Key Summary Points
As a first step in developing a Core Outcome Set, we performed a scoping review using a systematic methodology to identify used outcomes in nutritional intervention studies in malnourished older adults and those at risk.
AbstractSection FindingsA large variation in used outcomes and primary outcomes was identified, with considerable differences in the frequency of outcomes between settings. For most outcomes no preferred assessment method could be recognised.
AbstractSection MessageA large heterogeneity in used outcomes and methods to assess outcomes was observed, highlighting the need to develop a Core Outcome Set in order to facilitate future evidence syntheses (e.g. meta-analyses).
Abstract
Purpose
To conduct a scoping review to provide a systematic overview of outcomes used in nutritional intervention studies focused on the treatment of protein-energy malnutrition in older adults.
Methods
A systematic search of four electronic databases (Medline, EMBASE, CINAHL and Cochrane Central Register of Controlled Trials (CENTRAL) was performed to retrieve randomized controlled trials (RCTs), published until March 9, 2020, that evaluated the effect of nutritional interventions to treat protein-energy malnutrition in older adults and those at risk for malnutrition. Two authors screened titles, abstracts and full texts independently. One author extracted data that were cross-checked by another author.
Results
Sixty-three articles reporting 60 RCTs were identified. Most frequently used outcomes included body weight/body mass index (75.0% of RCTs), dietary intake (61.7%), functional limitations (48.3%), handgrip strength (46.7%), and body circumference (40.0%). The frequencies differed by setting (community, hospital and long-term care). For some outcomes there was a preferred assessment method (e.g., Barthel index for functional limitations), while for other outcomes (e.g., functional performance) a much greater variation was observed.
Conclusion
A large variation in outcomes, not only across but also within settings, was identified in nutritional intervention studies in malnourished older adults and those at risk. Furthermore, for many outcomes there was a large variation in the used assessment method. These results highlight the need for developing a Core Outcome Set for malnutrition intervention studies in older adults to facilitate future meta-analyses that may enhance our understanding on the effectiveness of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite increasing scientific interest in the topic of malnutrition in older persons over the last decades, many uncertainties remain regarding the effectiveness of nutritional interventions [1,2,3,4]. Individual randomized controlled trials (RCTs) show mixed findings, which could be caused by differences in sample size, selection of subjects, type and duration of the intervention, setting, selected outcome(s) and assessment, and overall quality of the conducted research.
The heterogeneity in treatment effects can be investigated using meta-analyses and its cause explored by performing subgroup analyses. Individual participant data (IPD) meta-analyses specifically allow the investigation of subject-level and study-level sources of this heterogeneity in treatment effects [5]. In IPD analyses potential interactions between treatment and factors such as study setting, type of intervention or participants’ characteristics can be thoroughly investigated. Thus, IPD meta-analyses are helpful to increase our understanding on the effectiveness of malnutrition interventions in older adults and to identify who will benefit most from which treatment and in what setting.
A previous IPD meta-analysis investigating the effectiveness of malnutrition intervention in older adults was hampered by several factors [6], including limited availability of individual-based datasets of previously conducted trials (e.g., data were destroyed or could not be shared), limited number of variables regarding subject characteristics (e.g., no information on malnutrition status or dietary intake at baseline), and a large variation in used outcome measures between trials. Thus, only a limited number of trials could be included in the pooled analysis for a specific outcome.
To overcome the latter problem and to support future meta-analyses, the idea to develop a Core Outcome Set (COS) for malnutrition intervention studies in older adults was raised within the Joint Action Malnutrition in the Elderly Knowledge Hub (MaNuEL) [7]. A COS is an agreed minimum set of outcomes that should be measured and reported in all clinical trials of a specific disease or trial population [8]. The development of a COS for malnutrition intervention studies in older adults serves two main purposes: first, stimulate the inclusion of relevant outcome variables to test the effectiveness of a nutritional intervention in malnourished older adults or those at risk; second, decrease the heterogeneity between malnutrition intervention studies in older adults that will benefit future (IPD) meta-analyses.
No COS for malnutrition intervention studies in older adults is yet available. However, three proposals for a Minimum Data Sets (MDS) for intervention studies in older adults were identified: the Geriatric Minimum Data Set [9], the Minimum Data Set 3.0 Resident Assessment Instrument [10], and a MDS for nutritional intervention studies in older adults [11]. Unfortunately, these previously published MDSs cannot serve as a basis for a COS. The main reasons include: not targeting malnourished older persons or those at risk; not targeting nutritional interventions; limited to one setting only; no focus on outcome variables; or the measurement instruments for assessing the outcome variables were not specified.
Therefore, within two Special Interest Groups (SIGs) of the European Geriatric Medicine Society (EuGMS) the work towards developing a COS was initiated. As a first step in this process [12], we performed a scoping review to provide an overview of outcomes and their assessment methods used in nutritional intervention studies focused on the treatment of protein-energy malnutrition in older adults.
Methods
This scoping review was conducted by interested members of the EuGMS SIG Nutrition, in close collaboration with the EuGMS SIG Systematic Reviews and Meta-analysis. All methods were prespecified. The protocol is available upon request.
Search strategy
One author (GT) developed the search strategy which was reviewed and commented by members of the study group. The final search consisted of keywords and text words and database-specific syntax. References were retrieved from Medline (via Ovid), Embase (via Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL via EbscoHost) and the Cochrane Central Register of Controlled Trials (CENTRAL via the Cochrane Library) from database inception through March 9, 2020. The full search strategies are shown in Supplementary Table S1. After removing duplicates, references of all retrieved items were uploaded in the systematic review web-application Covidence (Veritas Health Innovation, Melbourne, Australia; www.covidence.org).
Screening
Screening was undertaken by all authors. Two authors independently screened each title and abstract for eligibility. When in doubt due to limited information, the reviewers were instructed to include the reference. In case of a discrepancy between two reviewers, the involved reviewers discussed their opinions and tried to reach agreement. If the conflict was still unsolved, one reviewer (MV) made the final decision. When for a single RCT a results paper was available, as well as a protocol paper or a trial registration, only the results paper was included.
Inclusion criteria:
-
All languages
-
Participants:
-
o
Age 65 years and above or, when the age range was not reported, a mean age of at least 70 years
-
o
With malnutrition (based on (i) a screening/assessment capturing multiple aspects of undernutrition, or (ii) BMI < 22 kg/m2, or (iii) involuntary weight loss (as defined by study authors)) OR at risk of malnutrition (based on a malnutrition screening tool)
-
o
All health conditions (e.g., an RCT conducted in hip fracture patients only was also included)
-
o
-
All settings: community, hospital or long-term care/nursing home
-
Interventions: nutritional intervention focused on increasing the intake of protein and/or energy (e.g., through dietetic counselling, provision of oral nutritional supplements (ONS) or protein supplements)
-
Control condition: The contrast between the randomized groups is the increase in protein and/or energy (e.g., if the intervention contained ONS plus exercise and the control exercise only, the RCT was included)
-
Study design: Randomized Controlled Trial (RCT), including quasi-randomized, cluster-randomized and randomized cross-over design
-
Publication type: result paper, protocol paper, trial registration
-
All outcomes
Exclusion criteria:
-
Undernutrition defined as a micronutrient deficiency
-
Nutritional interventions focused on adding micronutrients only
-
Combined intervention, e.g., nutrition and exercise
-
Conference abstracts, conference proceedings
-
Not peer-reviewed publications (such as editorials)
Once all titles and abstracts were screened, full texts of the included references were uploaded in Covidence and screened for inclusion by two reviewers independently. When a reference was excluded, the reason for exclusion was indicated using the following fixed hierarchy to minimise potential conflicts: (1) full text not available, (2) wrong type of publication (e.g., conference abstract or conference proceeding), (3) wrong study type (e.g., not an intervention study or not randomized), (4) wrong population (e.g., not meeting age criterion or including well-nourished older adults), and (5) wrong contrast/comparator (e.g., intervention group included ONS plus exercise while control group received usual care). In case of discrepancies, the involved reviewers tried to reach agreement and if this was not feasible, a third reviewer (MV) made the final decision.
Data extraction
For all included full texts, one author (MV) extracted the data using a standardised data extraction sheet. Two authors (NM and GT) each checked half of the extracted data. Disagreements were resolved through discussion between the two authors and consensus of the third author.
Extracted data included bibliographic information (first author, year of publication, country), setting (community, hospital, long-term care, mixed), sample description (general or specific patient group (e.g., hip fracture patients)), sample size of intervention group(s) and control group, age (mean age, age range or age inclusion criterion), method to assess (risk of) malnutrition, type of intervention and control condition, duration of the intervention and duration of the primary outcome follow-up, primary and secondary outcome(s) and their assessment method, level of potential conflict of interest (based on funding source(s), funding of used supplements, and potential authorship of funders), and type of paper (effect paper, protocol paper or trial registration).
During this phase, one RCT included from a trial registry was replaced by the results paper published after the search date [74].
Descriptive synthesis
Characteristics of the studies and of the study sample, intervention type and control conditions, as well as funding information were categorized as indicated in Table 1.
For clarity reasons, several outcomes were categorized into an outcome domain: body circumference (including calf, thigh and mid-upper arm circumference), skinfold (including triceps, sub-scapula, supra-iliac and abdominal skinfold) and blood marker (including a wide variety of markers).
Outcomes were considered primary outcomes, when: (1) they were listed as primary outcomes in the article, or (2) they were not listed as primary outcomes but a power calculation for those outcomes was included. In case both criterion 1 and 2 could not be applied, all outcomes were considered as primary outcomes. The distinction between primary and secondary outcomes was made to highlight which outcomes were considered most critical by the investigators, as this information can be of potential help in the next steps of establishing a COS. In addition, by making this distinction we could also explore whether the heterogeneity in outcomes was potentially smaller for primary outcomes compared to secondary outcomes and whether the most frequently used outcomes across all RCTs were included as primary outcomes only.
The frequency of outcomes and outcome domains used in the included RCTs was determined, as well as the frequency of primary outcomes and outcome domains. In addition, the percentage of RCTs using a specific outcome (domain) was calculated. These analyses were repeated stratified by setting: community, hospital, long-term care, and other (i.e., RCTs with a mixed-setting or when no information about the setting was provided).
Results
From the 4277 identified references, 325 full articles were screened, and 63 articles [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] describing 60 RCTs were included in our review (Fig. 1).
Table 1 describes the main characteristics of the included RCTs. The main characteristics of each individual included RCT are shown in supplementary Table S2.
The majority of the included RCTs was conducted in Europe (67%), published in the past 10 years (62%) and included community-dwelling older adults (43%). Most RCTs used age > 65 years as the inclusion criterion (62%) and recruited a combination of older adults with malnutrition and those at risk (67%). For the inclusion criteria, assessment of nutritional status was mostly based on the MNA, either assessed alone (n = 17; 28%) or in combination with some other measurements (n = 11; 18%) such as BMI, weight loss or albumin concentration. Low BMI was used as the sole criterion in 4 RCTs (7%), or in combination with other measurements in 14 RCTs (23%). Recent weight loss was mostly used in combination with other measurements (n = 19; 32%) and only rarely as single criterion (n = 1; 2%).
Sample size was mainly between 50 and 200 (68%) and the intervention lasted 12 weeks or less for most RCTs (60%). For seven RCTs the intervention duration was not reported (7%). Most RCTs provided either ONS or dietary counselling as the nutritional intervention (78%), and most included a control group receiving usual care (62%). For two RCTs (3%) providing dietary counselling only it was explicitly stated that this could include the prescription of ONS when deemed necessary by the health care professional involved. For nine RCTs (15%) dietary counselling was combined with daily ONS for all older adults throughout the whole intervention period (the dietary counselling + ONS category).
Outcomes
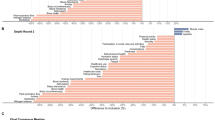
The outcomes assessed in each of the 60 RCTs can be found in supplementary Table S3. Five RCTs use a single outcome only, while most RCTs had multiple outcomes. The frequency of the outcomes and outcome domains used in all included RCTs is shown in supplementary Table S4. Figure 2 shows the frequency of the outcomes and outcome domains reported in at least two RCTs. The frequency of these outcomes and outcome domains used as primary outcome is also indicated in Fig. 2. Across all RCTs, the top five of most frequently used outcomes or outcome domains included body weight/BMI, dietary intake, functional limitation, handgrip strength and body circumference. When only primary outcomes were considered, the top five was slightly different: body weight/BMI, dietary intake, functional limitation, malnutrition status and handgrip strength. The most frequently used outcomes or outcome domains were about equally included as a primary or secondary outcome, with the exception of body weight/BMI which was mostly included as a primary outcome (66.7%) in the included RCTs.
The top ten of most frequently used outcomes and outcome domains differed by setting (Table 2). Some outcomes were not, or almost never, included in a specific setting, such as cost in the community setting, blood marker and quality of life in the hospital setting, and functional performance, (re)hospitalization, muscle mass and mortality in the long-term care setting (Table S4).
Specific outcome variables included in outcome domains
Table 3 shows the specific variables included within the three defined outcome domains and the frequency of these variables in the selected RCTs.
Assessment methods of outcomes
Table 4 shows the methods used to assess outcomes. Outcomes used in a minimum of 10 RCTs are listed in Table 4, while the assessment method for all other outcomes used in the RCTs are shown in table S3. For some outcomes there seemed to be a clear preference for a certain assessment method (e.g., dietary records or 24-h dietary recalls to assess dietary intake, the Barthel index to assess functional limitations, and the MNA to assess malnutrition status), while for other outcomes there was a greater variation in assessment methods used (e.g., the instruments used for measuring handgrip strength or test used to assess functional performance). The variation in handgrip strength instruments results in variation in the measurement unit (kPA or kg) and variation in the used protocol to test handgrip strength, as some dynamometers can only be used with the arm hanging down instead of at a 90-degree angle. In some RCTs multiple assessment methods were used for a certain outcome.
Discussion
This scoping review was performed to provide a systematic overview of outcomes used in nutritional intervention studies focused on the treatment of protein-energy malnutrition in older adults. The review shows a large variation in used outcomes, not only across settings but also within a certain setting. Furthermore, a large variation in the methods used to assess these outcomes was observed for many outcomes. These results confirm the need for developing setting-specific COS for malnutrition intervention studies in older adults, to facilitate the future conduct of meta-analyses investigating the effectiveness of nutritional interventions as basis for evidence-based recommendations for clinical practice.
The selection of a study outcome is influenced by many factors, including relevance and responsiveness to treatment according to the researchers involved, local equipment (e.g., presence of a DXA scanner), local data access (e.g., access to standardized data from electronic patient files or mandatory assessments needed for reimbursement), time, costs and expertise available, or demands of the study funder. The most frequently used outcomes according to this review, should therefore not be viewed as the outcomes considered most relevant, most feasible or most responsive to treatment in the different settings. Further research is needed to determine which outcomes are considered most relevant and feasible for each specific setting.
The majority (92%) of the included RCTs had multiple outcomes, reflecting the breath of effects expected by a nutritional intervention. A direct effect of the intervention on dietary intake was evaluated in 62% of the RCTs, and its subsequent effect on body weight or malnutrition status was evaluated in 75% and 38% of the RCTs. A better nutritional status induced by the intervention may lead to many functional and clinical improvements, which might explain the wide variation in outcomes observed across RCTs. Thirty-three RCTs (55%) defined primary and secondary outcomes, while five RCTs (8%) defined a single outcome. These primary and single outcomes are likely to be considered most relevant by the researchers involved. However, the frequency pattern of the primary and single outcomes was fairly similar to the frequency pattern of all outcomes, suggesting that there were no specific outcomes more likely to be defined as primary outcome(s).
The overview of used outcomes obtained in this review will serve as an important basis for the next steps in developing a COS [12], including a web-based survey using a Delphi approach to rank the identified outcomes in the current study based on specific criteria. The survey will be distributed to researchers involved in malnutrition research in older adults as well as to health care professionals such as geriatricians and dieticians treating older adults with malnutrition, to avoid discipline-related biases [76]. A further step will include research among (malnourished) older adults in different settings to identify outcomes that are considered most relevant to them. In a final step, a setting-specific COS will be developed and published.
In performing the current scoping review, some additional observations were made that are of interest. First, the included RCTs show a large variation in defining malnutrition as the inclusion criterion for recruitment. As we applied specific inclusion criteria for the assessment of malnutrition for our review, the actual variation across RCTs is probably even larger. Recent efforts to reach global consensus on how malnutrition should be assessed and defined [77, 78] most likely will contribute to a greater overlap in these methods in the future, also supporting future meta-analyses. Second, in 18 RCTs (30%) no primary outcome was defined nor was a power calculation for a relevant outcome provided, increasing the risk for underpowered studies. This observation also highlights the need for future meta-analyses in this field. Third, a potential conflict of interest was identified in 27 included RCTs (45%) as nutritional products and/or funding was provided by industry, or employees of industry funders were included as authors. For six RCTs (10%) no information regarding funding was provided. These last two observations could indicate an increased risk of bias in several nutritional intervention studies.
A strength of our scoping review is the very strict methodology used in searching and reviewing the literature, extracting the data and reporting the results (Table S5) [79]. Furthermore, the complementary expertise of the authors has strengthened the review process. Another strength is that we included trial protocols in our review to ensure including outcomes of recently designed RCTs, as outcomes may vary over time for example due to recent scientific insights or the development of new assessment methods. However, a limitation of including trial protocols is that they often lack specific information on the assessment methods used to measure study outcomes. In several RCTs no distinction was made between primary and secondary outcomes, suggesting that all outcomes were deemed equally critical and relevant by the investigators. For these RCTs, in the absence of further information, we considered all outcomes as primary outcomes, which may not have been a correct interpretation.
In conclusion, this scoping review highlights the wide variety of outcomes used in nutritional intervention studies conducted in malnourished older adults and those at risk. Furthermore, it shows that the most frequently used outcomes differ by setting and that some outcomes are not used in specific settings. Finally, for most outcomes the methods used to assess the outcome were heterogeneous. The information obtained in this scoping review provides the necessary basis for the next steps in developing a COS for nutritional intervention studies focused on the treatment of protein-energy malnutrition in older adults.
References
Milne AC, Potter J, Vivanti A, Avenell A (2009) Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev 2:CD003288
Health Council of the Netherlands (2011) Undernutrition in the elderly Publication no 2011/32E. Health Council of the Netherlands, The Hague
Baldwin C, Kimber KL, Gibbs M, Weekes CE (2016) Supportive interventions for enhancing dietary intake in malnourished or nutritionally at-risk adults. Cochrane Database Syst Rev 12:CD009840
De van der Schueren MA, Wijnhoven HA, Kruizenga HM, Visser M (2016) A critical appraisal of nutritional intervention studies in malnourished, community dwelling older persons. Clin Nutr 35:1008–1014
Riley RD, Lambert PC, Abo-Zaid G (2010) Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 340:221
Reinders I, Volkert D, de Groot LCPGM, Beck AM, Feldblum I, Jobse I, Neelemaat F, de van der Schueren MAE, Shahar DR, Smeets ETHC, Tieland M, Twisk JWR, Wijnhoven HAH, Visser M (2019) Effectiveness of nutritional interventions in older adults at risk of malnutrition across different health care settings: pooled analyses of individual participant data from nine randomized controlled trials. Clin Nutr 38:1797–1806
Volkert D, Visser M, Corish CA, Geisler C, de Groot L, Cruz-Jentoft AJ, Lohrmann C, Oonnor EM, Schindler K, de van der Schueren MAE, on behalf of MaNuEL consortium (2020) Joint action malnutrition in the elderly (MaNuEL) knowledge hub: summary of project findings. Eur Geriatr Med. 11:169–177
Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E et al (2012) Developing core outcome sets for clinical trials: issues to consider. Trials 13:132
Abellan Van Kan G, Sinclair A, Andrieu S, Olde Rikkert M, Gambassi G, Vellas B, on behalf of the Gerontonet Collaboration (2008) The geriatric minimum data set for clinical trials (GMDS). J Nutr Health Aging 12:198–200
Centers for Medicare & Medicaid Services. The Long-Term Care Facility Resident Assessment Instrument User’s Manual for Version 3.0. https://downloads.cms.gov/files/mds-3.0-rai-manual-v1.17.1_october_2019.pdf. Accessed March 3, 2021.
Salvà A, Corman B, Andrieu S, Salas J, Vellas B (2004) Minimum data set for nutritional intervention studies in elderly people. J Gerontol Med Sci 59:M724–M729
Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M et al (2016) How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”—a practical guideline. Trials 17:449
Breslow RA, Hallfrisch J, Guy DG, Crawley B, Goldberg AP (1993) The importance of dietary protein in healing pressure ulcers. J Am Geriatr Soc 41:357–362
Carver AD, Dobson AM (1995) Effects of dietary supplementation of elderly demented hospital residents. J Hum Nutr Dietet 8:389–394
Volkert D, Hubsch S, Oster P, Schlierf G (1996) Nutritional support and functional status in undernourished geriatric patients during hospitalization and 6-month follow-up. Aging Clin Exp Res 8:386–395
Lauque S, Arnaud-Battandier F, Mansourian R, Guigoz Y, Paintin M, Nourhashemi F et al (2000) Protein-energy oral supplementation in malnourished nursing-home residents. A controlled trial. Age Ageing 29:51–56
Bos C, Benamouzig R, Bruhat A, Roux C, Valensi P, Ferriere F et al (2001) Nutritional status after short-term dietary supplementation in hospitalized malnourished geriatric patients. Clin Nutr 20:225–233
Beck AM, Ovesen L, Schroll M (2002) Home-made oral supplement as nutritional support of old nursing home residents, who are undernourished or at risk of undernutrition based on the MNA. A pilot trial. Mini Nutritional Assessment. Aging Clin Exp Res 14:212–215
Payette H, Boutier V, Coulombe C, Gray-Donald K (2002) Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: a prospective randomized community trial. J Am Dietet Assoc 102:1088–1095
Gazzotti C, Arnaud-Battandier F, Parello M, Farine S, Seidel L, Albert A et al (2003) Prevention of malnutrition in older people during and after hospitalisation: results from a randomised controlled clinical trial. Age Ageing 32:321–325
Hampson G, Martin FC, Moffat K, Vaja S, Sankaralingam S, Cheung J et al (2003) Effects of dietary improvement on bone metabolism in elderly underweight women with osteoporosis: a randomised controlled trial. Osteop Int 14:750–756
Edington J, Barnes R, Bryan F, Dupree E, Frost G, Hickson M et al (2004) A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: clinical and health economic outcomes. Clin Nutr 23:195–204
Lauque S, Arnaud-Battandier F, Gillette S, Plaze JM, Andrieu S, Cantet C et al (2004) Improvement of weight and fat-free mass with oral nutritional supplementation in patients with Alzheimer’s disease at risk of malnutrition: a prospective randomized study. J Am Geriatr Soc 52:1702–1707
Price R, Daly F, Pennington CR, McMurdo ME (2005) Nutritional supplementation of very old people at hospital discharge increases muscle strength: a randomised controlled trial. Gerontology 51:179–185
Salas-Salvado J, Torres M, Planas M, Altimir S, Pagan C, Gonzalez ME et al (2005) Effect of oral administration of a whole formula diet on nutritional and cognitive status in patients with Alzheimer’s disease. Clin Nutr 24:390–397
Nutritional intervention in malnourished elderly patients. 2006. https://clinicaltrialsgov/show/NCT00417508
A clinical trial to determine outcomes and cost effectiveness of nutritional intervention in elderly patients. 2006. https://clinicaltrialsgov/show/NCT00316966
Persson M, Hytter-Landahl A, Brismar K, Cederholm T (2007) Nutritional supplementation and dietary advice in geriatric patients at risk of malnutrition. Clin Nutr 26:216–224
Rabadi MH, Coar PL, Lukin M, Lesser M, Blass JP (2008) Intensive nutritional supplements can improve outcomes in stroke rehabilitation. Neurology 71:1856–1861
Smoliner C, Norman K, Scheufele R, Hartig W, Pirlich M, Lochs H (2008) Effects of food fortification on nutritional and functional status in frail nursing home residents at risk of malnutrition. Nutrition 24:11–12
Chapman IM, Visvanathan R, Hammond AJ, Morley JE, Field JB, Tai K et al (2009) Effect of testosterone and a nutritional supplement, alone and in combination, on hospital admissions in undernourished older men and women. Am J Clin Nutr 89:880–889
McMurdo ME, Price RJ, Shields M, Potter J, Stott DJ (2009) Should oral nutritional supplementation be given to undernourished older people upon hospital discharge? A controlled trial. J Am Geriatr Soc 57:2239–2245
Bonnefoy M, Laville M, Ecochard R, Jusot JF, Normand S, Maillot S et al (2010) Effects of branched amino acids supplementation in malnourished elderly with catabolic status. J Nutr Health Aging 14:579–584
Ha L, Hauge T, Iversen PO (2010) Body composition in older acute stroke patients after treatment with individualized, nutritional supplementation while in hospital. BMC Geriatr 10:75
Ha L, Hauge T, Spenning AB, Iversen PO (2010) Individual, nutritional support prevents undernutrition, increases muscle strength and improves QoL among elderly at nutritional risk hospitalized for acute stroke: a randomized, controlled trial. Clin Nutr 29:567–573
A multi-component behavioral nutrition intervention for homebound elderly. 2010. https://clinicaltrialsgov/show/NCT01197768 2010
Cameron ID, Kurrle SE, Uy C, Lockwood KA, Au L, Schaafsma FG (2011) Effectiveness of oral nutritional supplementation for older women after a fracture: rationale, design and study of the feasibility of a randomized controlled study. BMC Geriatr 11:32
Feldblum I, German L, Castel H, Harman-Boehm I, Shahar DR (2011) Individualized nutritional intervention during and after hospitalization: the nutrition intervention study clinical trial. J Am Geriatr Soc 59:10–17
Effects of a nutritional supplementation on the functional status of frail elders with low socioeconomic status (ses). 2011. https://clinicaltrialsgov/show/NCT01404299
Arija V, Martin N, Canela T, Anguera C, Castelao AI, Garcia-Barco M et al (2012) Nutrition education intervention for dependent patients: protocol of a randomized controlled trial. BMC Publ Health 12:373
Holyday M, Daniells S, Bare M, Caplan GA, Petocz P, Bolin T (2012) Malnutrition screening and early nutrition intervention in hospitalised patients in acute aged care: a randomised controlled trial. J Nutr Health Aging 16:562–568
Beck AM, Kjaer S, Hansen BS, Storm RL, Thal-Jantzen K, Bitz C (2013) Follow-up home visits with registered dietitians have a positive effect on the functional and nutritional status of geriatric medical patients after discharge: a randomized controlled trial. Clin Rehab 27:483–493
Leslie WS, Woodward M, Lean ME, Theobald H, Watson L, Hankey CR (2013) Improving the dietary intake of under nourished older people in residential care homes using an energy-enriching food approach: a cluster randomised controlled study. J Hum Nutr Dietet 26:387–394
Kim CO, Lee KR (2013) Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: a community-based randomized controlled study. J Gerontol Biol Med Sci 68:309–316
Locher JL, Vickers KS, Buys DR, Ellis A, Lawrence JC, Newton LE et al (2013) A randomized controlled trial of a theoretically-based behavioral nutrition intervention for community elders: lessons learned from the Behavioral Nutrition Intervention for Community Elders Study. J Ac Nutr Dietet 113:1675–1682
Schilp J, Kruizenga HM, Wijnhoven HA, van Binsbergen JJ, Visser M (2013) Effects of a dietetic treatment in older, undernourished, community-dwelling individuals in primary care: a randomized controlled trial. Eur J Nutr 52:1939–1948
Schilp J, Bosmans JE, Kruizenga HM, Wijnhoven HAH, Visser M (2014) Is dietetic treatment for undernutrition in older individuals in primary care cost-effective? JAMDA 15:226
Stange I, Bartram M, Liao Y, Poeschl K, Kolpatzik S, Uter W et al (2013) Effects of a low-volume, nutrient- and energy-dense oral nutritional supplement on nutritional and functional status: a randomized, controlled trial in nursing home residents. JAMDA 14(628):e621-628
Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, Germain C, Blanc JF, Dauba J et al (2014) Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS ONE 9:e108687
Beck A, Andersen UT, Leedo E, Jensen LL, Martins K, Quvang M et al (2015) Does adding a dietician to the liaison team after discharge of geriatric patients improve nutritional outcome: a randomised controlled trial. Clin Rehab 29:1117–1128
Jobse I, Liao Y, Bartram M, Delantonio K, Uter W, Stehle P et al (2015) Compliance of nursing home residents with a nutrient- and energy-dense oral nutritional supplement determines effects on nutritional status. J Nutr Health Aging 19:356–364
Pouyssegur V, Brocker P, Schneider SM, Philip JL, Barat P, Reichert E et al (2015) An innovative solid oral nutritional supplement to fight weight loss and anorexia: open, randomised controlled trial of efficacy in institutionalised, malnourished older adults. Age Ageing 44:245–251
Pouyssegur V, Antoine V, Castelli C, Chkair S, Bouvet S (2017) Solid oral supplementation: economic assessment. Economic impact of the introduction of a solid oral nutritional supplement adapted to malnourished older adults with poor dental health. Eur Geriatr Med. 8:234–239
Stow R, Ives N, Smith C, Rick C, Rushton A (2015) A cluster randomised feasibility trial evaluating nutritional interventions in the treatment of malnutrition in care home adult residents. Trials 16:433
Tylner S, Cederholm T, Faxen-Irving G (2016) Effects on weight, blood lipids, serum fatty acid profile and coagulation by an energy-dense formula to older care residents: a randomized controlled crossover trial. JAMDA 17(275):e275–e281
Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL et al (2016) Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr 35:18–26
Evaluating the efficacy of a novel oral supplement in tackling malnutrition in the elderly. 2016. https://clinicaltrialsgov/show/NCT02683720
Pedersen JL, Pedersen PU, Damsgaard EM (2016) Early nutritional follow-up after discharge prevents deterioration of adl functions in malnourished, independent, geriatric patients who live alone—a randomized clinical trial. J Nutr Health Aging 20:845–853
Van Wymelbeke V, Brondel L, Bon F, Martin-Pfitzenmeyer I, Manckoundia P (2016) An innovative brioche enriched in protein and energy improves the nutritional status of malnourished nursing home residents compared to oral nutritional supplement and usual breakfast: FARINE+ project. Clin Nutr ESPEN 15:93–100
Buys DR, Campbell AD, Godfryd A, Flood K, Kitchin E, Kilgore ML et al (2017) Meals enhancing nutrition after discharge: findings from a pilot randomized controlled trial. J Acad Nutr Dietet 117:599–608
Fernandez-Barres S, Garcia-Barco M, Basora J, Martinez T, Pedret R, Arija V et al (2017) The efficacy of a nutrition education intervention to prevent risk of malnutrition for dependent elderly patients receiving Home Care: a randomized controlled trial. Int J Nursing Stud 70:131–141
Effects of protein and medium-chain triglycerides-enriched milk on rehabilitation outcomes in malnourished older patients with hip fracture: a randomized controlled trial. 2017. http://wwwwhoint/trialsearch/Trial2aspx?TrialID=JPRN-UMIN000028715
Lindegaard Pedersen J, Pedersen PU, Damsgaard EM (2017) Nutritional follow-up after discharge prevents readmission to hospital—a randomized clinical trial. J Nutr Health Aging 21:75–82
The nutritional health for the elderly reference centre study (The NHERC Study). 2017.https://clinicaltrialsgov/show/NCT03245047
Intervention for the elderly with malnutrition, hidden hunger and low skeletal muscle mass. 2018. https://clinicaltrialsgov/show/NCT03648580 2018
Vicente de Sousa O, Soares Guerra R, Sousa AS, Pais Henriques B, Pereira Monteiro A, Amaral TF (2017) Impact of nutritional supplementation and a psychomotor program on patients with Alzheimer’s disease. Am J Alzheim Dis Other Dementias 32:329–341
Ballesteros-Pomar MD, Martinez Llinas D, Goates S, Sanz Barriuso R, Sanz-Paris A (2018) Cost-effectiveness of a specialized oral nutritional supplementation for malnourished older adult patients in Spain. Nutrients 10:22
Park Y, Choi JE, Hwang HS (2018) Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 108:1026–1033
Terp R, Jacobsen KO, Kannegaard P, Larsen AM, Madsen OR, Noiesen E (2018) A nutritional intervention program improves the nutritional status of geriatric patients at nutritional risk-a randomized controlled trial. Clin Rehab 32:930–941
Buondonno I, Sassi F, Carignano G, Dutto F, Ferreri C, Pili FG et al (2019) From mitochondria to healthy aging: the role of branched-chain amino acids treatment: MATeR a randomized study. Clin Nutr 18:18
Loman BR, Luo M, Baggs GE, Mitchell DC, Nelson JL, Ziegler TR et al (2019) Specialized high-protein oral nutrition supplement improves home nutrient intake of malnourished older adults without decreasing usual food intake. J Parent Ent Nutr 43:794–802
A randomized controlled trial on the effect of pre-thickened oral nutritional supplements on nutritional status of nursing home residents with dysphagia and (at risk of) malnutrition. 2019. http://wwwwhoint/trialsearch/Trial2aspx?TrialID=NL7898
Yang PH, Lin MC, Liu YY, Lee CL, Chang NJ (2019) Effect of nutritional intervention programs on nutritional status and readmission rate in malnourished older adults with pneumonia: a randomized control trial. Int J Environ Res Public Health 16:27
Söderström L, Rosenblad A, Bergkvist L, Frid H, Thors AE (2020) Dietary advice and oral nutritional supplements do not increase survival in older malnourished adults: a multicenter randomised controlled trial. Ups J Med Sci 125:240–249
Otsuki I, Himuro N, Tatsumi H, Mori M, Niiya Y, Kumeta Y et al (2020) Individualized nutritional treatment for acute stroke patients with malnutrition risk improves functional independence measurement: a randomized controlled trial. Geriatr Gerontol Int 20:176–182
Correa-Pérez A, Lozano-Montoya I, Volkert D, Visser M, Cruz-Jentoft AJ (2018) Relevant outcomes for nutrition interventions to treat and prevent malnutrition in older people: a collaborative senator-ontop and manuel delphi study. Eur Geriatr Med 9:243–248
Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S et al (2015) Diagnostic criteria for malnutrition—an ESPEN Consensus Statement. Clin Nutr 34:335–340
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T et al (2019) GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr 38:1–9
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest and no competing interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Visser, M., Mendonça, N., Avgerinou, C. et al. Towards developing a Core Outcome Set for malnutrition intervention studies in older adults: a scoping review to identify frequently used research outcomes. Eur Geriatr Med 13, 867–879 (2022). https://doi.org/10.1007/s41999-022-00617-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-022-00617-5