, 2021, https://doi.org/10.1016/j.chest.2021.07.646). We present six additional cases of MDA5-DM that developed shortly after the administration of different kinds of COVID-19 vaccines. A review of other similar cases of myositis developing from the COVID-19 vaccine was also done. We aim to explore and discuss the evidence around recent speculations of a possible relation of MDA5-DM to COVID-19 infection and vaccine. The importance of vaccination during a worldwide pandemic should be maintained and our findings are not intended to discourage individuals from receiving the COVID-19 vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-MDA5 is a myositis-specific autoantibody (MSA) that is associated with DM and more specifically with Clinically Amyopathic Dermatomyositis (CADM). CADM is a subset of DM, which is defined by the presence of classic cutaneous features of DM without objective muscle weakness. The anti-MDA5 antibody has been shown to have a high risk of being complicated by life-threatening RP-ILD and poor survival [1,2,3,4,5,6,7,8]. Complications with spontaneous pneumothorax and pneumomediastinum have also been reported with the anti-MDA5 antibody [5, 9, 10]. There have been recent publications showing a possible relation of this rare disease to COVID-19 infection and/or vaccine, as well as other viruses although evidence for a direct correlation is insufficient, and hence, more is needed [4, 9, 11,12,13,14,15]. These findings warrant a further understanding of viral and vaccine interaction, particularly that of SARS-CoV-2 (COVID-19) with the host immune response and subsequent autoantibody development.
We herein report six cases of MDA5-DM that developed within days after administration of different kinds of COVID-19 vaccine. To date and our knowledge, there has been only one other case report (published in October 2021) suggesting the COVID-19 vaccine as a possible trigger of MDA5-DM [9]. This case identified a 58-year-old man who had developed respiratory failure and shock four days after receiving a novel mRNA COVID-19 vaccine (Carrasco et al. did not specify which vaccine type or whether symptoms started after the first or second dose). Noteworthy clinical findings included oral blisters, digital ischemia/ulceration, and computed tomography (CT) chest showing diffuse and sub-pleural ground-glass opacities (GGO). An extensive infectious workup, including COVID-19, was negative. The diagnosis was confirmed with serum antibodies against MDA5. The significance of this case is being the first clinical support for the relation between MDA5 activation by the COVID-19 vaccine with subsequent disease development. We present six additional cases along with a literature review with the aim to further support this relation.
Methods
Written and informed consent was obtained from all the patients for the cases as well as the images. The search strategy for writing review articles proposed by Gasparyan et al. [16] was followed. Using PubMed, Science Direct, and SCOPUS databases, we searched articles available anytime using the following search terms: (“MDA5” OR “CADM” OR “dermatomyositis”) AND “Covid”, “Covid” AND “myositis”, (“MDA5” OR “CADM” OR “dermatomyositis) AND (“ILD” OR “interstitial lung disease), and “Covid vaccine” AND (“myositis” OR “connective tissue disease”). Of the 388, 686, and 392 articles obtained, respectively, in each database, articles in a language other than English were excluded. Only one article describing MDA5-DM developing after COVID-19 vaccine administration was identified in our search [9]. After an initial screening of titles and abstracts, the remaining articles were retained according to their relevance to COVID-19 infection and vaccine with the MDA5 cytosolic sensor, viral etiologies of MDA5-DM, and MDA5-DM pathogenesis. Additional literature searches on clinical findings, diagnosis, and treatment of MDA5-associated disease were also done to inform this case-based review (Fig. 1).
Case reports
Case 1
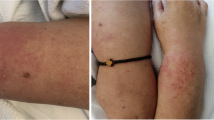
A 45-year-old-Hispanic male without any past medical history or routine medications developed acute onset of rashes and swelling two days after the 2nd dose of the Moderna (mRNA-1273) COVID-19 vaccine. The patient had the 1st dose 2 months prior without any complications. Two days after receiving the 2nd dose, he started developing swelling and redness surrounding his left eye that evolved into extensive edema and erythema. His symptoms progressed over the next 4–5 months eventually causing characteristic ulcerative lesions over bilateral metacarpophalangeal (MCP) regions (Gottron’s papules), erythematous maculopapular lesions over interphalangeal (IP) palmar creases (palmar papules), erythema over upper eyelids (heliotrope rash), chest (V-sign), back (Shawl sign), arms, lateral upper thigh, and knees. Erosive retiform lesions were also noted over his back (Fig. 2).
Case 1. Gottron’s papules seen over bilateral MCPs (A) with palmar erythema, and “reverse Gottron’s lesions” over PIP and DIP palmar creases (B). Diffuse edema of entire right arm extending to hand (C). Erosive retiform lesions over back (D). CT thorax showing bilateral ground opacities with nodular consolidate opacities that developed rapidly over a few days (E). CT of right upper extremity showing diffuse cutaneous thickening with circumferential subcutaneous fat stranding and soft tissue swelling (F)
He later developed intense swelling of the entire right arm extending from upper arm to hand. A CT scan of the arm showed diffuse subcutaneous changes concerning for myositis. He denied any muscle weakness but had obvious limited range of motion of the right arm and hand from the intense edema and pain. On exam, he had normal strength in all other extremities.
A skin biopsy showed interface dermatitis and sparse perivascular lymphocytic infiltrate consistent with dermatomyositis. Antinuclear antibody (ANA) was negative, erythrocyte sedimentation rate (ESR) 75 (0–10 mm/h), C-reactive protein (CRP) 8.2 (< 0.8 mg/dL) (increased later to 19), creatine kinase (CK) 336 (subsequently 1066) (< 194 U/L), aspartate aminotransferase (AST) 66 (< 40 U/L), alanine transaminase (ALT) 139 (< 50 U/L), lactate dehydrogenase (LDH) 1970 (300–600 U/L), and serum ferritin 5100 (15–464 ng/ml). Anti-Ro and rheumatoid factor (RF) tested positive while all other autoimmune serologies were negative (including anti-dsDNA, anti-Smith, anti-Jo-1, anti-SSB, anti-RNP, anti-Centromere, anti-CCP, and anti-Scl70).
The patient had no respiratory complaints and had a normal chest x-ray (CXR) on admission. He received intravenous (IV) methylprednisolone for suspected IIM, followed by prednisone taper with intravenous immune globulin (IVIG). He developed hypoxia with oxygen saturation as low as ~ 85% on room air on the third day of the IVIG regimen. A repeat CXR showed fluffy opacities and computed tomography (CT) thorax depicted bilateral ground-glass and nodular consolidated opacities as well as rapid progression of diffuse airspaces concerning for diffuse alveolar hemorrhage. At this point, Rituximab (RTX) was commenced (hospital day 9).
The extended myositis panel returned on day 16 with positive high-titer anti-MDA5 autoantibody at 168 (N < 15) and Ro-52 at 142 AU/mL (0–40). The patient’s hypoxia improved after commencing steroids, IVIG, and RTX over the next 2 weeks. This was affirmed by repeat CT thorax depicting an improvement in the ground glass opacities (GGOs). Additionally, his arm edema/pain resolved and thus was started on methotrexate (MTX) and daily folate with close outpatient monitoring. He returned for outpatient follow-up 5 months after his diagnosis. He was without any dyspnea, cough, or hypoxia. He had residual hyperpigmentation in the areas of his initial rash that were healing. No new rashes or ulcerative lesions were present. A repeat MDA5 serology was positive, however, the titer was lower at 65 (N < 15).
Case 2
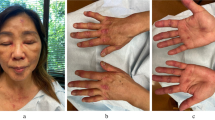
A 58-year-old-Asian female with well-controlled diabetes-mellitus developed rashes 1 week after the 2nd dose of the Covidshield (ChAdOx1 nCoV-19) COVID-19 vaccine. Prior to the vaccine the patient was in good overall health and received 1st dose 40 days before 2nd dose without any complications. At presentation, the patient had rashes involving the face and knees (Gottron’s sign) predominantly that were getting progressively worse. By the following week (~ 14 days since 2nd dose of vaccine), the patient developed polyarthralgia (mostly in hands and feet) severe enough that it kept her from doing her activities of daily living. She subsequently developed dyspnea-on-exertion in the 2nd week that later had progressed to at rest as well. Several ulcers over the ears (erythematous auricular papules) also appeared which later extended to the feet and hands (Fig. 3). The patient denied any muscle weakness.
Case 2. Ulcerative lesion on right ear (A) and numerous papules seen over left antihelix (B). Distal finger with erythematous maculopapular lesion. (C). CT thorax showing ground glass opacities in bilateral lower lobes (D). Several ulcerative lesions seen on medial right foot and ankle (E). Ichthyosis over left forearm (F)
She continued to progress with worsening rashes, arthralgias, and dyspnea, for which she eventually required hospital admission (3 months since the first symptom onset). Her lab work revealed a mostly normal complete blood count (CBC) only with mild anemia (Hgb 11.7). Ferritin was normal at 173.8 (11–307). A CK level was normal at 0.88 (0.6–1.2 mg/dL), LDH 498 (225–450 U/L) and AST 74 (< 35 U/L). Viral markers (CMV-IgM, HIV, hepatitis-B, and hepatitis-C) were negative. A CT chest confirmed GGOs, traction bronchiectasis, and subpleural increased reticulation. The serology panel showed strongly positive anti-MDA5, however, ANA, anti-Ro52, and the other myositis panel antibodies were negative.
She was initially treated with mycophenolate mofetil (MMF) and hydroxychloroquine (about 2 weeks after her symptoms had first started). The patient has since been on several different immunosuppressants including cyclophosphamide and RTX throughout her hospitalization. Her respiratory issues continued to worsen despite her skin lesions improving. A subsequent CT chest 2 months down the line suggested worsening ILD. In addition, multiple enlarged necrotic lymph nodes were seen at the bilateral supraclavicular region.
Tofacitinib 5 mg daily was commenced with initial improvement followed by secondary worsening with hypoxia culminating in intensive care unit admission. A trial of tacrolimus and IVIG with plasma exchange (PLEX) was eventually given. The patient’s hypoxia improved and was deemed stable for discharge home with oxygen supplementation (2 L/min), hydroxychloroquine, and nintedanib with close monitoring.
Case 3
A 45-year-old-Caucasian female with a past medical history of seropositive rheumatoid arthritis for nine years, previously treated with MTX and infliximab was in remission on upadacitinib. Three days after receiving her 2nd dose of the Pfizer (BNT162b2) COVID-19 vaccine, she noticed cracking of skin over the palms (mechanic hands), ulcerations of the fingertips, an erythematous facial rash along her cheeks, profound fatigue, and worsened arthralgias (Fig. 4). The patient had the 1st dose of the vaccine 21 days prior without any complications. Upadacitinib was switched to adalimumab due to worsening arthralgias, and she was given prednisone 20 mg daily with minimal response. She quickly began to develop fevers, chills with shortness of breath, dyspnea-on-exertion, and 13 kg weight loss over 2 weeks after the onset of rashes when adalimumab was discontinued whilst the prednisone dose increased to 60 mg daily. Her symptoms progressed, resulting in hospitalization when a CT chest showed bilateral GGOs in the upper lobes along with reticular interstitial opacities in the peripheral lower lobes. Bronchoscopy with bronchoalveolar lavage (BAL) was negative for bacteria, fungi, and pneumocystis-PCR. She was discharged on 2–3L/min home oxygen due to interim improvement in her dyspnea after prednisone 60 mg/day. The outpatient evaluation showed a positive ANA 1:640 (homogenous pattern), and a strongly positive anti-MDA5 autoantibody, with the rest of the myositis panel being negative. Her repeat RF (484) and CCP (> 250 U) both were highly positive as in the past when she was diagnosed with rheumatoid arthritis. Cutaneous biopsy at this juncture showed vacuolar interface dermatitis with focal interstitial granulomatous inflammation consistent with dermatomyositis. Electromyography (EMG) showed mild myopathic features but no muscle biopsy was performed. A CK level was normal as well as her muscle strength.
Case 3. Significant facial and lower eyelid edema bilaterally with overlying erythema (heliotrope rash) (A). Erythematous lesions over MCPs (Gottron’s papules) with periungal erythema bilaterally (B). Erythematous rash over forearm (C). Initial CT thorax showing ground glass and reticular interstitial infiltrates in peripheral lobes (D). Repeat CT thorax 5 months later showing progression with patchy bilateral consolidations, ground glass opacities, and “Atoll” sign (central GGO surrounded by circumferential consolidation) (E)
Based on these features, she was diagnosed with anti-MDA5 positive CADM, and RTX was commenced (1 g IV × 2, 2 weeks apart) whilst she received tapering doses of prednisone until 20 mg/day. She continued to worsen over the next 4 weeks to develop significant facial edema (heliotrope rash), worsening skin rashes over the hand joints, specifically the MCPs (Gottron’s papules), toes, elbows, scalp, neck, and shoulder with worsening nonproductive cough, dyspnea, and hypoxemia requiring oxygen up to 6 L/min. A repeat CT chest confirmed RP-ILD due to anti-MDA5 positive IIM with demonstrable patchy bilateral consolidation and GGOs, consistent with an organizing pneumonia pattern with the “Atoll” sign. BAL ruled out an underlying active infection, and high-dose IV methylprednisolone with PLEX was given for 5 days. Tofacitinib was started with improvement in the patient’s fatigue and dyspnea. One week later the patient experienced an acute worsening of her dyspnea with increased oxygen needs, as well as a worsening rash (periorbital edema and violaceous rash on extremities). A repeat CT chest angiography showed no pulmonary embolism but demonstrated worsening GGOs and thus she was readmitted to the hospital and treated for presumed ILD flare with pulse IV steroids. Tofacitinib was stopped and tacrolimus with steroid taper was done. Approximately 2 weeks later, she was diagnosed with COVID-19 infection primarily with respiratory symptoms. Tacrolimus was held during this time but restarted once she recovered from the acute COVID-19 infection. Her respiratory status remained stable, however, her worsening rash was the main concern, therefore, IVIG was added to her outpatient regimen with close monitoring.
Case 4
A 28-year-old-Caucasian female with a past medical history of hypothyroidism, and Brooke-Spiegler syndrome (a rare, autosomal dominant condition associated with nodular, adnexal tumors) diagnosed five years earlier, developed acute onset of cutaneous rashes on arms, face, elbows, hands, chest, and trunk approximately 2 weeks after receiving her 2nd dose of the Pfizer (BNT162b2) COVID-19 vaccine. She had the 1st dose 21 days prior without any complication. The rash became flaking and painful that continued to worsen despite treatment with topical nystatin-triamcinolone and oral methylprednisolone taper.
Two weeks later her symptoms had worsened with new-onset proximal symmetric muscle weakness of upper and lower extremities. Physical exam revealed erythema and scaling of entire scalp, forehead, cheeks, nose (diffuse Gottron’s sign) with V-sign over the chest, and Gottron’s papules seen overlying bilateral MCPs and IP joints of hands. She had objective muscle weakness with 4/5 on Medical Research Council (MRC) scale on deltoid as well as iliopsoas with moderate difficulty rising from a seated position with the rest of the muscle strength within normal limits. Dermoscopy of proximal nail fold revealed dilated capillary loops and ragged cuticles with periungual erythema (Fig. 5). Given this constellation of findings highest concern was for dermatomyositis and therefore a punch biopsy was done.
At a two-week follow-up, the rash had improved with topical steroids (oral steroids were avoided due to previous lack of response), however, she developed worsening muscle weakness requiring assistance getting in and out of vehicles, difficulty swallowing, and weight loss. Skin biopsy showed findings consistent with dermatomyositis and was started on hydroxychloroquine 300 mg daily and prednisone 1 mg/kg daily until seen by a rheumatologist. Subsequent lab work showed a normal metabolic panel aside from elevated ALT at 55. (< 40 U/L). CBC, CK, aldolase, LDH, and ESR all were normal, as well as a negative ANA. A myositis antibody panel resulted strongly positive for both MDA5 and TIF-1 gamma. Although the patient denied any respiratory symptoms, a CXR and CT chest were done confirming no lung involvement. MMF 1500 mg twice a day was later added to her treatment regimen as well as sulfamethoxazole-trimethoprim for pneumocystis pneumonia prophylaxis. She was recommended for medical exemption from the COVID-19 booster shot in light of this concern. She has remained stable with no known flaring of her disease.
Case 5
A 51-year-old-Asian female with a past medical history of COVID-19 infection developed a photosensitive rash over her face, neck, knuckles, and thighs as well as polyarthralgia and progressive dyspnea 1 week after the 2nd dose of the Pfizer (BNT162b2) COVID-19 vaccine. She received the 1st dose 28 days prior without any complications. Six months prior she was diagnosed with COVID-19 infection complicated by organizing pneumonia (OP) and subsegmental pulmonary embolism. During her hospitalization for COVID-19, she required intubation for 4 days as well as IV pulse methylprednisolone for 1 week. She improved and was able to be discharged on an oral steroid taper with follow-up in the respiratory clinic. A repeat high-resolution CT (HRCT) chest 5 months later showed significant improvement in the OP pattern previously seen. She symptomatically improved over several months and was able to return to her normal routine (until the symptoms after vaccination started).
The rash over her bilateral knuckles progressed to painful ulcerative lesions accompanied by fingertip ulcerations. She later developed a loss of appetite with a 13 lb weight loss over 3 months. She denied any muscle weakness or difficulty swallowing. At the time of the rheumatology visit, her physical examination was noteworthy for Gottron’s papules over bilateral MCPs with ulcerations, heliotrope rash, holster sign over bilateral thighs, and Shawl sign which were all suggestive of a dermatomyositis rash (Fig. 6). She also had active synovitis over bilateral knees and wrists. Lung examination revealed fine crepitations over bilateral mid-to-lower lobes. There was no muscle weakness, peripheral edema, or lymphadenopathy noted. The rest of the physical exam was unremarkable.
Case 5. Bilateral dorsal PIPs and MCPs with erythematous, ulcerative lesions (A). Reverse Gottron’s lesions on bilateral palms and over PIP and DIP palmar creases (B). Erythematous rash overlying lateral thigh (Holster sign) (C). Erythematous macular rash over upper back (Shawl sign) (D). High resolution CT of lungs showing organizing pneumonia pattern concerning for progressive ILD (E)
Lab work revealed a normal CBC, normal CK, normal CMP aside from AST 64 (< 35 U/L), elevated ESR 73 (0–20.0 mm/h), elevated CRP 6.8 (< 5.0 mg/L), and a negative ANA. Complement levels were normal. A repeat COVID-19 PCR was also negative. An extended myositis panel was strong-positive for anti-MDA5 and anti-Ro52. A repeat HRCT of the lungs showed a worsening appearance of OP pattern with mediastinal lymphadenopathy (Fig. 6). She underwent pulmonary function testing (PFT) which showed a restrictive pattern (FVC 44%, FEV1/FVC 89%). A thorough infectious workup was negative including tuberculosis (TB). She was subsequently diagnosed with MDA5-associated CADM with RP-ILD and initiated on prednisolone 1 mg/kg (60 mg daily). Since being diagnosed, she has undergone 4 out of 6 cycles of cyclophosphamide 0.6 g/m2 (monthly) in view of RP-ILD while tapering oral prednisolone. Immunosuppression was held recently in light of a soft tissue infection requiring incision and drainage, however, she has had marked improvement in terms of muscle weakness (MRC score decreased from 4 to currently 1). She is scheduled for repeat HRCT and PFT once her infection resolves.
Case 6
A 54-year-old Hispanic woman with a past medical history of well-controlled asthma and mild COVID-19 infection presented to the Rheumatology Clinic due to concerns of dermatomyositis after having received the first dose of the BioNTech/Pfizer (BNT162b2 mRNA) COVID-19 vaccine. She received the first dose of the Pfizer COVID-19 vaccine on June 1st, 2021. Approximately 2 weeks later she developed a burning erythematous rash at the external aspect of the left arm that progressed to the neck, face, anterior chest, metacarpophalangeal joints, and right arm. She also developed bilateral eyelid and facial edema, hyperkeratotic eruptions on the lateral surfaces of the digits, and polyarthralgia mainly in the hands and knees. She received the second dose of the vaccine on July 12th which exacerbated the previously mentioned symptoms. She was initially given oral daily prednisone for concerns for cutaneous adverse reaction to COVID-19 vaccine which provided some improvement, however, symptoms returned necessitating a skin biopsy. The biopsy showed findings consistent with interface dermatitis. Hydroxychloroquine was later started with continuation of oral prednisone.
Her symptoms continued to progress for the next 4 months until she was evaluated by a rheumatologist. A physical exam showed intense erythema over the forehead and cheeks (sunburn sign), eyelids (heliotrope rash), chest (V-sign), erythematous maculopapular lesions over the MCPs (Gottron’s papules), periungual erythema, and mechanic hands (Fig. 7). There was no synovitis or muscle weakness. Cardiopulmonary exam was also normal. A CBC, urinalysis, kidney, and thyroid tests were all normal. She had slightly increased AST 35 (N < 32 U/L) and LDH 234 (N < 214 U/L), and normal ALT 29 (N < 52), CPK 64 U/L (N < 223) and aldolase 4.4 U/L (N < 7.6). CRP (1.61 mg/dl (N < 0.30)) and ESR (26 mm/h (N < 20) were elevated. C3, C4, and immunoglobulin G were normal. ANA was positive at 1:640 with a fine speckled nuclear pattern, however, anti-dsDNA, anti-Smith, anti-RNP, and anti-La were negative. The myositis panel was positive for anti-MDA5 (54, N < 10) and anti-Ro-52 antibodies (23, N < 10). Thoracic, abdominal, and pelvic CT was done without signs of interstitial lung disease or malignancy. Electromyography and muscle biopsy were not performed. A diagnosis of anti-MDA5 and anti-Ro-52 positive amyopathic dermatomyositis temporally associated with COVID-19 vaccination was given. She was started on azathioprine (later changed to MMF) and oral prednisone with adequate clinical response and nearly complete disappearance of facial edema and rash at the most recent follow-up in March 2022. A few weeks later MMF was stopped due to concern for hair loss and replaced with MTX plus the continuation of tapering prednisone.
Limitations
The findings of our cases have to be seen in light of some limitations. The first and primary limitation is the number of reported cases of MDA5-associated disease from COVID-19 vaccination is scarce provided the number of people in the world who have been vaccinated. Recognition, as well as awareness of MDA5-associated disease by clinicians, may also be decreased for multiple reasons including its rarity, its lack of typical myositis features such as significant muscle weakness and elevated CK levels, and its clinical similarities to COVID-19 infection. COVID-19 infection itself is a new disease entity and we are yet to fully understand its full long-term implications. The cases we are presenting will have to be followed over a longer period of time to see if their outcomes are similar to previously reported cases of MDA5 prior to the COVID-19 era.
Literature review and discussion
We herein describe six serendipitous occurrences of anti-MDA5 CADM, a rare subtype of IIM, with temporality after COVID-19 vaccination. While association cannot be causation, especially in the setting of a global vaccination drive during a worldwide pandemic, we explore and discuss the possibilities and evidence around the specific interactions of the interferon pathway, the MDA5 gene, and the COVID-19 vaccine and its intersection with myositis. We further scrutinize the link of anti-MDA5-associated myositis with viral infections in general, draw parallels, and discuss the possibilities of an aetiopathological overlap from an immunologic standpoint.
The association of a positive anti-MDA5 autoantibody and CADM with RP-ILD and poor survival is well established in the literature. Objective muscle weakness is typically not common in this dermatomyositis subtype, although cutaneous and muscle manifestations may vary, ranging from minimal skin lesions to striking, widespread ulcerative lesions and/or characteristic palmar papules [1,2,3,4, 7, 8, 11, 12, 17,18,19,20,21,22,23,24]. CADM has also been used as an umbrella term that combines amyopathic and hypomyopathic DM patients. In some instances, the glaring absence of both cutaneous and muscle manifestations may culminate in delayed diagnosis of autoimmune RP-ILD with fatal outcomes within weeks of symptom onset in up to 50% [3, 8, 18, 21, 25, 26].
Viral infection has long been thought to be a potential etiology for autoimmune disease, including IIM [1, 3, 12, 27]. Development of autoimmune reaction following SARS-CoV-2 infection (including IIM) has been described extensively and the similarities with anti-MDA5 disease and COVID-19 infection have further increased this suspicion [1, 2, 4, 12,13,14,15, 28,29,30,31,32,33,34,35]. Firstly, the pulmonary damage from MDA5-DM RP-ILD is difficult to distinguish from the interstitial pneumonia and subsequent acute respiratory distress syndrome (ARDS) seen in COVID-19 infection. Both MDA5-DM and COVID-19 seem to share several pathogenic mechanisms by activation of the IFN-I signaling pathway [1,2,3, 14, 35]. Notably, COVID-19 infection activates downstream interferon signaling for viral clearance by host defense, and this is initiated by cytoplasmic viral RNA sensors such as the RIG-I and MDA5 [1, 2, 36,37,38]. This sets the tone for the suggestion that the production of anti-MDA5 antibodies during virus infection, including COVID-19, is an epiphenomenon associated with the onset of CADM and RP-ILD; namely, infection of the skin and lung epithelium that may up-regulate the expression of MDA5 in the infected tissues [1,2,3, 17, 36, 39]. The possible mechanisms of autoimmunity may be molecular mimicry, or the virus itself may activate antigen-presenting cells directly, activation of T-cells, and consequent cytokine storm with resultant tissue damage [13, 27]. This can further be favored by specific genetic variants predisposing individuals to loss of tolerance of the MDA5 antigen resulting in the production of anti-MDA5 autoantibodies [3, 39, 40]. In a 2020 study done by Megremis et al. on dermatomyositis patients, there were six distinct epitopes with high sequence affinity to human SARS-CoV2 virus and of these, three linear epitopes of six amino acid lengths were highly specific for SARS-CoV-2 [28]. Around this time, a case series by Gokhale et al. noted the “sudden spur” in dermatomyositis cases coinciding with the peak of COVID-19 infections. Among the five cases they saw in a span of 4–5 months, four were positive for MSAs, and one of those patients was positive for MDA5 [15]. Similar to the cytokine storm seen in severe COVID-19 infections, the increased levels of Cutaneous T cell Attracting Chemokine (CTACK), IFN-gamma, IL-8, monocyte chemoattractant protein-3 (MCP-3), and stem cell growth factor-beta (SCGF-beta) were observed in MDA5-DM RP-ILD. Liu et al. performed a retrospective study involving 3 hospitals across China (in Wuhan, Harbin, and Beijing) from December 2019 to April 2020 where they showed a 48.2% positivity rate (132/274 patients) for anti-MDA5 antibody among COVID-19 patients. They also showed that higher titers of anti-MDA5 antibodies were more prevalent among the severe and non-survival cases [34].
An additional hypothesis of a viral trigger of MDA5-DM is suggested by epidemiological studies that highlighted a seasonal distribution of the disease with an epidemic period in fall and winter, a peak in late winter and spring, and a dip in summer [3]. The clustered occurrence of cases of anti-MDA5 positive IIM in Japan along the Kiso River are further pointers to an environmental trigger, potentially in the genetically predisposed [41].
Another important aspect is the occurrence of severe COVID-19 in apparently healthy young individuals who were later found to have subtle IFN pathway defects and polymorphisms including those affecting the MDA5 gene [42]. It is also shown that Pangolins, the natural reservoir of COVID-19 lack the MDA5 gene, possibly providing them an evolutionary advantage [43]. Autoimmunity and immunodeficiency occur along a continuum, and the frequent occurrence of viral infections in patients with anti-MDA5 positive myositis raises the case for deregulated viral clearance due to polymorphisms in viral sensors [27, 44]. Besides, occasional reports of psoriasiform rashes and cutaneous features in patients with anti-MDA5 positive IIM, when such rashes are otherwise described in those with MDA5 mutations suggest that our current understanding of this disease may be just the tip of the iceberg, with several undiagnosed forms currently misunderstood in the absence of a timely genetic and autoimmune screen [45].
The mRNA COVID-19 vaccine was shown to exclusively induce type I IFN effects. RNA sensors such as Toll-like receptor 7 (TLR7) and MDA5 are triggered by the mRNA vaccine [46]. The upregulation of these immunological pathways is widely considered to be the basis of several immune-mediated diseases, especially in genetically predisposed subjects who have impaired clearance of nucleic acids [47, 48]. Some data suggest that these individuals may acquire the infection asymptomatically and the presence of autoreactive cells and autoantibodies cross-reacting against SARS-CoV-2 epitopes may provide natural protection against the infection [48]. Although more evidence is needed, the administration of nucleic acid vaccines may put these individuals at risk of developing immunological side effects via sensitization of pattern recognition particles (PRRs) or generation of cross-reactive cell clones and antibodies [47]. Additionally, the COVID-19 mRNA vaccine, like the COVID-19 infection mentioned above, may generate an unbalance in the cytokine pathways via stimulation of myeloid or plasmacytoid dendritic cells (DCs) which are known to have a crucial role in autoimmunity and autoinflammation [32, 49]. To what extent the novel mRNA COVID-19 vaccine plays a role in autoimmunity and subsequent disease are unanswered questions that need further attention.
We have introduced six cases to the previously documented single case (Carrasco et al.) of MDA5-associated CADM that developed within days of receiving the COVID-19 vaccine [9]. Although no specific diagnostic criteria for the MDA5 subtype of DM exists, all our patients did meet the 2017 EULAR/ACR (European League Against Rheumatism/American College of Rheumatology) classification criteria for adult IIMs and their subgroups [50]. See Table 1. Although our focus here is on MDA5-associated disease with the COVID-19 vaccine (and infection), our literature review also included other forms of myositis from the COVID-19 vaccine, including four cases of non-autoimmune myositis [51,52,53], two cases of myositis with positive myositis-associated antibodies (MAAs) [54, 55], and two MSA cases (anti-Jo-1 and SAE1-associated dermatomyositis) [55, 56].
All our cases of MDA5-DM demonstrated rashes consistent with classic DM with varying degrees of muscle weakness and lung disease. No recommendations for the management of anti-MDA5-DM exist at this time, however, a combination of immunosuppressive therapy has been used exemplified by the variety seen in our cases. In addition to the case reported by Carrasco et al., 3 out of our 6 cases developed RP-ILD. Interestingly, one of our cases, Case 4, was positive for two different MSAs, TIF-1 gamma and MDA5, which is very rare [57]. Case 1 presented with intense, unilateral, limb swelling, which was concerning as some case reports have suggested that unilateral/bilateral limb edema can be associated with more aggressive types of IIM [58, 59]. This patient, like Case 5 and Case 6, was also positive with SSA-52 (Ro-52), a MAA that when co-present with MSAs can correlate with increased disease intensity [60].
In summary, we bring attention to the relationship seen between MDA5-DM and COVID-19 vaccination and infection. Due to the severity and rapid course of anti-MDA5-associated CADM, timely diagnosis and therapy initiation is crucial. As previously mentioned, while association cannot be causation, especially in the setting of a global vaccination drive during a worldwide pandemic, our findings highlight the continued need to understand viral and vaccine interaction with the host immune response and subsequent autoantibody development, particularly in patients presenting with symptoms of myositis. Our findings are not intended to dissuade clinicians against recommending COVID-19 vaccination for patients, especially those with known autoimmunity and/or immunocompromised, but rather to sensitize medical providers to this rare but potentially life-threatening disease that may arise in the post-vaccine period.
References
Rebendenne A et al (2021) SARS-CoV-2 triggers an MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells. J Virol. https://doi.org/10.1128/jvi.02415-20
Yin X et al (2021) MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep 34(2):108628. https://doi.org/10.1016/j.celrep.2020.108628
Nombel A, Fabien N, Coutant F (2021) Dermatomyositis with anti-MDA5 antibodies: bioclinical features, pathogenesis and emerging therapies (in eng). Front Immunol 12:773352. https://doi.org/10.3389/fimmu.2021.773352
Wang Y, Du G, Zhang G, Matucci-Cerinic M, Furst DE (2020) Similarities and differences between severe COVID-19 pneumonia and anti-MDA-5-positive dermatomyositis-associated rapidly progressive interstitial lung diseases: a challenge for the future, (in eng). Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2020-218594
Wu W et al (2021) Interstitial lung disease in anti-MDA5 positive dermatomyositis (in eng). Clin Rev Allergy Immunol 60(2):293–304. https://doi.org/10.1007/s12016-020-08822-5
Gupta R, Kumar S, Gow P, Hsien-Cheng Chang L, Yen L (2020) Anti-MDA5-associated dermatomyositis (in eng). Intern Med J 50(4):484–487. https://doi.org/10.1111/imj.14789
Li Y et al (2020) Predictors of poor outcome of Anti-MDA5-associated rapidly progressive interstitial lung disease in a Chinese cohort with dermatomyositis (in eng). J Immunol Res 2020:2024869. https://doi.org/10.1155/2020/2024869
Huang K et al (2019) Clinical spectrum and therapeutics in Canadian patients with anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis: a case-based review (in eng). Rheumatol Int 39(11):1971–1981. https://doi.org/10.1007/s00296-019-04398-2
Carrasco L, Arthur A, Gaitan Rueda J, Case C (2021) A rapidly progressive and rare illness: autoantibodies against melanoma differentiation-associated protein 5 (anti-MDA5): amyopathic dermatomyositis with progressive interstitial lung disease that developed after covid-19 vaccine. Chest J. https://doi.org/10.1016/j.chest.2021.07.646
Kotsiou OS, Daniil Z, Gourgoulianis KI (2017) Pneumomediastinum in MDA5-associated clinically amyopathic dermatomyositis. Rheumatol Adv Pract 1(1):rkx003. https://doi.org/10.1093/rap/rkx003
McNamara L, Brady VA (2021) Is COVID-19 the culprit? A case of anti-MDA5 antibody positivity in a rapidly progressive organizing pneumonia. In: TP31. TP031 INTERESTING CASES ASSOCIATED WITH SARS-COV-2 INFECTION, (American Thoracic Society International Conference Abstracts: American Thoracic Society, pp A2002-A2002. DOI: https://doi.org/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A2002
Saud A, Naveen R, Aggarwal R, Gupta L (2021) COVID-19 and myositis: what we know so far (in eng). Curr Rheumatol Rep 23(8):63. https://doi.org/10.1007/s11926-021-01023-9
Mehta P, Machado PM, Gupta L (2021) Understanding and managing anti-MDA 5 dermatomyositis, including potential COVID-19 mimicry (in eng). Rheumatol Int 41(6):1021–1036. https://doi.org/10.1007/s00296-021-04819-1
Giannini M, Ohana M, Nespola B, Zanframundo G, Geny B, Meyer A (2020) Similarities between COVID-19 and anti-MDA5 syndrome: what can we learn for better care? Eur Respir J. https://doi.org/10.1183/13993003.01618-2020
Gokhale Y et al (2020) Dermatomyositis during COVID-19 pandemic (a case series): is there a cause effect relationship? (in eng). J Assoc Phys India 68(11):20–24
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors (in eng). Rheumatol Int 31(11):1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Kuwana M (2009) RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease (in eng). Arthritis Rheum 60(7):2193–2200. https://doi.org/10.1002/art.24621
Sakamoto N et al (2019) Clinical features of Anti-MDA5 antibody-positive rapidly progressive interstitial lung disease without signs of dermatomyositis (in eng). Intern Med 58(6):837–841. https://doi.org/10.2169/internalmedicine.1516-18
Tsuji H et al (2020) Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis (in eng). Arthritis Rheumatol 72(3):488–498. https://doi.org/10.1002/art.41105
So H, Wong VTL, Lao VWN, Pang HT, Yip RML (2018) Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis (in eng). Clin Rheumatol 37(7):1983–1989. https://doi.org/10.1007/s10067-018-4122-2
Allenbach Y et al (2020) Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases (in eng). Neurology 95(1):e70–e78. https://doi.org/10.1212/WNL.0000000000009727
Borio G et al (2021) Skin rash and interstitial pneumonia can be a fatal combination: a rare case of anti-melanoma differentiation-associated gene 5 (MDA5)-associated interstitial lung disease (in eng). Eur J Case Rep Intern Med 8(10):002860
Dunga SK, Kavadichanda C, Gupta L, Naveen R, Agarwal V, Negi VS (2021) Disease characteristics and clinical outcomes of adults and children with anti-MDA-5 antibody-associated myositis: a prospective observational bicentric study (in eng). Rheumatol Int. https://doi.org/10.1007/s00296-021-04897-1
Gupta L, Naveen R, Gaur P, Agarwal V, Aggarwal R (2021) Myositis-specific and myositis-associated autoantibodies in a large Indian cohort of inflammatory myositis (in eng). Semin Arthritis Rheum 51(1):113–120. https://doi.org/10.1016/j.semarthrit.2020.10.014
Chen L, Owens K, Murina A (2020) Anti-MDA5 antibody-positive dermatomyositis presenting as unilateral eyelid edema. JAAD Case Rep 6(9):909–911. https://doi.org/10.1016/j.jdcr.2020.07.019
Aoyama J et al (2019) Anti-MDA5 antibody-positive rapidly progressive interstitial pneumonia without cutaneous manifestations. Respir Med Case Rep 26:193–196. https://doi.org/10.1016/j.rmcr.2019.01.012
Huang L et al (2021) Association of cytomegalovirus infection with anti-MDA5 Antibody-positive dermatomyositis: a prospective cohort study (in eng). Front Med (Lausanne) 8:740154. https://doi.org/10.3389/fmed.2021.740154
Megremis S et al (2020) Antibodies against immunogenic epitopes with high sequence identity to SARS-CoV-2 in patients with autoimmune dermatomyositis. Ann Rheum Dis 77(10):1383–1386. https://doi.org/10.1136/annrheumdis-2020-217522
Aschman T et al (2021) Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died (in eng). JAMA Neurol 78(8):948–960. https://doi.org/10.1001/jamaneurol.2021.2004
Cao M et al (2020) COVID-19 or clinical amyopathic dermatomyositis associated rapidly progressive interstitial lung disease? A case report (in eng). BMC Pulm Med 20(1):304. https://doi.org/10.1186/s12890-020-01335-z
Wang G et al (2021) Presence of Anti-MDA5 antibody and its value for the clinical assessment in patients with COVID-19: a retrospective cohort study (in eng). Front Immunol 12:791348. https://doi.org/10.3389/fimmu.2021.791348
Ehrenfeld M et al (2020) Covid-19 and autoimmunity (in eng). Autoimmun Rev 19(8):102597. https://doi.org/10.1016/j.autrev.2020.102597
Rodríguez Y et al (2020) Autoinflammatory and autoimmune conditions at the crossroad of COVID-19 (in eng). J Autoimmun 114:102506. https://doi.org/10.1016/j.jaut.2020.102506
Liu C et al (2020) Analysis of the correlation between anti-MDA5 antibody and the severity of COVID-19: a retrospective study. medRxiv. https://doi.org/10.1101/2020.07.29.20164780
Qian J, Xu H (2021) COVID-19 disease and dermatomyositis: a mini-review (in eng). Front Immunol 12:747116. https://doi.org/10.3389/fimmu.2021.747116
Thorne LG et al (2021) SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. Embo J 40(15):e107826. https://doi.org/10.1038/s41590-021-01091-0
Sampaio NG et al (2021) The RNA sensor MDA5 detects SARS-CoV-2 infection. Sci Rep 11(1):13638. https://doi.org/10.1038/s41598-021-92940-3
Dias Junior AG, Sampaio NG, Rehwinkel J (2019) A balancing act: MDA5 in antiviral immunity and autoinflammation. Trends Microbiol 27(1):75–85. https://doi.org/10.1016/j.tim.2018.08.007
Sadler AJ (2018) The role of MDA5 in the development of autoimmune disease. J Leukoc Biol 103(2):185–192. https://doi.org/10.1189/jlb.4MR0617-223R
Caso F et al (2020) Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? (in eng). Autoimmun Rev 19(5):102524. https://doi.org/10.1016/j.autrev.2020.102524
Muro Y, Sugiura K, Hoshino K, Akiyama M, Tamakoshi K (2011) Epidemiologic study of clinically amyopathic dermatomyositis and anti-melanoma differentiation-associated gene 5 antibodies in central Japan (in eng). Arthritis Res Ther 13(6):R214. https://doi.org/10.1186/ar3547
Liu G et al (2021) ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity (in eng). Nat Microbiol 6(4):467–478. https://doi.org/10.1038/s41564-021-00884-1
Fischer H, Tschachler E, Eckhart L (2020) Pangolins lack IFIH1/MDA5, a cytoplasmic RNA sensor that initiates innate immune defense upon Coronavirus infection (in eng). Front Immunol 11:939. https://doi.org/10.3389/fimmu.2020.00939
Gupta P, Kharbanda R, Lawrence A, Gupta L (2021) Systemic flare and cutaneous ulceration following cytomegalovirus infection in a patient with anti-melanoma differentiation-associated protein 5 (MDA5) associated myositis: diagnostic challenge during the time of coronavirus disease (COVID-19) pandemic. The Egyptian Rheumatologist 43(4):271–274. https://doi.org/10.1016/j.ejr.2021.06.001
Rathore U, Haldule S, Gupta L (2021) Psoriasiform rashes as the first manifestation of anti-MDA5 associated myositis (in eng). Rheumatology (Oxford) 60(7):3483. https://doi.org/10.1093/rheumatology/keaa821
Teijaro JR, Farber DL (2021) COVID-19 vaccines: modes of immune activation and future challenges (in eng). Nat Rev Immunol 21(4):195–197. https://doi.org/10.1038/s41577-021-00526-x
Talotta R (2021) Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases” (in eng). Clin Immunol 224:108665. https://doi.org/10.1016/j.clim.2021.108665
Vojdani A, Kharrazian D (2020) Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases (in eng). Clin Immunol 217:108480. https://doi.org/10.1016/j.clim.2020.108480
Goriely S, Goldman M (2007) From tolerance to autoimmunity: is there a risk in early life vaccination? (in eng). J Comp Pathol 137(Suppl 1):S57-61. https://doi.org/10.1016/j.jcpa.2007.04.013
Lundberg IE et al (2017) 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups (in eng). Ann Rheum Dis 76(12):1955–1964. https://doi.org/10.1136/annrheumdis-2017-211468
Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N (2021) COVID-19 vaccine-related myositis (in eng). QJM 114(6):424–425. https://doi.org/10.1093/qjmed/hcab043
Ramalingam S et al (2021) COVID-19 vaccine-induced cellulitis and myositis (in eng). Cleve Clin J Med 88(12):648–650. https://doi.org/10.3949/ccjm.88a.21038
Maramattom BV, Philips G, Thomas J, Santhamma SGN (2021) Inflammatory myositis after ChAdOx1 vaccination (in eng). Lancet Rheumatol 3(11):e747–e749. https://doi.org/10.1016/S2665-9913(21)00312-X
Capassoni M et al (2021) AstraZeneca (AZD1222) COVID-19 vaccine-associated adverse drug event: a case report. J Med Virol 93(10):5718–5720. https://doi.org/10.1002/jmv.27175
Kaulen LD et al (2021) Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series (in eng). Eur J Neurol. https://doi.org/10.1111/ene.15147
Gupta K, Sharma GS, Kumar A (2021) COVID-19 vaccination-associated anti-Jo-1 syndrome (in eng). Reumatologia 59(6):420–422. https://doi.org/10.5114/reum.2021.111836
McHugh NJ, Tansley SL (2018) Autoantibodies in myositis (in eng). Nat Rev Rheumatol 14(5):290–302. https://doi.org/10.1038/nrrheum.2018.56
Duchesne M et al (2020) Edematous myositis: a clinical presentation first suggesting dermatomyositis diagnosis (in eng). Brain Pathol 30(5):867–876. https://doi.org/10.1111/bpa.12844
Milisenda JC, Doti PI, Prieto-González S, Grau JM (2014) Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature (in eng). Semin Arthritis Rheum 44(2):228–233. https://doi.org/10.1016/j.semarthrit.2014.04.004
Zheng YM et al (2020) Correlation study on anti-Ro52 antibodies frequently co-occur with other myositis-specific and myositis-associated autoantibodies (in chi). Beijing Da Xue Xue Bao Yi Xue Ban 52(6):1088–1092. https://doi.org/10.19723/j.issn.1671-167X.2020.06.016
Author information
Authors and Affiliations
Contributions
DG carried out the literature review, study design, and wrote the initial manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All co-authors are familiar with the revised, final version of the work and take full responsibility for the integrity and accuracy of all aspects of the work. No part of the manuscript, including photographs, has been copied or published elsewhere.
Informed consent
All patients gave their written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonzalez, D., Gupta, L., Murthy, V. et al. Anti-MDA5 dermatomyositis after COVID-19 vaccination: a case-based review. Rheumatol Int 42, 1629–1641 (2022). https://doi.org/10.1007/s00296-022-05149-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05149-6