Abstract
The development of environmentally friendly control methods to mitigate the severe damages caused by Phytophthora cinnamomi in the Mediterranean climate-type ecosystems is essential. In this way, crop waste and by-products which represent between 13 and 65% of agriculture production, are a rich source of bioactive compounds with antifungal and biocide activity. The main objective of this work was to determine the biocide activity against P. cinnamomi of three organic extracts. These extracts enriched in bioactive compounds come from residues of asparagus (Asp) and olive crops (Oliv and OH, from fruits and leaves respectively). They were evaluated at two doses (0.15 and 0.10%) on the mycelial growth and sporangial production of P. cinnamomi by in vitro experiments. Mycelial growth and sporangial production were significant reduced from the three plant extracts at the two doses tested, reaching a total inhibition with Asp at both doses. In general, no phytotoxicity symptoms were observed on seed germination and plant development, except for a plant yield reduction in the substrate treated with Oliv and Asp at the highest dose. In experiments performed in artificially infested soil, Asp induced a reduction of chlamydospores viability greater than 75% compared to unamended soil. Additionally, in planta experiments showed a significant reduction in plant mortality in substrate amended with OH. These results suggest that soil application of Asp and OH can limit P. cinnamomi infectivity and survival, setting the first steps to develop a sustainable method to control the root disease based on agricultural waste circular economy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytophthora cinnamomi is one of the most harmful plant pathogens, included among the 100 worst invasive alien species (Lowe et al. 2004) based on its severe impact on biological diversity in both agricultural and natural ecosystems worldwide (Hardham and Blackman 2018; Sena et al. 2018). Ecosystem-level damages are caused by this soilborne oomycete in Mediterranean climate-type ecosystems such as Jarrah forests of Western Australia (Shearer et al. 2004; Hardham and Blackman 2018), native shrubs found in the Ione Formation of California (Swiecki et al. 2003; Swiecki and Bernhardt 2017), the fynbos vegetation in the Cape Floristic Region of South Africa (Nagel et al. 2013; Bose et al. 2018), and the oak and chestnut woodlands in the Mediterranean Basin (Brasier 1996; Balci and Halmschlager 2003; Vettraino et al. 2005; Scanu et al. 2013; Jung et al. 2018).
In the Iberian Peninsula, the most severe damages are caused by P. cinnamomi in the dehesa ecosystems (montados in Portugal) which are open woodland forests created and maintained by humans and their livestock (Serrano et al. 2011). The control of P. cinnamomi in dehesa systems is difficult firstly, because the pathogen can occupy the soil profile to a great depth (50 cm, Shearer and Tippett 1989), spread quickly in poorly drained or waterlogged soils and attack a large range of woody and herbaceous hosts (Erwin and Ribeiro 1996; Serrano et al. 2010, 2017). Secondly, by the seminatural character of dehesa systems (Serrano et al. 2017). For those reasons, an integrated control strategy has been developed for this disease management (Serrano et al. 2017) including cultural actions, such as the application of calcium soil amendments (Serrano et al. 2012a) and the restriction on the cultivation of susceptible crops such as Lupinus luteus (Serrano et al. 2010, 2012b); as well as biofumigation using species of the genus Brassica with high content in Sinigrin (Ríos et al. 2016) to reduce the infectivity of P. cinnamomi in soil. Although these actions are practical, they cannot eradicate the pathogen (Serrano et al. 2017) and, consequently, they should be complemented with the use of resistant (asymptomatic and uninfected trees) or tolerant (infected trees but asymptomatic or with low severity of disease symptoms) trees against the pathogen. Tolerant trees can be obtained by the application of systemic phosphonate fungicides by trunk injection (resistance inducers) (González et al. 2017, 2020; Romero et al. 2019) or increasing Ca2+ content into the tree (Serrano et al. 2013). However, because of the current demand for green and safe food production without the use of any synthetic agrochemicals, it is necessary to carry on searching for new environmentally friendly and sustainable management alternatives that may be applied in affected dehesas against the soilborne pathogen P. cinnamomi.
In this way, bioactive compounds from plants (phenolic derivates as flavonoids and saponins), are secondary metabolites with a broad range of biological properties directly associated with their chemical structure (Sparg et al. 2004), including antibacterial and antifungal activities (Sparg et al. 2004; Rosado-Álvarez et al. 2014; Okoro and Onaebi 2020; Do et al. 2021). These phytochemical compounds are present in a wide variety of plant species broadly distributed worldwide (Sparg et al. 2004). Several studies have demonstrated that wild plant extracts rich in saponins, and flavonoids have shown in vitro and in vivo antifungal activity against several Phytophthora species, including P. capsici (Ansary et al. 2016; Švecová et al. 2017), P. infestans (Choi et al. 2004; Goufo et al. 2010; Pham et al. 2021), P. agathidicida and P. cinnamomi (Lawrence et al. 2019). In other cases, phytochemicals were obtained from crops and wastes from agro-industry (Deacon and Mitchell 1985; Rosado-Álvarez et al. 2014). For example, saponins present in root extracts of Avena sativa might reduce pathogenic oomycete populations in soil, including P. cinnamomi, by zoospore lysis (Deacon and Mitchell 1985). Rosado-Álvarez et al. (2014) recorded the antifungal activity of ‘triguero’ asparagus (wild landrace from Huétor-Tájar, southern Spain) extract, rich in flavonoids, which reduced the mycelial growth and conidia germination of three Fusarium species, pathogenic on asparagus, carnation, and strawberry. Preliminary in vitro works also concluded that lyophilized asparagus spear extract generally achieves 100% of mycelial growth inhibition of P. cinnamomi and Pythium spiculum (Basallote-Ureba et al. 2016). Commercial and wild asparagus, including ‘triguero’ asparagus, have a high content of saponins and flavonoids whose composition and concentration depend on species, varieties (Fuentes-Alventosa et al. 2008; Jaramillo-Carmona et al. 2017; Hamdi et al. 2017), and even plant organs (Jaramillo-Carmona et al. 2017). Olive crop by-products are also a suitable source of phenolic compounds, such as hydroxytyrosol which is one of the most important natural antioxidants, with effective antifungal and -bacterial activities (Medina et al. 2013).
Agriculture is one of the main productive activities in Spain, with more than 16 million ha dedicated to a broad variety of horticultural, cereal and fruit crops (MAPA 2021). This high level of productivity also implies a great production of organic waste and by-products derived from crop cultivation, and fruits and vegetables processed for selling (Jiménez-Moreno et al. 2020). The residues, which represent between 13 and 65% of the total agriculture production, are discarded due to their lack of economic value (Ross and Rogoff 2012). However, the removal and/or recycling of these remnants must be correctly managed according to European Regulation (European Directive 2018/850). As proposed the European Commission in the New Circular Economy Action Plan (COM 2020/98), another option is the valorization of the agricultural residues by obtaining useful biocide compounds (Ross and Rogoff 2012; Jiménez-Moreno et al. 2020), enhancing waste prevention, reusability and circularity. In this way, organic wastes and by-products from agricultural systems may be one of the greatest biological renewable resources, essential for obtaining high-value bioproducts and bioenergy (Jiménez-Moreno et al. 2020). Thereby, the main objective of this study was to determine the biocide activity against P. cinnamomi of three organic extracts rich in bioactive compounds that comes from organic waste and by-products of asparagus and olive crops.
Material and methods
Obtention of extracts from agricultural by-products
Three different organic extracts were used in all the experiments (Table 1). The first extract was obtained from asparagus by-products which consisted in the basal portions of the spears that are discarded prior to its industrial canning. The other two extracts were obtained from olive-tree crop residues, which consist in discarded fruits and leaves acquired from pruning activities.
The functional organic extract from asparagus (Asp) with potential anti-oomycete activity was achieved as described by Fuentes-Alventosa et al. (2013). Briefly, the extraction methods consisted in a hydrothermal treatment of a mix of agricultural by-products: water in a proportion 1:2 w/v at 121 °C for 2 h in an autoclave (Presoclave 75, Selecta, Barcelona, Spain). Two fractions were obtained, consisting of an aqueous functional extract containing most of the soluble bioactive compounds and a fibrous residue mainly integrated by fiber. The global aqueous extract was fractionated by adsorption chromatography and then eluted with 40% ethanol aqueous solution (Rosado-Álvarez et al. 2014). Afterwards, it was concentrated under vacuum and freeze-dried.
The olive fruit extract (Oliv) was prepared as described by Rodríguez et al. (2007). Briefly, the aqueous liquors generated during olive oil obtaining process was purified by anion exchange chromatography. The phenolic fraction was eluted with water and dried by spray-drying.
The olive leaf extract (OH) was obtained from dried leaves by aqueous extraction at 90 °C for 40 min. No additional purification step was needed due to its high phenolic content. The extract was concentrated under vacuum and freeze-dried.
Fungal material
Four P. cinnamomi isolates that had previously been isolated from the rhizosphere of symptomatic Quercus ilex subsp. ballota trees, located in a declining open woodland (dehesa) in the Andalusian region (south Spain), were used in the experiments. These isolates are stored in the oomycete collection of the University of Córdoba and showed a 99–100% homology with their expected ITS sequences in GenBank Blast database (Accession No. GU111591.1).
In vitro experiments
Effect on mycelial growth
The ability of the organic extracts rich in bioactive compounds to inhibit the mycelial growth of P. cinnamomi was evaluated. Corn Meal Agar medium (CMA) was amended with each organic extract at 0.10 and 0. 15% and then 20 ml per plate of amended CMA was poured into Petri dishes (9 cm diameter). CMA medium free of extracts was used as a control.
According to the methodology described by Serrano et al. (2012a), agar plugs (5 mm diameter), taken from actively growing colonies of each P. cinnamomi isolate on CA medium (Carrot-Agar 20%, Dhingra and Sinclair 1995), were placed in the center of Petri dishes containing amended or control CMA medium. Four dishes (replicates) per isolate, organic extract, and dose, plus the controls, were prepared. All the plates were incubated at 24 °C in the dark. Daily, mycelial growth (diameter) of colonies was measured until control colonies covered the full surface of the plates, in 9 days. The experiment was repeated at three different times.
The evolution of the mycelial growth (diameter) over the experiment was expressed as the relative area under the progress curve (rAUPC) calculated as follows (Campbell and Madden 1990):
Where, si = mycelial growth value for observation number i, smax = maximum value, ti = number of days between evaluation i, te = total evaluation period, and n = number of evaluations. Data of rAUPC did not fit normality even after applying transformations, and they were analyzed using a Kruskal-Wallis non-parametric test for the combination treatment-dose, repetition and isolate as three independent factors. Mean values were compared by Dunn’s test for P < 0.05. All the statistical analysis were done using Statistic software 10.0 (Analytical Software, Tallahassee, USA).
Effect on sporangial production
To induce sporangial production, the organic extracts were added separately to a soil extract (0.1% soil-water) previously prepared as described by Ribeiro (1978). Unamended soil extract was used as the control. Three plates (replicates) were prepared per extract and dose, plus controls. Agar plugs (5 mm diameter) of two P. cinnamomi isolates growing on PA medium (Pea-Agar 2%) for 4 days at 24 °C in the dark, were placed in the center of the Petri dishes containing amended or control soil extracts, and all of them were incubated at 23 °C in the light until sporangial production reached the maximum in control soil extract, which happened at 50 h. At this time, sporangia were counted by direct observation under an inverted microscope (Nikon, magnification ×400). The experiment was repeated three times. Data of total sporangial production did not fit normality even after applying transformations, and they were analyzed using a Kruskal-Wallis non-parametric test for the combination (1) treatment-dose, (2) repetition and (3) isolate as three independent factors. Mean values were compared by Dunn’s test for P < 0.05.
Plant experiments
Phytotoxicity
The phytotoxic activity was evaluated for Asp, Oliv, OH, in regards to seed germination and seedling growth. The herbaceous plant L. luteus is considered a model species of dehesa ecosystems and was used in phytotoxicity tests due to its susceptibility to P. cinnamomi (Serrano et al. 2010). Agar medium (1.5%) was amended with each organic extract at 0.10 and 0.15%, using unamended agar medium as control. The L. luteus seed surfaces were disinfested following the process described by Serrano et al. (2010), then six seeds were transferred to each Petri dish containing amended or control agar medium. Six Petri dishes (replicates) were prepared per treatment and dose. All plates were incubated at 23 °C with a 12 h photoperiod. The percentage of seed germination was recorded per day for each experimental unit (plate) over a 6 day period. Then (Phytotoxicity test 1) 18 germinated seeds of each treatment, including controls (3 seeds per plate) were transferred to 75 ml seedbed trays containing clean peat. All plants were incubated in a growth chamber (23 °C with 12 h photoperiod) and watered as required (approx. 25 ml water each 2–3 days). Weekly, phytotoxicity symptoms, such as plant organ deformation, discoloration and/or necrosis, were evaluated. At the end of the experiments, 4 weeks after transfer, the plant yield was assessed as total plant length and fresh and dry weight (dried at 60 °C until constants weight was achieved).
Additionally, the direct effects of organic extracts in the substrate on the growth of L. luteus plants were also tested (Phytotoxicity test 2). For that, 10-day-old seedlings obtained from pregerminated seeds of L. luteus (Serrano et al. 2010) were transferred to seedbed trays containing peat amended with the three organic extracts (Asp, Oliv, OH) at 0.10 and 0.15%. Unamended peat was used as the control. Twelve seedlings (replicates) per product and dose were planted. All plants were incubated in a growth chamber at 23 °C with a 12 h photoperiod and watered as required (approx. 25 ml water each 2–3 days). Phytotoxicity symptoms and plant yield were evaluated as described in the previous experiment (Phytotoxicity test 1).
All phytotoxicity experiments were repeated twice. After applying transformations data of percentage of seed germination, total plant length, fresh and dry weight did not fit normality, and they were analyzed using a Kruskal-Wallis non-parametric test for the combination treatment-dose and repetition as independent factors. Mean values were compared by Dunn’s test for P < 0.05.
Effect on root rot development
Based on the results of in vitro experiments, the most effective dose that inhibited P. cinnamomi mycelial growth and sporangial production without causing phytotoxicity was tested against pathogen survival and root rot disease. A substrate (peat: sand 1:1 vol.) was infested with an aqueous suspension of resistance spores of P. cinnamomi (chlamydospores) according to the methodology described by Sánchez et al. (2002) and adjusted to 65 viable chlamydospores per g of dry soil when added to the substrate (Serrano et al. 2015). Twelve containers (4 L) were prepared with infested substrate plus another three containers with uninfested substrate used as negative control. All containers were incubated in a growth chamber at 23 °C with a 12 h photoperiod. Four days after soil infestation, each organic extract at 0.15% was separately applied to the infested substrate of three replicate containers. All containers, including controls with substrate infested and untreated (positive control) and uninfested and untreated (negative control) were watered with 400 ml of tap water each and incubated in a growth chamber with the same conditions described before. After seven days, sixteen 10-days-old L. luteus seedlings produced as described Serrano et al. (2010) were transferred to each container, including positive and negative controls. All containers were incubated at 23 °C with a 12 h photoperiod and watered as required to maintain the substrate wet. Lupinus luteus mortality was evaluated weekly, and 4 weeks after plants transference, segments of lupin roots were plated on Pea-Agar-NARPH medium (modified from Hüberli et al. 2000) for P. cinnamomi re-isolation (Romero et al. 2007). Furthermore, at the end of the experiment, the viable spore density of P. cinnamomi in each treated and control substrate was also determined. For that, ten grams of homogenized and air-dry substrate was suspended in 100 ml sterilized water-agar (0.2%) and shaken. One ml aliquots of this mixture substrate-water-agar were plated on Petri dishes containing selective medium Pea-Agar-NARPH and distributed over the agar surface with a glass spreader. For each soil sample, a total of 20 Petri dishes were prepared. Dishes were incubated at 23 °C in the dark for 24 h, and then the agar surface of each plate was washed with sterile water and the substrate-water-agar mix removed. Dishes were re-incubated for another 24 h at the same conditions and the growing colonies were morphologically identified and counted. Chlamydospore viability was expressed as CFU g− 1 (colonies forming units per g of dry soil).
The full experiment was repeated at two times. The evolution of L. luteus survival over the experiment was expressed as the relative area under the progress curve (rAUPC) calculated using the formula described before. rAUPC and density data converted to (CFU g− 1)1/2 were tested for homoscedasticity using Levene’s test and then, two-way ANOVA tests were performed considering treatment, replicate and their interactions as factors. When significance was achieved (p < 0.05), mean values were compared (Fisher’s LSD test).
Results
In vitro experiments
Colonies growing on control plates (unamended media) covered the entire agar surface in 9 days. Figure 1a showed the average values of diametrical mycelial growth measured after 8 days. The statistical analysis showed significant differences in relative area under the mycelial growth progression curve depending on organic extracts and concentration (F = 346.28, P < 0.0001), while no differences were observed among isolates (F = 1.57, P = 0.1959), nor repetitions (F = 0.48, P = 0.6216). Mycelial growth was significantly inhibited by the three organic extracts (Asp, Oliv, OH) at all concentrations tested (0.1 and 0.15%), reaching an inhibition over the experimental period of 100% for Asp extract at both doses (Fig. 1b).
Average and standard error of P. cinnamomi mycelial growth (diameter) in media amended with the three organic extracts (Asp, Oliv and OH) at 0.10 and 0.15% and in unamended media (control) for 8 days (A) and the relative area under progression curve (rAUPC) for mycelial growth (B). Bars with different letters significantly differ according to Dunn’s test at P < 0.05
The average values of sporangial production under the influence of each organic extract are shown in Fig. 2. The statistical analysis showed significant differences among treatments and doses (F = 51.36, P < 0.0001), but not between isolates (F = 1.61, P = 0.2067) and repetitions (F = 0.10, P = 0.9038). All organic extracts regardless of the dose tested stimulated a production of P. cinnamomi sporangia significantly lower than the unamended control treatment. It is also notable that all the extracts and doses inhibited sporangial production over 83%, reaching a total inhibition for Asp extract even at the lowest dose tested.
In vivo experiments
Phytotoxicity
Results indicated that the three organic extracts at the two doses tested did not differ in the percentage of seed germination in comparison with untreated control (Fig. 3). After seed germination under the influence of the different organic extracts, L. luteus plants grew without showing phytotoxicity symptoms, such as organ deformation, discoloration and/or necrosis of plant tissue, except for a slight reduction in the yield of plants germinated under Asp and Oliv extracts at the highest dose (Table 2. Phytotoxicity test 1). In the same way, both organic extracts (Asp and Oliv) at 0.15%, as well as Asp at 0.1% induced a reduction in total plant length and dry weight when seedlings were grown in peat treated with them relative to controls (Table 2. Phytotoxicity test 2). Similar results were found between repetitions in phytotoxicity experiments.
Effect in root rot development
At the end of the experiment, 4 weeks after L. luteus transference, the statistical analysis showed significant differences in the viable resistance spore density for the different treatments (F = 22.32, P = 0.0025). Comparison of means showed that the average number of viable chlamydospores was significantly inhibited in substrate treated with Asp in comparison with untreated control (Fig. 4).
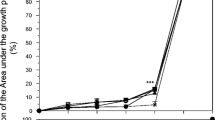
The percentage of L. luteus seedlings alive along the evaluation period, and average values of rAUPC obtained for plant survival progression are shown in Fig. 5a, b, respectively. The statistical analysis of this rAUPC showed significant differences depending on treatment (F = 9.46, P = 0.0002). As shown in Fig. 5b, only plants growing in inoculated substrate treated with OH extract showed an evolution of survival significantly higher than inoculated and untreated plants (Control +).
Phytophthora cinnamomi was always reisolated from plants growing in infested substrate, amended, or not amended with organic extracts, but never from plants growing in uninfested soil (Control −). For the different variables analyzed not significant differences were found between repetitions.
Survival percentage of Lupinus luteus growing in substrate treated with three organic extracts (Asp, Oliv and OH) at 0.15%, plants growing in unamended and infested substrate (Control +) and in unamended and uninfested substrate (Control −) during the experiment (A) and the relative area under the progress curve (rAUPC) for plant survival percentage (B). Bars with different letters significantly differ according to Fisher’s LSD test at P < 0.05
Discussion
Extracts from wild and crop plants have been described to have antimicrobial activity showing a potential for the control of phytopathogenic fungi (Pizzolitto et al. 2013; Sales et al. 2016; Ahmad and Matsubara 2020; Desoukey et al. 2020) and oomycetes (Del Rı́o et al. 2003; Ansary et al. 2016; Lawrence et al. 2019; Pham et al. 2021). Our in vitro experiments showed that extracts from the selected species, asparagus, and olive tree, exhibited effective anti-oomycete activity against P. cinnamomi. Although, the results showed that the different extracts tested varied in their effectiveness in inhibiting the infective and survival proficiency of the pathogen.
In this way, the aqueous extract obtained from the basal portions of asparagus spears showed the highest biocide action in vitro against P. cinnamomi, completely inhibiting its mycelial growth and zoospore release (sporangial production), as well as significantly reducing the viability of chlamydospores in soil. These facts were partially confirmed by two previous works which demonstrated that aqueous and ethanolic extracts from similar by-products of triguero asparagus from Huétor-Tájar landrace as we used (it is an endemic variety from southern Spain close to A. officinalis and Asparagus maritimus), showed antifungal activity against the mycelial growth of different isolates of P. cinnamomi and Py. spiculum (Basallote-Ureba et al. 2016) and against Fusarium oxysporum pathogenic to carnation, strawberry, and asparagus (Rosado-Álvarez et al. 2014). However, this work approach did not focus on other key steps of pathogen life cycle, such as infective spore production or its viability. Additionally, in concordance with these results, different root and shoot extracts of fresh and dried plant material from A. officinalis had antifungal properties against Alternaria tenuissima, Botrytis cinerea, F. oxysporum, Macrophomina phaseolina, Rhizoctonia solani (Desoukey et al. 2020). In the same way, some fractions of A. officinalis extracts were able to reduce the synthesis of fumonisin, a mycotoxin produced by Fusarium proliferatum during the plant infection process (Witaszak et al. 2020).
Few data are available on the activity of plant extracts against pathogen inoculum density. Bowers and Locke (2004) reported that two Cassia extracts and different oil formulations were able to strongly reduce Phytophthora nicotianae populations in soil compared with the untreated control soil, which resulted in suppression of plant disease development. In our experiments, asparagus extract reached a significant inhibition of chlamydospores viability in soil, with inoculum density over 4 times lower than in the untreated control and soil amended with both olive extracts. Moreover, the anti-oomycete activity of asparagus extract was robust enough to counter the strong ability of L. luteus to stimulate P. cinnamomi resistance spore production (Serrano et al. 2012b). Unfortunately, the high susceptibility of L. luteus to P. cinnamomi did not allow us to discriminate the effectiveness of asparagus extract on root rot development, obtaining a plant mortality similar to the untreated control. Therefore, the next step in our investigations will be to confirm the potential of asparagus extract to protect seedlings of woody species from P. cinnamomi root disease.
On the other hand, a previous study found that an aqueous extract from a semisolid olive-mill by-product called alpeorujo resulted in a total inhibition of the mycelial growth of Phytophthora cactorum, as well as reduction over 50% of Alternaria sp. and B. cinerea (Medina et al. 2011). However, our experiments reported that although olive extracts from leaves and fruits effectively reduced the mycelial growth of P. cinnamomi regardless of the dose tested, inhibition reached only a maximum of 36% for Oliv at 0.15%. In concordance with our results, Lawrence et al. (2019) reported that to inhibit mycelial growth of Phytophthora agathidicida and P. cinnamomi the required dose of kanuka extract was around 20 times higher than to inhibit zoospore germination and mobility. Nevertheless, the capacity of P. cinnamomi to infect does not depend on its saprophytic growth, but on its ability to produce infective zoospore through sporangial production (Erwin and Ribeiro 1996; Hardham and Blackman 2018). In this way, we demonstrate the ability of both olive extracts to effectively inhibit sporangial production (84–98%, highlighting OH at the highest dose) and consequently to prevent infective zoospore release.
It was also observed in the in vivo experiments that although no olive extracts affected chlamydospore viability, the mortality of L. luteus seedlings was significantly reduced by OH extract in comparison with untreated and infested soil (positive control), probably due to the higher inhibition of P. cinnamomi infectivity (sporangial production) by OH extract compared to Oliv. In general, the results of the current study confirm the effectiveness of the extract from olive leaves (OH) to control both P. cinnamomi infectivity and root rot development, also demonstrating a differential behavior associated to the biomass used to obtain the extract. These findings agree with Desoukey et al. (2020) who described that root extracts of Asparagus officinalis showed a higher inhibitory activity against Alternaria tenuissima than shoot extracts, while the effect of both root and shoot extracts varied against other fungal species, such as B. cinerea and Rhizoctonia solani (Desoukey et al. 2020).
A possible explanation of the differential behaviors reported for the three organic extracts used in this study could be that their phenolic compound profiles are considerably different, even between both olive extracts (Supplemental information). According to Ahmad and Matsubara (2020), the antimicrobial activity of plant extracts is directly related to the diversity in their chemical compound profile. However, the composition and concentration of bioactive compounds in plant extracts are highly dependent not only on plant species (Jaramillo-Carmona et al. 2017; Lawrence et al. 2019; Okoro and Onaebi 2020), but also on the vegetable organ (Fuentes-Alventosa et al. 2008; Lawrence et al. 2019; Desoukey et al. 2020), phenological phase (Bahraminejad et al. 2012), geographic location, cultivar, and plant nutrition (Borjan et al. 2020), as we confirmed in our study. Thus, the flavonoid-3-O-glycoside rutin (quercetin-3-O-rutinoside) was the major secondary metabolite in the aqueous extracts of the basal portions of triguero asparagus spears (Fuentes-Alventosa et al. 2008), which was also one of the most abundant flavonoid component in vegetative organs of grapevines (Goufo et al. 2020; Buzón-Durán et al. 2022), whereas the bottom of asparagus stem had a much higher content in saponins than in flavonoids (Fuentes-Alventosa 2009). In agreement with these results, previous studies reported that several extracts from plant species with high rutin content, such as Vitis vinifera, Hedera helix, Ferocactus species, Acacia saligna and Ruta graveolens, have also shown significant growth inhibition of pathogenic fungi and bacteria, including Neofusicoccum, Dothiorella, Fusarium, Aspergillus, Penicillium, Botrytis, etc. (Parvu et al. 2015; Elansary et al. 2020a, b, c; Buzón-Durán et al. 2022). In the same way, different plant extracts from Cassia alata and Kunzea robusta both with high content in flavonoid effectively inhibited the mycelial growth of Phytophthora infestans (Pham et al. 2021), as well as the mycelial growth and zoospore production and mobility of P. agathidicida and P. cinnamomi (Lawrence et al. 2019). Three isoflavonoids isolated of Dalbergia odorifera, a Chinese legume plant, overall reduced zoospores mobility and viability of the oomycete Aphanomyces cochlioides (Islam 2008). Consequently, based on the results from this study, rutin might be a good candidate to be tested against P. cinnamomi to determine its potential implication on asparagus extract anti-Phytophthora activity.
In contrast the prevailing phytochemicals in the aqueous extract obtained from olive waste were polyphenolic compounds (Liu et al. 2017). Thus, the leaves extract (OH) is essentially composed by dihydroxyphenylglycol, and mainly oleuropein, reaching a total richness of 4.46%. Meanwhile, the total phenolic concentration in the olive fruit (Oliv) extracts was 6.20%, being hydroxytyrosol and tyrosol the most frequent phytochemicals (Rodríguez et al. 2007). The higher anti-oomycete activity shown by OH extract than Oliv is supported by the presence of oleuropein as main active secondary metabolite of olive leaves. This fact was confirmed by Korukluoğlu et al. (2008) who demonstrated that among all the phenolic components present in the olive leaf extract, including tyrosol, oleuropein showed the best antifungal activity against several genera of pathogenic fungi (Alternaria, Aspergillus, Fusarium, etc.). In contrast with it, tyrosol was a more active agent than oleuropein against Phytophthora sp. (Del Rı́o et al. 2003). Although both studies suggested an increase in the antimicrobial activity induced by a synergistic effect of the phenolic components of olive leaves (Del Rı́o et al. 2003; Korukluoğlu et al. 2008).
Most of those studies, in agreement with results of the present work, reported a direct effect of plant extracts enriched in bioactive compounds against various key steps of pathogenic species life cycles (Lawrence et al. 2019; Pham et al. 2021; Buzón-Durán et al. 2022). However, different mechanisms of antimicrobial actions have been described for flavonoids (Al Aboody and Mickymaray 2020) and phenolic compounds (Kumar et al. 2020). Thus, rutin did not show direct effect against the bacteria Xanthomonas perforans but acting as a resistance inducer on tomato plants (Safaie Farahani and Taghavi 2018). In contrast, olive leaf extracts with high content in oleuropein showed direct antimicrobial activity by inducing changes in the permeability of the cell membrane of pathogenic fungi (Korukluoğlu et al. 2008). Meanwhile, Moushib et al. (2013) described a doble action against P. infestans of sugar-beet extracts, significantly inhibiting sporangia production and inducing defense response against the pathogen. In this way, several Lamiaceae herb aqueous extracts showed direct effect inhibiting mycelial growth, inoculum density and severity of Fusarium root rot, but authors also suggested an induction of SAR by the antioxidants present in plant extracts (Ahmad and Matsubara 2020). Our findings demonstrated the direct action of high bioactive compounds content asparagus and olive extracts against P. cinnamomi however, further investigations are needed to determine if these extracts are also able to act as plant resistance inductors.
On the other hand, the antifungal activity is also related to the solvents, water, or organic products, such as acetone, ethanol, or methanol, used for the extraction process or the subsequent dilution (Sales et al. 2016; Korukluoğlu et al. 2008). In this manner, Korukluoğlu et al. (2008) reported that aqueous olive leaf extracts showed a higher antifungal activity than organic solvents extracts, reaching a completely growth inhibition of Aspergillus spp. and Fusarium semitectum. Similarly, although comparisons should be taken with caution due to differences in the pathogens and doses tested, we achieved a total inhibition of P. cinnamomi mycelial growth with the aqueous extract of asparagus, while fungi inhibition lower than 88% was obtained with similar asparagus extract eluted in ethanol against Fusarium oxysporum (Rosado-Álvarez et al. 2014) at a dose 200 times higher than in our experiment. Moreover, ethanol asparagus extract significantly affect seed germination and plant development (Rosado-Álvarez et al. 2014), while we reported no significant phytotoxicity symptoms, except for a slight reduction of plant size for Asp and Oliv at the highest dose. Therefore, because of the most prominent fungicide and anti-oomycete activity of extracts diluted in water, their easy preparation and low-cost, aqueous extracts enriched in bioactive compounds may be more interesting to agriculture (Korukluoğlu et al. 2008) and forest ecosystems applications.
In conclusion, the findings of the present study demonstrated that asparagus and both olive extracts, from fruit and leaves, have a significant effect on the epidemiology of the root disease caused by P. cinnamomi, highlighting the suppression of the infectivity capacity of the pathogen by the inhibition of sporangial production induced by all extracts regardless of the dose tested. Additionally, the application of asparagus and olive leaf extracts to soil infested by P. cinnamomi negatively affected the viability of chlamydospore and reduced root rot mortality, respectively. Consequently, the soil application of both asparagus and olive leaf extracts should be taken into account to prevent or limit pathogen survival potential and disease severity, being an effective tool for the integrated control of the root disease caused by P. cinnamomi. Further studies under controlled conditions will be conducted to confirm obtained results on susceptible woody species seedlings (Quercus spp.) and to determine the best way of extracts application under field conditions. So, this study set the first steps to develop a sustainable method to control the root disease based on the circular economy of agricultural waste contributing to their valorization.
References
Ahmad H, Matsubara YI (2020) Antifungal effect of Lamiaceae herb water extracts against Fusarium root rot in Asparagus. J Plant Dis Prot 127:229–236. https://doi.org/10.1007/s41348-019-00293-x
Al Aboody MS, Mickymaray S (2020) Anti-fungal efficacy and mechanisms of flavonoids. Antibiot 9:45. https://doi.org/10.3390/antibiotics9020045
Ansary MWR, Hoque E, West HM, Rahman MM, Akanda AM, Wang Y, Islam MT (2016) Medicinal plant extracts and protein kinase C inhibitor suppress zoosporogenesis and impair motility of Phytophthora capsici zoospores. Plant Prot Sci 52:113–122. https://doi.org/10.17221/103/2015-PPS
Bahraminejad S, Abbasi S, Maassoumi SM, Tabein S (2012) Evaluation of inhibitory effects of extracts of plants from western Iran against Phytophthora drechsleri. Aust J Crop Sci 6:255–260
Balci Y, Halmschlager E (2003) Phytophthora species in oak ecosystems in Turkey and their association with declining oak trees. Plant Pathol 52:694–702. https://doi.org/10.1111/j.1365-3059.2003.00919.x
Basallote-Ureba MJ, Rodríguez-Arcos R, Barrau García C (2016) Actividad de un liofilizado de espárrago frente a oomicetos asociados con el decaimiento del arbolado de la dehesa. In: Actas XVIII Congreso SEF, Palencia, pp 326
Borjan D, Leitgeb M, Knez Ž, Hrnčič MK (2020) Microbiological and antioxidant activity of phenolic compounds in olive leaf extract. Molecules 25:5946. https://doi.org/10.3390/molecules25245946
Bose T, Wingfield MJ, Roux J, Vivas M, Burgess TI (2018) Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecol 36:17–25. https://doi.org/10.1016/j.funeco.2018.09.001
Bowers JH, Locke JC (2004) Effect of formulated plant extracts and oils on population density of Phytophthora nicotianae in soil and control of Phytophthora blight in the greenhouse. Plant Dis 88:11–16. https://doi.org/10.1094/PDIS.2004.88.1.11
Brasier CM (1996) Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann Sci 53:347–358. https://doi.org/10.1051/forest:19960217
Buzón-Durán L, Langa-Lomba N, González-García V, Casanova-Gascón J, Sánchez-Hernández E, Martín-Gil J, Ramos PM (2022) Rutin-stevioside and related conjugates for potential control of grapevine trunk diseases. Phytopathol Mediterr 61:65–77. https://doi.org/10.36253/phyto-13108
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. John Wiley & Sons, NC
Choi GJ, Jang KS, Kim JS, Lee SW, Cho JY, Cho KY, Kim JC (2004) In vivo antifungal activities of 57 plant extracts against six plant pathogenic fungi. Plant Pathol J 20:184–191. https://doi.org/10.5423/PPJ.2004.20.3.184
Deacon JW, Mitchell RT (1985) Toxicity of oat roots, oat root extracts, and saponins to zoospores of Pythium spp. and other fungi. Trans Br Mycol Soc 84:479–487. https://doi.org/10.1016/S0007-1536(85)80010-3
Del Rı́o JA, Báidez AG, Botı́a JM, Ortuno A (2003) Enhancement of phenolic compounds in olive plants (Olea europaea L.) and their influence on resistance against Phytophthora Sp. Food Chem 83:75–78. https://doi.org/10.1016/S0308-8146(03)00051-7
Desoukey SF, El-Nahas SE, Sabh AZ, Taha ZK, El-Shabrawi HM (2020) Antimicrobial effect of Asparagus officinalis L. extracts. Plant Arch 20:9253–9264
Dhingra OD, Sinclair JB (1995) Basic plant pathology methods, 2nd edn. CRC, Boca Raton
Do HTT, Nguyen TH, Nghiem TD, Nguyen HT, Choi GJ, Ho CT, Le Dang Q (2021) Phytochemical constituents and extracts of the roots of Scutellaria baicalensis exhibit in vitro and in vivo control efficacy against various phytopathogenic microorganisms. S Afr J Bot 142:1–11. https://doi.org/10.1016/j.sajb.2021.05.034
Elansary HO, Szopa A, Kubica P, Ekiert H, Al-Mana FA, Al-Yafrsi MA (2020a) Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants 9:908. https://doi.org/10.3390/plants9070908
Elansary HO, Szopa A, Klimek-Szczykutowicz M, Ekiert H, Barakat AA, Al-Mana FA (2020b) Antiproliferative, antimicrobial, and antifungal activities of polyphenol extracts from Ferocactus species. Processes 8:138. https://doi.org/10.3390/pr8020138
Elansary HO, Szopa A, Kubica P, Ekiert H, El-Ansary DO, Al-Mana F, Mahmoud EA (2020c) Polyphenol content and biological activities of Ruta graveolens L. and Artemisia abrotanum L. in northern Saudi Arabia. Processes 8(5):531. https://doi.org/10.3390/pr8050531
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide, 1st edn. American Phytopathological Society (APS Press), St. Paul
Fuentes-Alventosa JM (2009) Caracterización de componentes bioactivos del esparrago verde: obtención de ingredientes funcionales a partir de los subproductos generados durante su transformación industrial. Dissertation, Universidad de Córdoba
Fuentes-Alventosa JM, Jaramillo S, Rodríguez-Gutiérrez G, Cermeño P, Espejo JA, Jiménez-Araujo A, Guillén-Bejarano R, Fernández-Bola?os J, Rodrñguez-Arcos R (2008) Flavonoid profile of green asparagus genotypes. J Agric Food Chem 56:6977–6984. https://doi.org/10.1021/jf8009766
Fuentes-Alventosa JM, Jaramillo-Carmona S, Rodríguez-Gutiérrez G, Guillén-Bejarano R, Jiménez-Araujo A, Fernández-Bolaños J, Rodríguez-Arcos R (2013) Preparation of bioactive extracts from asparagus by-product. Food Bioprod Process 91:74–82. https://doi.org/10.1016/j.fbp.2012.12.004
González M, Caetano P, Sánchez ME (2017) Testing systemic fungicides for control of Phytophthora oak root disease. Pathol 47:e12343. https://doi.org/10.1111/efp.12343
González M, Romero MA, Serrano MS, Sánchez ME (2020) Fosetyl-aluminium injection controls root rot disease affecting Quercus suber in southern Spain. Eur J Plant Pathol 156:101–109. https://doi.org/10.1007/s10658-019-01865-1
Goufo P, Fontem DA, Ngnokam D (2010) Evaluation of plant extracts for tomato late blight control in Cameroon. N Z J Crop Hortic Sci 38:171–176. https://doi.org/10.1080/01140671.2010.495374
Goufo P, Singh RK, Cortez I (2020) A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 9:398. https://doi.org/10.3390/antiox9050398
Hamdi A, Jaramillo-Carmona S, Beji RS, Tej R, Zaoui S, Rodríguez-Arcos R, Guillén-Bejarano R (2017) The phytochemical and bioactivity profiles of wild Asparagus albus L. plant. Food Res Int 99:720–729. https://doi.org/10.1016/j.foodres.2017.06.027
Hardham AR, Blackman LM (2018) Phytophthora cinnamomi. Mol Plant Pathol 19:260–285. https://doi.org/10.1111/mpp.12568
Hüberli D, Tommerup IC, Hardy GSJ (2000) False-negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. Australas Plant Pathol 29:164–169. https://doi.org/10.1071/AP00029
Islam T (2008) Secondary metabolites from nonhost plants affect the motility and viability of phytopathogenic Aphanomyces cochlioides zoospores. Z Naturforsch C J Biosci 63:233–240. https://doi.org/10.1515/znc-2008-3-413
Jaramillo-Carmona S, Rodriguez‐Arcos R, Jiménez‐Araujo A, López S, Gil J, Moreno R, Guillén‐Bejarano R (2017) Saponin profile of wild asparagus species. J Food Sci 82:638–646. https://doi.org/10.1111/1750-3841.13628
Jiménez-Moreno N, Esparza I, Bimbela F, Gandía LM, Ancín-Azpilicueta C (2020) Valorization of selected fruit and vegetable wastes as bioactive compounds: opportunities and challenges. Crit Rev Environ Sci Technol 50:2061–2108. https://doi.org/10.1080/10643389.2019.1694819
Jung T, Pérez-Sierra A, Durán A, Jung MH, Balci Y, Scanu B (2018) Canker and decline diseases caused by soil-and airborne Phytophthora species in forests and woodlands. Pers Mol Phylogeny Evol Fungi 40:182–220. https://doi.org/10.3767/persoonia.2018.40.08
Korukluoğlu M, Şahan Y, Yiğit A (2008) Antifungal properties of olive leaf extracts and their phenolic compounds. J Food Saf 28:76–87. https://doi.org/10.1111/j.1745-4565.2007.00096.x
Kumar S, Abedin MM, Singh AK, Das S (2020) Role of phenolic compounds in plant-defensive mechanisms. In: Lone R, Shuab R, Kamili A (eds) Plant Phenolics in Sustainable Agriculture. Springer, Singapure, pp 517–532. https://doi.org/10.1007/978-981-15-4890-1_22
Lawrence SA, Burgess EJ, Pairama C, Black A, Patrick WM, Mitchell I, Gerth ML (2019) Mātauranga-guided screening of New Zealand native plants reveals flavonoids from kānuka (Kunzea robusta) with anti-Phytophthora activity. J R Soc N Z 49:137–154. https://doi.org/10.1080/03036758.2019.1648303
Liu Y, McKeever LC, Malik NS (2017) Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front Microbiol 8:113. https://doi.org/10.3389/fmicb.2017.00113
Lowe S, Browne M, Boudjelas S, De Poorter M (2004) 100 of the World’s worst invasive alien species. A selection from the Global Invasive Species Database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN). http://www.iucngisd.org/gisd/100_worst.php. Accessed 2 Mar 2023
MAPA (2021) Producciones agrícolas. https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/. Accessed 2 Mar 2023
Medina E, Romero C, de Los Santos B, de Castro A, García A, Romero F, Brenes M (2011) Antimicrobial activity of olive solutions from stored alpeorujo against plant pathogenic microorganisms. J Agric Food Chem 59:6927–6932. https://doi.org/10.1021/jf2010386
Medina E, de Castro A, Romero C, Ramírez E, Brenes M (2013) Effect of antimicrobial compounds from olive products on microorganisms related to health, food and agriculture. In: Méndez-Vilas A (ed) Microbial pathogens and strategies for combating them: science. Technology and education. Formatex Research Center Publishing, Badajoz, pp 1087–1094
Moushib LI, Witzell J, Lenman M, Liljeroth E, Andreasson E (2013) Sugar beet extract induces defense against Phytophthora infestans in potato plants. Eur J Plant Pathol 136:261–271. https://doi.org/10.1007/s10658-012-0160-9
Nagel JH, Gryzenhout M, Slippers B, Wingfield MJ (2013) The occurrence and impact of Phytophthora on the African continent. In: Lamour K (ed.) Phytophthora: a global perspective, CABI Plant Protection Series, CABI International, pp 204–214. https://doi.org/10.1079/9781780640938.0204
Okoro CA, Onaebi CN (2020) Efficacy of methanolic leaf extract of Hyptis suaveolens and Moringa oleifera in the control of soil-borne pathogens. Annu Res Rev Biol 35:56–63. https://doi.org/10.9734/arrb/2020/v35i730246
Parvu M, Vlase L, Parvu AE, Rosca-Casian O, Gheldiu AM, Parvu O (2015) Phenolic compounds and antifungal activity of Hedera helix L. (Ivy) flowers and fruits. Not Bot Horti Agrobot Cluj-Napoca 43:53–58. https://doi.org/10.15835/nbha4319644
Pham DQ, Pham HT, Han JW, Nguyen TH, Nguyen HT, Nguyen TD, Le Dang Q (2021) Extracts and metabolites derived from the leaves of Cassia alata L. exhibit in vitro and in vivo antimicrobial activities against fungal and bacterial plant pathogens. Ind Crops Prod 166. https://doi.org/10.1016/j.indcrop.2021.113465
Pizzolitto RP, Dambolena JS, Zunino MP, Larrauri M, Grosso NR, Nepote V, Zygadlo JA (2013) Activity of natural compounds from peanut skins on Fusarium verticillioides growth and fumonisin B1 production. Ind Crops Prod 47:286–290. https://doi.org/10.1016/j.indcrop.2013.03.020
Ribeiro OK (1978) A source book of the genus Phytophthora. Vaduz, Liechtenstein
Ríos P, Obregón S, de Haro A, Fernández-Rebollo P, Serrano MS, Sánchez ME (2016) Effect of Brassica biofumigant amendments on different stages of the life cycle of Phytophthora cinnamomi. J Phytopathol 164:582–594. https://doi.org/10.1111/jph.12482
Rodríguez G, Rodríguez R, Fernández-Bolaños J, Guillén R, Jiménez A (2007) Antioxidant activity of effluents during the purification of hydroxytyrosol and 3,4- dihydroxyphenylglycol from olive oil waste. Eur J Food Res Technol 224:733–741. https://doi.org/10.1007/s00217-006-0366-1
Romero MA, Sánchez JE, Jiménez JJ, Belbahri L, Trapero A, Lefort F, Sánchez ME (2007) New Pythium taxa causing root rot on Mediterranean Quercus species in South-West Spain and Portugal. J Phytopathol 155:289–295. https://doi.org/10.1111/j.1439-0434.2007.01230.x
Romero MA, González M, Serrano MS, Sánchez ME (2019) Trunk injection of fosetyl-aluminium controls the root disease caused by Phytophthora cinnamomi on Quercus ilex woodlands. Ann Appl Biol 174:313–318. https://doi.org/10.1111/aab.12503
Rosado-Álvarez C, Molinero-Ruiz L, Rodríguez-Arcos R, Basallote-Ureba MJ (2014) Antifungal activity of asparagus extracts against phytopathogenic Fusarium oxysporum. Sci Hortic 171:51–57. https://doi.org/10.1016/j.scienta.2014.03.037
Ross DE, Rogoff MJ (2012) What a waste… the World Bank’s call for action. Waste Manag Res 30:755–757. https://doi.org/10.1177/0734242X12455401
Safaie Farahani A, Taghavi SM (2018) Rutin promoted resistance of tomato against Xanthomonas perforans. Eur J Plant Pathol 151. https://doi.org/10.1007/s10658-017-1374-7.:527– 53
Sales MDC, Costa HB, Fernandes PMB, Ventura JA, Meira DD (2016) Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac J Trop Biomed 6:26–31. https://doi.org/10.1016/j.apjtb.2015.09.026
Sánchez ME, Caetano P, Ferraz J, Trapero A (2002) Phytophthora disease of Quercus ilex in south-western Spain. Pathol 32:5–18. https://doi.org/10.1046/j.1439-0329.2002.00261.x
Scanu B, Linaldeddu BT, Franceschini A, Anselmi N, Vannini A, Vettraino AM (2013) Occurrence of Phytophthora cinnamomi in cork oak forests in Italy. Pathol 43:340–343. https://doi.org/10.1111/efp.12039
Sena K, Crocker E, Vincelli P, Barton C (2018) Phytophthora cinnamomi as a driver of forest change: implications for conservation and management. Ecol Manag 409:799–807. https://doi.org/10.1016/j.foreco.2017.12.022
Serrano MS, Fernández-Rebollo P, De Vita P, Carbonero MD, Trapero A, Sánchez ME (2010) Lupinus luteus, a new host of Phytophthora cinnamomi in Spanish oak-rangeland ecosystems. Eur J Plant Pathol 128:149–152. https://doi.org/10.1007/s10658-010-9652-7
Serrano MS, Fernández-Rebollo P, De Vita P, Carbonero MD, Sanchez ME (2011) The role of yellow lupin (Lupinus luteus) in the decline affecting oak agroforestry ecosystems. Pathol 41:382–386. https://doi.org/10.1111/j.1439-0329.2010.00694.x
Serrano MS, De Vita P, Fernández-Rebollo P Sánchez Hernández ME (2012a) calcium fertilizers induce soil suppressiveness to Phytophthora cinnamomi root rot of Quercus ilex. Eur J Plant Pathol 132:271–279. https://doi.org/10.1007/s10658-011-9871-6
Serrano MS, Fernández-Rebollo P, De Vita P, Sánchez ME (2012b) Susceptibility of common herbaceous crops to Phytophthora cinnamomi and its influence on Quercus root rot in rangelands. Eur J Plant Pathol 134:409–414. https://doi.org/10.1007/s10658-012-9999-z
Serrano MS, Fernández-Rebollo P, De Vita P, Sánchez ME (2013) Calcium mineral nutrition increases the tolerance of Quercus ilex to Phytophthora root disease affecting oak rangeland ecosystems in Spain. Agrofor Syst 87:173–179. https://doi.org/10.1007/s10457-012-9533-5
Serrano MS, Rios P, Gonzalez M, Sanchez ME (2015) Experimental minimum threshold for Phytophthora cinnamomi root disease expression on Quercus suber. Phytopathol Mediterr 54:461–464. https://doi.org/10.14601/Phytopathol_Mediterr-15128
Serrano MS, Ríos P, González M, Romero MA, Fernández-Rebollo P, Sánchez ME (2017) A review of integrated control of Phytophthora cinnamomi root rot in oak rangeland ecosystems. IOBC/WPRS Bull 127:110–114
Shearer BL, Tippett JT (1989) Jarrah dieback: the dynamics and management of Phytophthora cinnamomi in the jarrah (Eucalyptus marginata) forest of south-western Australia, vol 3. Department of Conservation and Land Management, Como, WA
Shearer BL, Crane CE, Cochrane A (2004) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Aust J Bot 52:435–443. https://doi.org/10.1071/BT03131
Sparg S, Light ME, Van Staden J (2004) Biological activities and distribution of plant saponins. J Ethnopharmacol 94:219–243. https://doi.org/10.1016/j.jep.2004.05.016
Švecová E, Colla G, Crinò P (2017) Antifungal activity of Boerhavia diffusa L. extract against Phytophthora spp. in tomato and pepper. Eur J Plant Pathol 148:27–34. https://doi.org/10.1007/s10658-016-1065-9
Swiecki TJ, Bernhardt EA (2017) Testing and implementing methods for managing Phytophthora root diseases in California native habitats and restoration sites In: U.S.D.A. (ed.) Proceedings of the Sudden Oak Death Sixth Science Symposium, San Francisco, pp 20–23
Swiecki TJ, Bernhardt EA, Garbelotto M (2003) First report of root and crown rot caused by Phytophthora Cinnamomi affecting native stands of Arctostaphylos myrtifolia and A. Viscida in California. Plant Dis 87:1395–1395. https://doi.org/10.1094/PDIS.2003.87.11.1395B
Vettraino AM, Morel O, Perlerou C, Robin C, Diamandis S, Vannini A (2005) Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with ink disease and crown decline. Eur J Plant Pathol 111:169–180. https://doi.org/10.1007/s10658-004-1882-0
Witaszak N, Lalak-Kańczugowska J, Waśkiewicz A, Stępień Ł (2020) The impacts of asparagus extract fractions on growth and fumonisins biosynthesis in Fusarium proliferatum. Toxins 12:95. https://doi.org/10.3390/toxins12020095
Acknowledgements
We deeply thank Dr. M. González Romero (Institute for Sustainable Agriculture-CSIC) for the critical review of this manuscript. This study was funded by Leonardo Grants for Researcher and Cultural Creators 2021 of BBVA Foundation and by an Emergia Project (Andalusian Government—Call 2021).
Funding
Funding for open access publishing: Universidad de Córdoba/CBUA
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of Competing Interests
The authors declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martín, M.Á.R., García, R.L., Rodríguez-Arcos, R. et al. Anti-oomycete activity of asparagus and olive by-products with potential to control Phytophthora cinnamomi root rot. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01696-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01696-y