Abstract
The adsorption of carbon dioxide on the surfaces of zinc oxide doped with cerium was studied. For this, a theoretical study was carried out using computational simulations based on density functional theory to determine possible improvements in photocatalytic activity. for the capture and dissociation of carbon dioxide. Calculations were performed using density functional theory within the Perdew-Burke-Ernzerhof generalized gradient approximation, also the Hubbard correction together with ultrasmooth atomic pseudopotentials and a basic plane wave implemented in the Quantum-ESPRESSO package. The doping concentration level considered in this work was 6.25%, among the results, the semiconducting character of zinc oxide was evident from the density of states calculations, it was also found that by adding cerium impurities to zinc oxide, the values of the lengths and angles of the bonds vary a little, this may be due to the small difference in covalent radius that the zinc atom has with respect to the cerium atom, consequently, changes occurred in the electronic properties that consist in intermediate states in the energy bandgap located around the Fermi energy. This may suggest that the cerium-doped zinc oxide system can probably absorb visible light, which could lead to possible improvements in the photocatalytic properties of the material. Furthermore, we found that by adding carbon dioxide to the cerium-doped zinc oxide surface, the carbon dioxide is activated and this could suggest the dissociation or reduction of this contaminant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Environmental contamination is one of the biggest problems in the world, as it represents a constant threat to human health and ecological balance. Therefore, green technologies are a source of sustainable resources, in the photocatalysis process is one of the most used and promising, serving mainly to capture, convert, and store pollutants [1].
In particular, zinc oxide is a semiconductor material that has many applications in medicine, the ceramics industry, the manufacture of ultraviolet radiation shields, coatings, electronics, and spintronics [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Therefore, it is acquiring great interest due to its photocatalytic activity for the treatment of environmental pollution due to its oxidation and degradation capacity, an interest that has been increasing considerably in recent years. This semiconductor is one of the best photocatalysts due to its photochemical stability, low dielectric constant, high chemical stability, high optical activity, and above all its photocatalytic activity at room temperature and low cost [17]. However, zinc oxide has a relatively large band gap (\(\sim \)3.3 eV [18]), which does not allow it to absorb visible light and for this reason, the photocatalytic properties of zinc oxide could be limited.
Three crystalline structures can be found for zinc oxide, which are hexagonal wurtzite type, cubic zinc blende, and rock salt (or Rochelle salt). Under ambient conditions, the thermodynamically stable phase is that of wurtzite symmetry [19]. Each zinc atom is surrounded by 4 oxygen atoms at the corners of a tetrahedron (typical coordination of bonds with \(sp^{3}\) hybridization) and vice versa to form the wurtzite-type structure belonging to the P63mc space group which is made up of alternating layers of atoms along the Z axis [20].
Zinc oxide shows four preferential growth surfaces: (101\(\overline{0}\)), (112\(\overline{0}\)), (0001), and (000\(\overline{1}\)), the latter two being categorized as polar surfaces and playing an important role in the applications of this material. These two structures grow along the C axis and are found in the literature as Zn-polar (0001) and O-polar (000\(\overline{1}\)) [21]. Furthermore, quantized zinc oxide, with several alternating Zn-polar (0001) and O-polar (000\(\overline{1}\)) facets, is a typical polar crystal, and many extensive studies have indicated that zinc oxide with a large proportion of 0001 planes exhibits enhanced photocatalytic, such as degradation of organic contaminants [22,23,24].
Studies such as that of Yufei et al. [25] show that the energy of the polar Zn surface is higher than that of its counterpart, which means that it is more unstable and active. However, the (0001) surface has been shown to have the highest photocatalytic activity of all four surfaces.
McLaren et al. [24] discovered that OH ions have preferential adsorption on the (0001) surface due to the positive charge, which reacts with the holes of the semiconductor and generates OH radicals, promoting the photocatalytic reaction. E. Cerrato et al. [26] studied the properties of zinc oxide by doping it with different rare earth ions (lanthanum, erbium, praseodymium, ytterbium, and cerium), where they observed that the cations of lanthanum, erbium, praseodymium, and ytterbium could be within the crystalline structure of zinc oxide, while cerium creates a new phase of dioxide cerium. Furthermore, this type of action generated an influence on the optical absorption of pure zinc oxide, since by incorporating lanthanide ions in its structure there is a small absorption of visible light.
On the other hand, it is known that the properties of materials can be improved by doping or adding impurities [27], therefore, it is valid to ask if it is possible to improve the photocatalysis properties of zinc oxide surfaces by doping or addition of cerium impurities. To answer this question, we carried out a theoretical study of the structural and electronic properties of the pure zinc oxide 0001 surface and the cerium-doped system using density functional theory to determine possible improvements in the photocatalytic activity that cerium produces to zinc oxide.
In this study, we found that by adding cerium impurities to zinc oxide, the structural parameters slightly changed, changes were also presented in the electronic properties, which consist of intermediate states in the energy bandgap, located around the Fermi energy. The above may suggest that the zinc oxide system doped with cerium under a concentration of 6.25% can probably absorb visible light, which could lead to possible improvements in the photocatalytic properties of the material. It was also found that when carbon dioxide is absorbed on the surfaces of zinc oxide and cerium-doped zinc oxide, the bond lengths of this molecule increase and its bond angle decreases, which may signify a start in the process of reduction of this polluting gas.
In the next section, the computational methods are described, then the results are presented and discussed. Finally, a summary closes the article.
2 Methodology
Density functional theory was used to study the structural and electronic properties of the pure zinc oxide 0001 surface and the doped system. The Quantum-Espresso computational package implementing the Perdew-Burke-Ernzerhof Generalized Gradient Approximation was used to optimize the input parameters for the construction of the pure zinc oxide 0001 surface and the cerium-doped zinc oxide system.
To create our surface, the atomic positions and network parameters were taken, where the convergence of the total energy for the Bulk of zinc oxide, which was achieved using a cutoff energy of 85 and 850 Ry for the wave functions and a k-point mesh (6 \(\times \) 6 \(\times \) 3) as seen in Fig. 1.
After having the optimal parameters, we created the structure of the bulk of zinc oxide in the Wurtzite phase. Where the (0001) surface of zinc oxide has been modeled with a vacuum study as a function of the total energy as shown in Fig. 2.
Where the vacuum was increased by one angstrom by one angstrom until a vacuum of 10 \({\text{\AA }}\) was determined, thus guaranteeing that there is no atomic interaction between periodic images.
Subsequently, a study of the surface energy is carried out as a function of the number of layers as shown in Fig. 3, where 4 layers were taken, because the variation is no greater than 200 \(eV/A^2\), which is little compared to the average value, then it can be taken that from the fourth layer a trend towards the stability value is seen.
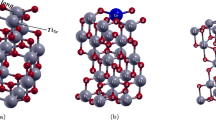
After optimizing the vacuum and the number of layers, we proceeded to create the four-layer \(2\times 2\) supercell, where we fixed the bottom layer, and a relaxation calculation was made. This \(2\times 2\) supercell has a thickness of 8.306 \({\text{\AA }}\) consisting of 32 atoms, where 16 atoms are zinc (gray color) and 16 oxygen atoms (red color) as shown in Fig. 4.
3 Results and discussion
This section focuses on the detailed characterization of the structural and electronic properties, as well as the adsorption phenomenon in the system under study. Through techniques such as Density Functional Theory within the Perdew-Burke-Ernzerhofl generalized gradient approximation implemented in the Quantum-ESPRESSO package, bond lengths and angles are measured; thus evidencing the structural response to the presence of cerium impurities. Simultaneously, electronic parameters, such as density of states, are explored. Additionally, adsorption processes are analyzed, providing crucial information on how impurities and structure affect the interaction of the material with the adsorbate.
3.1 Structural properties
The structural properties of the surface (0001) of ZnO are studied, as shown in Fig. 5a, which is a \(4-\)layer \(2\times 2\) supercell with a thickness of 8.306 \({\text{\AA }}\) consisting of 32 atoms, where 16 atoms are zinc. (Gray color) and 16 oxygen atoms (red color). oxygens and zinc are presented three times coordinated with bond lengths \(Zn-O\), \(O-Zn\), and \(Zn-Zn\); also, an angle \(\alpha \) formed by \(Zn-O-Zn\).
Next, the structural properties of the ZnCeO surface doped in the top layer are studied as shown in Fig. 6a, which is a (\(2\times 2\)) \(4-\)layer supercell with a thickness of 8.948 \({\text{\AA }}\), consisting of 32 atoms, where 15 atoms are zinc (gray color), 16 oxygen atoms (red color), and one cerium atom (blue color), three times coordinated oxygens with bond lengths \(Zn-O\), \(O-Zn\), \(Zn-Zn\), \(O-Ce\), and \(Zn-Ce\) where the zincs are located at the same distance; there is also an angle \(\alpha \) formed by \(Zn-O-Zn\) and an angle \(\beta \) formed by \(Zn-O-Ce\).
Finally, the structural properties of the ZnCeO surface doped in the middle layer are studied as shown in Fig. 7a, which is a \(4-\)layer \(2\times 2\) supercell with a thickness of 8.908 \({\text{\AA }}\), consisting of 32 atoms, where 15 atoms are zinc (grey color), 16 oxygen atoms (red color), and one cerium atom (blue color), there are three times coordinated oxygens with \(O-Zn\) bond lengths of 1.9604 \({\text{\AA }}\) in the doping, \(O-Zn\) of 3.3762 \({\text{\AA }}\) in the Z direction, \(Zn-O\), \(Zn-Zn\), \(O-Ce\), and \(Zn-Ce\); there is also an angle \(\alpha \) formed by \(Zn-O-Zn\).
It can be seen in Table 1 the bond lengths and angles between the atoms reported.
We decided to make a comparison of the data obtained for the Hubbard value \(U=0\,eV\) and \(U=7\,eV\) since in the literature we do not find work on zinc oxide surfaces doped with cerium and by making this comparison we can see that in Table 1, The values of the bond lengths and angles vary a little, this may be due to the small difference in covalent radius that the zinc atom has with respect to the cerium atom, the covalent radius of the zinc atom is 1.417 \({\text{\AA }}\) and the of cerium is 1.627 \({\text{\AA }}\), these covalent radii are those reported by the program used Quantum ESPRESSO.
3.2 Electronic properties
Next, we present the electronic properties for the systems studied with a Hubbard value \(U=0 \, eV\) and \(U=7 \, eV\).
3.2.1 Electronic properties for Hubbard (U=0 eV)
The electronic properties of the surface (0001) of ZnO are studied. Figure 8 shows the density of states (DOS) and PDOS contributions, where the fermi level is located at the origin of the plot.
From Fig. 8, we can see that there is an overlap between the bands, which indicates that it is a conductor, where the orbitals that contribute the most are the p orbitals of the oxygen atom and the d orbitals of the zinc atom, it also presents a small 0.21 Bohr mag/cell magnetization.
Next, the electronic properties of the ZnCeO surface doped in the top layer are studied. Figure 9 shows the density of states (DOS) and PDOS contributions, where the fermi level is located at the origin of the plot.
From Fig. 9, we can see that there is an overlap between the bands, which indicates that it is a conductor, where the orbitals that contribute the most are the 2p orbitals of the oxygen atom and the 5d orbitals of the cerium atom, where it presents symmetry for the spin up and spin down which indicates that it does not have magnetic properties.
Finally, the electronic properties of the ZnCeO surface doped in the penultimate layer are studied. Figure 10 shows the density of states (DOS) and PDOS contributions, where the fermi level is located at the origin of the plot.
In the same way, we can see that in Fig. 10 there is an overlap between the bands, which indicates that it is a conductor, where the orbitals that contribute the most are the d orbitals of the cerium atom and the p orbitals of the oxygen atom, where it presents symmetry for spin up and spin down which indicates that it does not have magnetic properties.
Because in the literature we only found studies of monolayers of zinc oxide doped with cerium, we decided to make a comparison between the electronic properties of the zinc oxide surface when it is doped with cerium in the upper layer and when it is doped in an intermediate layer, thus finding that there is little difference between the layers, since it continues to present the same symmetry for the up and down spin, thus indicating that it does not have magnetic properties. Furthermore, the orbitals that contribute the most to the valence band and the conduction band remain the same for both shells.
3.2.2 Electronic properties for Hubbard (U=7 eV)
From Fig. 11, we can see that there is an overlap between the bands, which indicates that it is a conductor, where the orbitals that contribute the most are the p orbitals of the oxygen atom and the p orbitals of the zinc atom, where not presents symmetry for the spin up and spin down which indicates that it has a small magnetization of −0.11 Borh mag/cell.
Next, the electronic properties of the ZnCeO surface doped in the top layer are studied. Figure 12 shows the density of states (DOS) and PDOS contributions, where the fermi level is located at the origin of the plot.
From Fig. 12, we can see that there is an overlap between the bands, which indicates that it is a conductor, where the orbitals that contribute the most are the p orbitals of the oxygen atom and the d orbitals of the cerium atom, there is not symmetry for the spin up and spin down it, which indicates that it has a small magnetization of −0.12 Borh mag/cell
Finally, the electronic properties of the ZnCeO surface doped in the middle layer are studied. Figure 13 shows the density of states (DOS) and PDOS contributions, where the fermi level is located at the origin of the plot.
In the same way, we can see that in Fig. 13 there is an overlap between the bands, which indicates that it is a conductor, where the orbitals that contribute the most are the d orbitals of the cerium atom and the p orbitals of the oxygen atom, there is no symmetry for the spin up and spin down it, which indicates that it has a small magnetization of 0.10 Borh mag/cell
Comparing the electronic properties of the cerium-doped zinc oxide surfaces in the upper layer and in an intermediate layer with different Hubbard values \(U=0\,eV\) and \(U=7\,eV\), we observe that for a value of \(U=0\,eV\) it does not present magnetization, while that when adding the value of \(U=7\,eV\), it does not present symmetry in the spin up and the spin down, which indicates that there is a magnetization.
3.3 Study of the adsorption of carbon dioxide on the surface of zinc oxide and zinc oxide doped with cerium
In Fig. 14, we show the configurations in which carbon dioxide adsorption occurred for the clean and doped systems in the top layer.
Thus, in the systems studied, the activation of carbon dioxide is observed, because it bends the carbon dioxide molecule. Where a charge transfer occurs towards the surface Table 2, and the lengths of the bonds increase Table 3, being in accordance with Reimers, W.G. [28]
3.3.1 Electronic properties for carbon dioxide adsorption
For Fig. 15, we observe that there is an overlap between the bands, which indicates that they behave like a conductor, where the orbitals that contribute the most to the valence band are the p of oxygen while the orbitals that contribute the most to the conduction band are the p of titanium. We also note that the upward spin is not symmetrical with the downward spin, therefore it presents a magnetization of −0.60 Borh mag/cell.
For Fig. 16, we again observe an overlap between the bands, which indicates that they behave like a conductor, where the orbitals that contribute the most to the valence band are the p of oxygen while the orbitals that contribute the most to the band conduction are the p of titanium and the d orbitals of cerium. We also note that the upward spin is symmetrical with the downward spin, therefore it does not have magnetic properties.
4 Conclusion
A study on the effects of adding cerium impurities on the structural and electronic properties of the 0001 zinc oxide surface has been successfully carried out, from which the following conclusions can be made:
Cerium doping on the zinc oxide surface slightly distorts the lattice parameters and bond lengths due to the small difference in covalent radius that the cerium atom has concerning zinc.
Having a concentration of 6.25% of cerium on the zinc oxide surface, the orbitals that make the greatest contribution to the valence band are the p orbitals of the zinc atoms and the p orbitals of the oxygen atoms, obviously, in the conduction band presents a great change, this is due to the effect produced by the impurity of the cerium atom on the zinc oxygen surface, thus being the d orbitals of the cerium atom the one that makes the greatest contribution in the conduction band, where it presents symmetry for the spin up and the spin down which indicates that it does not have magnetic properties.
It was found that when adding cerium impurities to zinc oxide, there were changes in the electronic properties, which consist of intermediate states in the forbidden energy band, located around the Fermi energy. This may suggest that the \(Zn_{0,9375}\) \(Ce_{0,0625} O\) system can probably absorb visible light, which could lead to possible improvements in the photocatalytic properties of the material.
When dioxide of carbon is adsorbed on the surfaces of zinc oxide and zinc oxide doped of cerium, the bond lengths of this molecule increase, and its bond angle decreases, which can mean a beginning in the process of reduction of this polluting gas.
References
S.M. Rodríguez, J.B. Gálvez, Procesos fotocatalíticos para la destrucción de contaminantes orgánicos en agua. In: Recursos Naturales Y Medio Ambiente en el Sureste Peninsular, pp. 49–62 (1997)
G.F. Delgado Calderon, E. Rucana Guadalupe, et al, Influencia de nanomaterial (Óxido de zinc) en la durabilidad del concreto convencional (2023)
L.W.M. Deeb, et al, Estudio comparativo de las propiedades bio-físicas entre los ementos a base de óxido de zinc-eugenol y los nuevos bioceramicos como material de obturación a retro en la cirugía periapical: una revisión sistemática (2023)
J.J. Rocha Cuervo, Producción y caracterización de películas delgadas de zinc/óxido de zinc obtenidas por evaporación catódica reactiva sobre polímeros fabricados por estereolitografía y su rendimiento bajo condiciones de desgaste (2021)
L.W.M. Deeb, et al, Estudio comparativo de las propiedades bio-físicas entre los cementos a base de óxido de zinc-eugenol y los nuevos bioceramicos como material de obturación a retro en la cirugía periapical: una revisión sistemótica (2023)
S.L. Rodríguez, K.O.J. Moreno, Hierarchical assembly of ZnO nanostructures on SnO2 backbone nanowires: low-temperature hydrothermal preparation and optical properties. Revista de Farmacología
B. Murguía Martínez, et al, Síntesis y caracterización de nano y micropartículas de óxido de zinc: un estudio morfológico desde partículas densas hasta mesoporosas sin utilizar plantillas orgánicas (2021)
J.O. Alamilla Pérez, A.U. Trejo González, Obtención de un catalizador oxidativo (pentóxido de vanadio/Óxido de zinc) para la esterificación de aldehídos (2016)
A. Sanz Serrano, et al, Nanopartículas de zinc para la mejora de los dispositivos de almacenamiento de energía (2006)
H.P. Carranza Meza, Técnica del tratamiento restaurador atraumático (tra) utilizando pasta de óxido de zinc y eugenol. B.S. thesis, Universidad de Guayaquil. Facultad Piloto de Odontología. (2015)
D.R. Martínez, G.G. Carbajal, Hidróxidos dobles laminares: arcillas sintéticas con aplicaciones en nanotecnología. Avances en química 7(1), 87–99 (2012)
D.L. Ojeda-Barrios, E. Perea-Portillo, O.A. Hernández-Rodríguez, D.J. Escudero-Almanza, J.J. Martínez-Téllez, G.R. López-Ochoa, El zinc como promotor de crecimiento y fructificación en el nogal pecanero. Tecnociencia Chihuahua 4, 64–71 (2010)
P. Capper, S.O. Kasap, A. Willoughby, Zinc oxide materials for electronic and optoelectronic device applications. Wiley, (2011)
Z.L. Wang, J. Song, Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 312(5771), 242–246 (2006)
P.X. Gao, Z.L. Wang, Nanoarchitectures of semiconducting and piezoelectric zinc oxide. J. Appl. Phys. 97(4) (2005)
Cervantes J.L. López, et al, Películas de óxidode zinc impurificado (zn1-xmxo) con propiedades fotoluminicentes, producidas a través del método de depósito atómico en capas (2014)
D.A. Ochoa Fajardo, Desarrollo de un material compósito de óxido de zinc impregnado en un soporte granular que presente actividad fotocatalítica. B.S. thesis, Quito: EPN, 2015. (2015)
L.A. Castillo Jauregui, Efecto de la temperatura de síntesis en el tamaño y ancho de banda prohibida de nanopartículas de zno producidas por sol-gel (2011)
Ü. Özgür, Y.I. Alivov, C. Liu, A, Teke, M.A. Reshchikov, S. Doğan, V. Avrutin, S.-J. Cho, H. Morkoç, A comprehensive review of zno materials and devices. J. Appl. Phys. 98(4) (2005)
J. Márquez Álvarez, et al, Estudio por primeros principios de propiedades estructurales, electrónicas y magnéticas para el compuesto zno codopado con titanio y vanadio. Master’s thesis, Universidad del Norte (2017)
C.E. Rocha Diaz, Cálculo de las propiedades electrónicas del pd/zno (0001) por primeros principios (2023)
X. Liu, L. Ye, S. Liu, Y. Li, X. Ji, Photocatalytic reduction of \(CO_2\) by \(ZnO\) micro/nanomaterials with different morphologies and ratios of \(\{\)0001\(\}\) facets. Sci. Rep. 6(1), 38474 (2016)
S.G. Kumar, K.K. Rao, Zinc oxide based photocatalysis: tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 5(5), 3306–3351 (2015)
A. Mclaren, T. Valdes-Solis, G. Li, S.C. Tsang, Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc. 131(35), 12540–12541 (2009)
Z. Yufei, G. Zhiyou, G. Xiaoqi, C. Dongxing, D. Yunxiao, Z. Hongtao, First-principles of wurtzite ZnO (0001) and (0001) surface structures. J. Semicond. 31(8)(2010)
E. Cerrato, C. Gionco, I. Berruti, F. Sordello, P. Calza, M.C. Paganini, Rare earth ions doped ZnO: synthesis, characterization and preliminary photoactivity assessment. J. Solid State Chem. 264, 42–47 (2018)
L. Schmidt-Mende, J.L. MacManus-Driscoll, ZnO-nanostructures, defects, and devices. Mater. Today 10(5), 40–48 (2007)
W.G. Reimers, Estudio teórico de la adsorción y reacción de co/co2/h2para la obtención de metanol utilizando catalizadores de ga2o3, ceo\(_2\) y zno. Master’s thesis, Universidad nacional del sur (2015)
Acknowledgements
Los autores agradecemos a la vicerrectoría de investigación y extensión de la universidad de Córdoba por haber financiado este trabajo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doria, A., Ponnefz Durango, R.E. & Alcalá Varilla, L.A. Activation of carbon dioxide on zinc oxide surfaces doped with cerium. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00662-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00662-9