Abstract
Background
Among various nutrients branched amino acids (BCAAS) have been shown to be the most responsible for the stimulation of protein synthesis in various situations including catabolic states.
Objectives
We evaluated the effect of a small amount of proteins enriched with BCAAs (0.4g/kg/day and 0.2g/kg/day BCAAs) on body weight and composition; nitrogen balance, energy intake and inflammation after 2 weeks of supplementation in acute elderly with catabolic status.
Design
Two weeks randomized controlled trial.
Setting
Geriatric department of teaching hospital.
Subjects
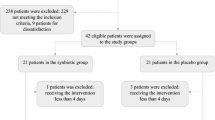
Thirty patients with malnutrition and inflammatory process (MNA < 24, albumin < 30 g/l and CRP ≥ 20 mg/l) who agreed to participate in the study were consecutively included.
Methods
Body composition was determined by labelled water dilution method; resting energy expenditure (REE) was determined by indirect calorimetry; energy intake was calculated for a 3 days period at D1 and D12. Nutritional and inflammatory proteins and cytokines (IL-6 and TNF) were measured at day 1 and 14.
Results
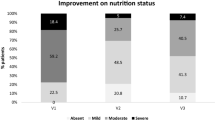
No difference was observed at day 14 between supplemented (S) and control (C) group for weight (S: 58.0 ± 11.8 kg and C: 60.0 ± 15.9 kg); fat free mass (S: 40.7 ± 8.3 kg and C: 40 ± 8.2 kg); nitrogen balance (S: 1.34 ± 2.21 g/day and C: 0.59 ± 4.47 g/day); and energy intake (S: 20 ± 3.6 kcal/day and C: 20.5 ± 8.6 kcal/day). Energy intake was at similar level than REE and clearly less than energy requirement in C and S. A significant decrease was observed for orosomucoid and Prognostic Inflammatory and Nutritional Index (PINI) in S.
Conclusion
Our results do not confirm improvement of nutritional status with enriched BCAAs supplementation as suggested in the literature. Persistence of inflammatory condition may be an explanation despite an improvement of inflammatory status was observed in the supplemented group. Those results show clearly that energy requirements are not covered in acute hospitalized elderly people. The fact that not only energy intake but also REE are decreased brings a new insight on catabolic situations.
Similar content being viewed by others
References
Gaillard C, Alix E, Boirie Y, Berrut G, Ritz P. Are elderly hospitalized patients getting enough protein? J Am Geriatr Soc 2008, 56:1045–1049
Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 2006, 91:4836–4841.
Attaix D, Mosoni L, Dardevet D, Combaret L, Patureau Mirand P, Grizard J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. The International Journal of Biochemistry & Cell Biology 2005, 37:1962–1973.
Morley JE. Anorexia and weight loss in older persons. J Gerontol Ser A Biol Sci Med Sci 2003; 58(2):131–137.
Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care 2005; 8:61–65.
Pasini A, Aquilani R, Dioguardi FS. Amino acids: chemistry and metabolism in normal and hypercatabolic states. Am J Cardiol 2004; 93:3–5.
Choudry HA, Pan M, Karinch AM, Souba WW. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr 2006; 136:314–318.
Fiatarone MA, O’Neil EF, Doyle R, Clements KM, Solares GR, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine 1994; 330:1769–1775.
Fujita S, Volpi E. Amino acids and muscle loss with aging. J Nutr 2006, 136:277–280.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006; 291:381–387.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005; 82:1065–1073.
Boelens PG, Nijveldt RJ, Houdjik AP, Meijer S, van Leeuwen PA. Glutamine alimentation in catabolic state. J Nutr 2001; 131:2569S–2577S.
Poon RT, Yu WC, Fan ST, Wong J. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Aliment Pharmacol Ther 2004; 19(7):779–788.
Stein TP, Donaldson MR, Leskiw MJ, Schluter MD. Branched-chain amino acid supplementation during bed rest: effect on recovery. J Appl Physiol 2003; 94:1345–1352.
Vellas B, Guigoz Y, Baumgartner M, Garry PJ, Lauque S, Albarede JL. Relationship between nutritional markers and the mini-nutritional assessment in 155 older persons. Journal of the American Geriatrics Society 2000; 48:1300–1309.
Bonnefoy M, Ayzac L, Ingenbleek Y, Kostka T, Boisson RC, Bienvenu J. Usefulness of the prognostic inflammatory and nutritional index (PINI) in hospitalized elderly patients. Internat. J. Vit. Nutr. Res. 1998; 68:189–195.
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198.
Fuentes-Orozco C, Anaya-Prado R, Gonzalez-Ojeda A, Arenas-Marquez H, Cabrera-Pivaral C, Cervantes-Guevara G, Barrera-Zepeda L, L-Alanyl-Lglutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clinical Nutrition 2004; 23:13–21
Harris JA, Benedict FG. A biometric study of basal metabolism. Publication N 279. Carnegie Bulletin of Washington, Washington, DC, 1919.
Arock M, Desnault H, Viars P & Guillosson J. Dosage of total N2 in biological environments by chemiluminescence comparison with the reference method). Annales de Biologie Clinique 1985, 43:872–874.
Frayn K. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Applied Physiology 1983; 55:628–634.
Schoeller D. A, Van Santen E., Peterson D.W, DIETZ W., Jaspan J., Klein P.D: Total body water measurement in humans with 18O and 2H labeled water. Am.J.Clin.Nutr 1980; 33:2686–2693.
Arkouche W., Fouque D., Pachiaudi C., Normand S, Laville M., Delawari E., Riou J.P., Traeger J., Laville M.: Total body water and body composition in chronic peritoneal dialysis patients. J. Am.Soc. Nephrology 1997; 8:1906–1914.
Coward WA. Calculation of pool size and flux rates. In the doubly labelled water method for meauring energy expenditure [AM Prentice, editor]. Vienna, Austria: International Atomic Agency.
Blanc S, Normand S, Ritz P, Pachiaudi C, Vico L, Gharib C, Gauquelin-Koch G: Energy and water metabolism, body composition and hormonal changes induced by 42 days of head down bed rest. J. Clin. Endocrinol Metab 1998; 83:4289–4297.
Monk DN, Plank LD, Franch-Arcas G, Finn PH, Streat SJ, Hill GL. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg 1996; 223:395–405.
Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab 2004, 10. 1210/jc2004-1702.
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A 1997; 94:14930–14935.
Mahe S, Roos N, Benamouzig R, Davin L, Luengo C, Gagnon L, Gausserges N, Rautureau J, Tome D. Gastrojejunal kinetics and the digestion of [15N] betalactoglobulin and casein in humans: the influence of the nature and quantity of the protein. Am J Clin Nutr 1996; 63:546–552.
Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a doseresponse study. J Physiol 2003; 552:315–324.
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signalling deficits underlie amino acid resistance of wasting, aging muscle. The FASEB Journal 2004; 10.1096/fj.04-2640fje.
Rennie MJ, Bohé J, Smith K, Wackerhage H, Greenhaff P. Branched-chain amino acids as fuels and anabolic signals in human. J Nutr 2006, 136:264–268.
Borsheim E., Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol 2004; 96:674–678.
Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000; 85:4481–4490.
Guillet Ch, Prod’homme M, Balage M; Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impairment anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004; 18:1586–1587.
Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Experimental Gerontology 2006; 41:215–219.
Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol Dial Transplant 2001; 16:1856–1862.
Gaillard C; Alix E, Sallé A, Berrut G, Ritz P. Energy requirements in frail elderly people: a review of the literature. Clinical Nutrition 2007, 26:16–24.
Das SK, Moriguti JC, McCrory MA, et al. An underfeeding study in healthy men and women provides further evidence of impaired regulation of energy expenditure in old age. J Nutr 2001; 131(6):1833–1838.
WHO 1985. Energy and protein requirements: report of a joint FAO/WHO/UNU expert consultation. WHO Technical Report Series No 724.
Alix E, Berrut G, Boré M, Bouthier-Quintard F, Buia JM, Chlala A, Cledat Y, d’Orsay G, Lavigne C, Levasseur R, Mouzet JB, Ombredanne MP, Sallé A, Gaillard C, Ritz P. Energy requirements in hospitalized elderly people. J Am Geriatr Soc 2 2007, 55:1085–1089.
Ohno T, Tanaka Y, Sugauchi F, Orito E, Hasegawa I, Nukaya H, Kato A, Matunaga S, Endo M, Tanaka Y, Sakakibara K, Mizokami M. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatol Res. 2008; 38(7):683–688.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonnefoy, M., Laville, M., Ecochard, R. et al. Effects of branched amino acids supplementation in malnourished elderly with catabolic status. J Nutr Health Aging 14, 579–584 (2010). https://doi.org/10.1007/s12603-010-0090-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-010-0090-1