Abstract
This research investigates the structural and electronic properties of titanium dioxide (\( TiO_2 \)) surfaces with the addition of cerium (Ce) impurities using first principles calculations within the framework of density functional theory, employing the pseudopotential method and the computational package Quantum ESPRESSO. Density of states (DOS) calculations reveal that the clean surface exhibits semiconductor behavior. In contrast, the titanium dioxide surface with Ce impurities on the fifth layer generates intermediate states within the energy band gap, resulting in a reduction in the band, with an intraband energy gap of 0.7882 eV induced. Moreover, the inclusion of a Ce atom on the fourth layer of the surface results it is observed that the surface an important characteristic is the appearance of intermediate states in the band gap, which are close to the valence band with a magnetic moment of 0.91 \( \mu \beta /cell \). The ntraband gap can enhance the utilization of the visible spectrum. Additionally, the Ce impurity on the \( TiO_2 \) surface could promote greater photocatalytic activity in pollutant degradation and increase the oxidative capacity of titanium dioxide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Global warming is a worldwide issue that has been affecting humanity, it is well known that one of the main causes of this phenomenon is the greenhouse effect [1], which in turn is known to be caused by gases such as \( CO_2 \). Therefore, any effort that contributes to reducing \( CO_2 \) emissions into the atmosphere is welcome.
Hence, researchers constantly consider viable solutions to this problem both theoretically and experimentally, making significant progress in their investigations and thus contributing to the development of materials science. Theoretical studies emphasize the use of computational codes to model many-body problems, which has contributed to the science of new materials and their potential applications in the field of emerging technologies.
The increase in environmental pollution and the accelerated consumption of fossil fuels have had a significant impact on nature, prompting the scientific community to develop chemical alternatives for air purification, water disinfection, and the reduction of hazardous waste. This is where photocatalysis emerges as a green method, meaning environmentally friendly, addressing the aforementioned issues [2].
In the quest to create new materials that replace old compounds for the benefit of humanity and the environment, condensed matter physicists are engaged in constant research to contribute to this purpose. Therefore, it becomes necessary to opt for theoretical works that allow them to determine the physical properties and establish prospective applications.
In this regard, the scientific community has developed methods and strategies such as the capture of \( CO_2 \) by semiconductor surfaces to achieve this purpose [3]. In particular, titanium dioxide (\( TiO_2 \)) has shown to be a very promising material for use in photocatalysis. Additionally, it is non-toxic, abundant, and cost-effective. However, titanium dioxide in its anatase phase has a relatively large energy bandgap (\( \sim 3.2\) eV), causing it to absorb only a limited portion of the available visible light, mainly in the ultraviolet region [4]. Another issue encountered with \( TiO_2 \) is that the recombination times between photogenerated electron-hole pairs are very short (\( \sim 30\) ns), leading to the recombination of the photo-excited electron before it can interact with \( CO_2 \) or any other substance [5].
Currently, Density Functional Theory (DFT) [6] has been used to calculate the electronic structure of atoms, molecules, solids, surfaces, and their interactions due to its efficiency and satisfactory results.
The main objective of this article is to study the effect of incorporating cerium impurities on the structural and electronic properties of titanium dioxide surfaces through computational modeling, aiming to identify possible photocatalytic enhancements of this material, Since cerium is a donor atom, intermediate states are expected to appear in the energy bandgap. These states could improve the photocatalytic activity of \( TiO_2 \) in two ways, the first by reducing the bandgap so that \( TiO_2 \) could take advantage of the greatest amount of energy visible light and the second with empty states in the bandgap that can act as traps that capture the photogenerated electrons, thus increasing the recombination times.
2 Computational method
All computational calculations were performed within the framework of density functional theory [6], along with the pseudopotential method [7], while the correlation and exchange effects among the electrons were included using the generalized gradient approximation by Perdew, Burke, and Ernzerhof (\( GGA-PBE \)) [8], as implemented in the Quantum Espresso computational package [9]. The Hubbard correction was taken into account with a value of \( U=4.2 \) eV [10] for the titanium atoms.
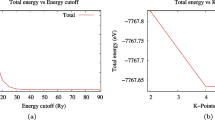
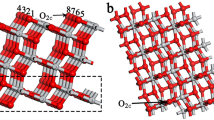
For the plane wave expansion of the wave function, a cutoff energy of 60 Ry was used, which was obtained after optimizing the total energy differences with respect to the cutoff energies. The integration over the first Brillouin zone was performed using a \( k-point \) mesh of \(4\times 4\times 2\) obtained with the Monkhorst-Pack method [11]. The titanium dioxide 001 surface was modeled using the periodic terrace method. To avoid interaction between adjacent surfaces, a vacuum region of 10 \({\text{\AA }}\) was included, and a \(2\times 2\) and 5 atomic layers geometry was employed, which consists of a total of 60 atoms (40 oxygen and 20 titanium). As shown in Fig. 1a, while Fig. 1b and c depict the surface with a Ce impurity in the fifth and fourth atomic layers, respectively.
All structural relaxation processes were terminated when convergence criteria for energy (\(10^{-4}\) eV) and forces (\(10^{-4}\) \({eV/\text{\AA }}\)) were achieved. Finally, as we are interested in looking for the ground state of the systems under study, we set the following parameters for temperature and pressure (\(T=0\) K, \(P=0\) Atm), respectively. And relativistic effects were not taken into account because the time-independent Schrodinger equation and the Born-Oppenheimer approximation were used.
3 Results and discussion
This section presents the results of computational calculations for the main parameters and electronic properties. The key parameters characterizing the structural relaxation of both the clean and \( Ce- \)doped surfaces were obtained through a vc-relax calculation, where atoms were allowed to move in all three Cartesian directions. Additionally, the electronic properties were analyzed by calculating the density of states and band structure.
3.1 Structural properties
The results of the main structural parameters, such as the lattice constant \(a=7.635\) \({\text{\AA }}\), the angle \( \alpha \) between \( Ti-O-Ti \), the \( Ti-O \) and \( Ti-Ti \) bond lengths (as shown in Fig. 1a), and the surface energy. For the clean surface, are listed alongside values reported by other authors in Table 1. The calculated values in this study are in excellent agreement with those reported by other authors and obtained using different approximations. This ensures the reliability of the computational calculations performed in this work.
Table 2 displays the key structural parameters for the \( Ce- \)doped \( TiO_2 \) surface, including the lattice constant a, the angles between \( Ti-O-Ce \), \( Ce-Ti-O \), and \( O-Ce-O \),5 the bond lengths \( O_y-Ce \), \( Ce-O_x \), and \( Ce-O_y \). The \( Ti-O-Ce \), \( Ce-Ti-O \), and \( O-Ce-Ti \) angles, as depicted in Fig. 1, are also presented. The last column in Tables 2 and 3 presents the Ce impurity concentration employed in this study, as well as the concentrations reported by other authors. The bond lengths \( O_y-Ce \), \( Ce-O_x \), and \( Ce-O_y \) agree well with values reported by other authors. Conversely, the values for the \( Ti-O-Ce \), \( Ce-Ti-O \), and \( O-Ce-Ti \) angles have not been previously reported. Finally, Table 3 reveals novel values for the structural parameters of the \( TiO_2 \) surface with Ce impurities in the fourth layer.
Acronyms
The following acronyms were used in this work: sp (spin-polarized), PP (pseudo potentials), GGA (generalized gradient approximation), PBE (Perdew-Burke-Ernzerhof), U (Hubbard term, all values in eV), PW (hybrid DFT method), PWA (projected wave augmented), J (effective exchange parameter for magnetic interactions, all values in eV), LCAO (linear combinations of atomic orbitals), PBE0 (hybrid functional), PXRD (X-ray diffraction).
3.2 Electronic properties
The electronic properties of the clean \( TiO_2 \) surface and \( TiO_2 \) surface with Ce impurities were analyzed by calculating the density of states (DOS). In all cases, the equilibrium structural parameters obtained after the optimization process were used to calculate the DOS. The Fermi level was chosen as the zero energy reference.
Figure 2a and b show the density of states of the clean \( TiO_2 \) surface and \( TiO_2 \) surface with Ce impurities, respectively.
As depicted in Fig. 2a, the clean \(TiO_2\) surface exhibits a semiconductor behavior with an indirect energy gap of 1.8686 eV. Additionally, it is observed that the \( O-2p \) orbital contributes predominantly to the valence band behavior, while the \( Ti-3d \) orbitals contribute significantly to the conduction band behavior. Figure 2b illustrates the density of states (DOS) on the surface of \( Ce- \)doped \(TiO_2\). It can be seen that the inclusion of Ce impurity in the \(TiO_2\) surface matrix alters its semiconductor behavior, Ce impurities on the fifth layer generates intermediate states within the energy band gap, resulting in a reduction in the band, with an intraband energy gap of 0.7882 eV induced. Transforming it into a metallic character. An intraband gap of 0.7882 eV is observed, which is smaller than the value of the gap in the clean \(TiO_2\) surface. Furthermore, it is noted that both the clean \(TiO_2\) surface and the \( Ce- \)doped surface exhibit non-magnetic properties, this is due to the pairing of the electrons which leads to the state density having symmetry between the states with spins up and down.
Figure 3 displays the total density of states and the orbitals that contribute the most on the surface of \( Ce-\)doped \( TiO_2 \) in the fourth layer. It is observed that the surface an important characteristic is the appearance of intermediate states in the band gap, which are close to the valence band. These intermediate states are empty or unoccupied and are primarily generated by an oxygen atom in the S-up, resulting in a small magnetization of \( - \)0.91 \( \mu \beta /cell \), This magnetic moment arises from the hybridization between \( O-2p \) states, primarily, and \( Ti-3d \) and \( Ce-2p \) states, to a lesser extent. and a polarization of -0.3314. This was confirmed by measuring the charge of oxygen on the clean surface (6.7326 |e| ) and its charge in the fourth layer (6.6302 |e| ), which gives a difference of 0.1024 |e| . This suggests that the oxygen atom is gaining charge. In Fig. 3, it can also be observed that introducing the cerium impurity breaks the bond with the oxygen atom located above it at a distance of 2.475 \({\text{\AA }}\) with an angle of 171.851\( ^\circ \) formed by \( Ti-O-Ti \), as shown in Table 3. It is worth noting that when the surface is free of impurities, the bond length between oxygen and titanium is 2.002 \({\text{\AA }}\) with an angle of 156.472\( ^\circ \). In addition, \( O-2p \) orbitals contribute significantly to the valence band. On the other hand, \( Ti-3d \) orbitals are the main contributors to the conduction band. In this case, the intraband space has a value of 1.7673 eV, which is quite close to the energy space of the clean surface of \( TiO_2 \).
4 Conclusion
In conclusion, employing first-principles calculations within the framework of density functional theory (DFT), we determined that the clean surface of \(TiO_2\) exhibits semiconductor behaviour with an indirect band gap of 1.8686 eV. When a Ce atom is introduced into the the fifth layer of the \( TiO_2 \) surface, equivalent to a concentration of 5%, shows that cerium creates intermediate states within the band gap, resulting in a reduction in the band width. From the latter, it could be inferred that the \( TiO_2-Ce \) system is capable of absorbing visible light, which can be interpreted as a possible enhancement of the photocatalytic activity of anatase. Furthermore, the generated intermediate states are hole states located near the valence band, originating from p orbitals of an oxygen atom located on the top of the surface above the Ce atom, with an intra-band gap of 0.7882 eV. This characteristic can be exploited in photocatalytic applications for the degradation of contaminants. Here, the system does not exhibit magnetic behaviour. In contrary, the introduction of a Ce atom in the fourth layer of \( TiO_2 \) results in an intra-band gap of 1.7673 eV. In this case, the system exhibits magnetic behaviour with a magnetic moment of 0.91 \( \mu \beta /cell \).
References
D.A. Gómez Díaz, El cambio climático y la respuesta de las grandes potencias. El caso de Estados Unidos y China. Análisis Pol’itico 33(99), 121–142 (2020)
N.y.S.E. Robert, Didier y Keller, Fotocatálisis y fotoquímica ambiental para un mundo sostenible: un gran desafío
R. Kun, S. Tarján, A. Oszkó, T. Seemann, V. Zollmer, M. Busse, I. Dékány, Preparation and characterization of mesoporous N-doped and sulfuric acid treated anatase TiO2 catalysts and their photocatalytic activity under UV and Vis illumination. J. Solid State Chem. 182(11), 3076–3084 (2009)
C.-G. Wu, L.-F. Tzeng, Y.-T. Kuo, C.H. Shu, Enhancement of the photocatalytic activity of TiO2 film via surface modification of the substrate. Appl Catal A Gen 226(1–2), 199–211 (2002)
F.M. Hossain, G. Murch, L. Sheppard, J. Nowotny, Ab initio electronic structure calculation of oxygen vacancies in rutile titanium dioxide. Solid State Ionics 178(5–6), 319–325 (2007)
P. Hohenberg, W. Kohn, Inhomogeneous electron gas. Phys. Rev. 136(3B), 864 (1964)
J. Kohanoff, N. Gidopoulos, Density functional theory: basics, new trends and applications. Handb. Mol. Phys. Quantum. Chem. 2(Part 5), 532–568 (2003)
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865 (1996)
S. Scandolo, P. Giannozzi, C. Cavazzoni, S. Gironcoli, A. Pasquarello, S. Baroni, First-principles codes for computational crystallography in the quantum-espresso package. Z. Kristallographie-Crystalline Mater. 220(5–6), 574–579 (2009)
B.J. Morgan, G.W. Watson, A DFT+ U description of oxygen vacancies at the TiO2 rutile (1 1 0) surface. Surf. Sci. 601(21), 5034–5041 (2007)
H.J. Monkhorst, J.D. Pack, Special points for Brillouin-zone integrations. Phys. Rev. B 13(21), 5188–5192 (1976)
E. Araujo-Lopez, L.A. Varilla, N. Seriani, J.A. Montoya, \( TiO_2 \) anatase’s bulk and (001) surface, structural and electronic properties: a DFT study on the importance of Hubbard and van der Waals contributions. Surf. Sci. 653(21), 187–196 (2016)
T.R. Esch, I. Gadaczek, T. Bredow, Surface structures and thermodynamics of low-index of rutile, brookite and anatase-a comparative DFT study. Appl. Surf. Sci. 288(21), 275–287 (2014)
Y. Ortega, D.F. Hevia, J. Oviedo, M. San-Miguel, A DFT study of the stoichiometric and reduced anatase (0 0 1) surfaces. Appl. Surf. Sci. 294, 42–48 (2014)
A. Roldan, M. Boronat, A. Corma, F. Illas, Theoretical confirmation of the enhanced facility to increase oxygen vacancy concentration in TiO2 by iron doping. J. Phys. Chem. C 114(14), 6511–6517 (2010)
A. Vittadini, M. Casarin, Ab initio modeling of TiO2 nanosheets. Theor. Chem. Accounts. 120, 551–556 (2008)
T. Shittu, M. Altarawneh, Investigative properties of CeO2 doped with niobium: a combined characterization and DFT studies. Nanotechnol. Rev. 11(1), 191–203 (2021)
A.R. Albuquerque, A. Bruix, J.R. Sambrano, F. Illas, Theoretical study of the stoichiometric and reduced Ce-doped TiO2 anatase (001) surfaces. J. Phys. Chem. C 119(9), 4805–4816 (2015)
Acknowledgements
The authors would like to express their gratitude to the Vice-Rectory for Research and Extension of the University of Córdoba for providing financial support (project FCB-08-19).
Author information
Authors and Affiliations
Contributions
This manuscript is original and has been approved by all the authors.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiménez, G.C., Alcalá Varilla, L.A. & Torres Hoyos, F. Potential enhancements in the photocatalytic activity of the anatase 001 surface by adding cerium impurity. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00717-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00717-x