Abstract
Background
Antimicrobial resistance (AMR) is one of the most serious public health challenges worldwide, including in Japan. Globally, research and development of new antimicrobials has stalled due to unfavorable market conditions, which undervalue antimicrobials. Furthermore, Japan faces the additional challenge of delayed commercialization for a number of recently approved treatments.
Objective
This study aims to examine the impact on AMR of introducing a new anti-infective treatment, ceftazidime/avibactam, into current treatment strategies. It reports the resulting clinical and economic outcomes from the perspective of healthcare payers in Japan.
Methods
A previously published and validated dynamic disease transmission model was adapted to the Japanese setting. The model estimated health economic outcomes for treating three Gram-negative hospital-acquired infections, under different treatment strategies, from a healthcare payers’ perspective. Outcomes were assessed over a 10-year time horizon with a willingness-to-pay threshold of ¥5,000,000 (US$45,556) per quality-adjusted life-year (QALY) gained and an annual discount rate of 2% applied to costs and benefits.
Results
Introducing ceftazidime/avibactam in the framework of a diversification strategy with piperacillin/tazobactam is associated with reducing 798,640 bed days, equating to ¥21.0 billion (US$190.9 million) savings in hospitalization costs, and a gain of 363,034 life-years, or 308,641 QALYs. This translates into a monetary benefit of ¥1.56 trillion (US$14.3 billion) to Japanese healthcare payers.
Discussion
Introducing a new antimicrobial agent into clinical practice is associated with considerable clinical and economic benefits. This analysis demonstrates that the approach taken to incorporate a new antimicrobial agent into clinical practice impacts on the scale of these clinical and economic benefits; greater benefits are associated with earlier use of antimicrobials as part of an antimicrobial stewardship program.
Conclusion
This analysis shows that changing the way in which a new antimicrobial is used within a treatment strategy has the potential for additional significant clinical and economic value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Antimicrobial resistance (AMR) is a significant and growing healthcare problem, globally and in Japan. The development and effective introduction of new antimicrobials into clinical practice is essential in combatting increasing AMR. |
The greatest economic and clinical benefits, from the perspective of healthcare payers, can be realized by introducing new antimicrobials such as ceftazidime/avibactam as a first-line treatment within an antimicrobial stewardship scheme. |
Both health economic benefits and resistance development need to be considered by decision makers when making recommendations regarding the use of new as well as currently available antimicrobials. |
1 Introduction
Antimicrobial resistance (AMR) poses one of the biggest global threats to modern healthcare, as greater levels of resistance increasingly limit the options to treat and prevent infections. If current trends in AMR continue, the annual number of deaths from infections globally is estimated to rise from 700,000 to 10 million, surpassing deaths from cancer, by 2050 [1].
In response to the WHO Global Action Plan on Antimicrobial Resistance [2], in 2016 the Japanese government published the National Action Plan on AMR which has contributed to the reduction of antimicrobial use in Japan [3]. However, globally, the development of antimicrobials has slowed and the current clinical pipeline is insufficient to address the demands of increasing AMR [4]. In Japan, this is further exacerbated by the slower uptake of newly approved antimicrobials following approval, compared with the USA and other high income European countries [5]. Antimicrobial stewardship (AMS) schemes promote appropriate use of antimicrobials, whereby more effective treatments are often reserved for highly resistant infections, resulting in low sales volumes and revenues. Therefore, manufacturers may decide to defer commercialization until it becomes financially viable or choose to abandon particular markets completely [5].
To stimulate research and development (R&D) and effectively integrate new antimicrobials into clinical practice, incentive structures are required. Incentives are broadly described as either ‘push’ or ‘pull’ measures [6]. ‘Push’ mechanisms provide financial support for preclinical R&D of new antimicrobials, and ‘pull’ mechanisms are characterized as a reward for successfully developing a new treatment [5]. Novel reimbursement models, such as those proposed in England and the USA, act as ‘pull’ incentives to ensure sufficient returns on investment. These models see payments made via a subscription between the government and pharmaceutical company, in which payments are fully de-linked from volume of sales and are instead based on the estimated value to patients and the health service [7, 8]. Sweden have introduced a partially de-linked model, where a minimum annual revenue is confirmed to guarantee limited stock. This deal can be described as a minimum supply agreement; however, it is unclear if such a model would incentivize R&D or enable antimicrobial stewardship as effectively as fully de-linked methods [9].
In Japan, such novel reimbursement models are absent, but there are growing calls to introduce them [10]. Understanding the true value of adding a new antimicrobial to existing treatment options can help health policy makers to arrive at a more informed assessment of their costs and benefits. In previous research we have demonstrated that there is potential for significant economic and clinical benefits through reducing projected AMR in Japan [11]. It has been widely documented that the use of heterogeneous antimicrobial agents has the capacity to reduce the rate of AMR through reduced selection pressure on organisms [12,13,14,15,16,17]. However, there is a paucity of evidence to show the impact of increasing antimicrobial diversity on healthcare costs, especially in Japan.
In this study, we aim to examine the effect of introducing ceftazidime/avibactam, currently unavailable in Japan, as part of an AMS strategy, in improving clinical and economic outcomes from the perspective of healthcare payers in Japan.
2 Methods
2.1 Overview
In the current study, we adapted a previously published and validated dynamic model of AMR [18] to estimate the impact of introducing a new antimicrobial into the current treatment strategy for the management of hospital acquired infections (HAIs) in Japan. The model considered complicated urinary tract infections (cUTIs), complicated intra-abdominal infections (cIAIs), and hospital-acquired pneumonia including ventilator-associated pneumonia (HAP/VAP). Causal pathogens in the model were Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), and Pseudomonas aeruginosa (P. aeruginosa). These pathogens were selected as they are often responsible for cUTIs, IAIs, and HAP/VAP infections [19,20,21], and are at risk for critical resistant organisms such as carbapenem-resistant Enterobacterales and multi-drug resistant P. aeruginosa (MDRP). Clinical and economic outcomes (life-years [LY], quality-adjusted life-years [QALYs], hospital length of stay [LOS], defined daily dose [DDD] of antibiotics, hospitalization costs, and monetary benefit) were estimated. The model also estimated the development of future AMR levels in modelled treatments. The perspective was that of healthcare payers in Japan, with QALYs valued at payers’ willingness to pay.
2.2 Model Structure
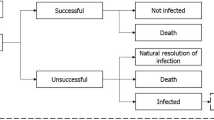
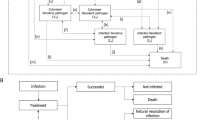
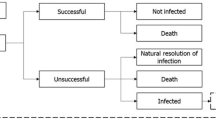
The dynamic disease transmission (a compartmental multi-state disease transmission model) and deterministic treatment pathway (decision tree) components of the model were used to produce regression equations of outcomes (time-on-treatment, death and resistance) based on an input-output dataset. The dynamic disease transmission component considered health state transitions within an infectious environment based on the prevalence and incidence of infection and colonization of patients with either resistant or sensitive pathogens. Newly infected patients enter the deterministic treatment pathway component (Supplementary Fig. 1), which considers the health economic impact based on the specified treatment strategy. All infected patients receive the first-line antimicrobial as they enter the treatment pathway where they are either cured (via successful treatment or natural resolution of the infection), remain infected, or die. It was assumed that patients who have exhausted all available antimicrobials, and their infections remain unresolved, die from infection 3 days after receiving their last treatment. Changes to resistance rates are captured within the regression equations based on the dynamic AMR model. Resistance development was based on exposure to treatment; resistance of pathogens to each treatment was considered individually and cross-resistance mechanisms aren’t specifically modelled. Detailed methodology of the previously published and validated dynamic model of AMR is available in published literature [18]. The input-output dataset was generated through over 1 million model permutations of model input combinations (population, baseline resistance, treatment strategy, treatment duration, and treatment efficacy) as outlined in Supplementary Table 1 (see electronic supplementary material [ESM]). Key model inputs including the probability of naturally resolving infection (3%) and daily mortality rates (cUTI: 0.0090939; cIAI: 0.0680878; HAP/VAP: 0.0296135; general population: 0.0000279) were kept consistent with the original AMR dynamic model and previous research, respectively [18]. The dynamic disease transmission component accounts for two non-traditional value components associated with antimicrobials, transmission value (value of avoiding the spread of AMR infection) and diversity value (the value of additional antimicrobials and AMS to lower resistance development by reducing selection pressure). Regression equations that embed the underlying transmission dynamics and prevalence of infection of the Japanese population were estimated using UK data; in this analysis it was assumed that the transmission dynamics in the UK are comparable with those in Japan.
The model considered outcomes in infected patients treated with up to three lines of treatment, in a specified order, with or without treatment diversification. It enabled comparisons to be made between alternative strategies with the introduction of a new antimicrobial agent, testing the impact of positioning and diversification strategies on health economic outcomes. In treatment diversification, the patient population was split evenly between either the first two or all three treatments, as their first-line treatment. Patients who are unsuccessfully treated then progress to receive the next treatment in the sequence.
2.3 Model Setting
The treatment scenario assumed to best represent the current management of the pathogens and indications being modelled was piperacillin/tazobactam as first-line treatment and meropenem as second-line treatment. These treatments were selected to best align with clinical practice in Japan, according to previous research [11], where piperacillin/tazobactam and meropenem are frequently used to manage Gram-negative HAIs. Analyses considered the introduction of ceftazidime/avibactam, which is currently unavailable in Japan, as a new antimicrobial agent. Ceftazidime/avibactam was considered due to the availability of publicly accessible data and, with clinical trials currently ongoing in Japan [22], there is an expectation that it will be launched in Japan within the next 2-3 years.
2.4 Data Sources
The annual number of infections was estimated through the use of a hospital-based claims database, Medical Data Vision (MDV) [23]. MDV has been covering Japanese public and private healthcare institutes since April 2003, accumulating >30 million patients. Data was extracted from inpatients aged 15 years and older on the MDV database with a diagnosis of interest (cUTI, cIAI and HAP/VAP). The total number of HAIs within each indication was multiplied by the probability of a HAI being due to each pathogen of interest (E. coli, K. pneumoniae, and P. aeruginosa) to estimate pathogen-specific infection incidence. Pathogen distributions for each infection indication were sourced from the literature [19,20,21], and are provided in Supplementary Table 2 (see ESM). The annual incidence of HAIs identified via MDV was scaled to account for the number of infections across Japan using a scaling factor of 6.039. This scaling factor was determined by the ratio of new hospitalized patients from the MDV database, compared with the number of new hospitalizations for the whole of Japan, over the period January to December 2019 [24]. Real-world data from 2019 was used to avoid the impact of the COVID-19 pandemic on healthcare data. The annual infected population and the distribution of pathogens is outlined in Table 1.
Pathogen-specific resistance levels in Japan for piperacillin/tazobactam and meropenem were informed by the Japan Nosocomial Infections Surveillance (JANIS) [25]. Due to the current lack of availability of ceftazidime/avibactam in Japan, resistance estimates for this combination were taken from Ko and Stone, an antimicrobial surveillance study in the Asia-Pacific region, including Japan (Table 2) [26]. For each intervention, treatment efficacy in treating infections with susceptible pathogens was estimated as a weighted average across the three indications (Table 1 and Supplementary Table 3, see ESM); treatment efficacy against susceptible infections was consistent regardless of treatment line. Data was sourced from randomized controlled trials identified in literature searches [27,28,29,30,31,32]. It was assumed that successful treatment of infections would require a LOS of 7 days, with 4 days for unsuccessful treatment followed by the next line of therapy (where available). Mortality was associated with an additional 3-day LOS. Assumptions regarding LOS were informed by the opinion of a local expert in infectious disease. Indication-specific hospitalization costs were estimated from the Japanese diagnosis procedure combinations (DPC) costs for 2020 [33]. DPC costs relate to diagnosis, treatment, and daily hospitalization costs specific to each indication. Acquisition and administration costs of treatments and the additional costs of managing adverse events were not considered in this analysis. Patient life expectancy was estimated as that of the general population with age equal to the mean age of the infected population, as given by Japanese life tables (22.13 years at 65–69 years old) [34, 35]. The mean population age (65–68 years old) was estimated as a weighted mean age of the indication-specific infected population using UK hospital admission data [35]; UK data was used as Japanese data was not available. The uninfected utility value was derived from Japanese population norms for a population aged 70 years and older [36]; the utility value during infection was estimated as a weighted average of values for each of the three indications, as sourced from the literature (Supplementary Table 3, see ESM) [37,38,39]. QALYs were calculated as life expectancy multiplied by utility.
Key model inputs are outlined in Table 1 and additional indication-specific inputs are available in Supplementary Table 3. Local inputs were sourced, where possible, to best reflect the AMR burden and clinical practice in Japan.
2.5 Cost-Effectiveness Analysis
The impact of introducing ceftazidime/avibactam was explored in two scenarios, comparing treatment strategies where the new agent was introduced at first and last (third)-line with and without diversification (50% of patients each initiating treatment with either first- or second-line treatments) as described in Table 3. Scenario 1 compared outcomes for ceftazidime/avibactam last-line without diversification (Strategy A) versus first-line without diversification (Strategy B) and scenario 2 compared ceftazidime/avibactam last-line without diversification (Strategy A) vs first-line with diversification (Strategy C). Analyses considered clinical and economic outcomes (LY, QALYs, LOS, hospitalization costs, DDD of antibiotics, and monetary benefit) over a 1-year and 10-year disease transmission horizon. Lifetime LYs and QALYs lost between treatment strategies over the disease transmission horizon were estimated based on LYs and QALYs lost due to infection (death and infection disutility), informed by the average life expectancy of the patient population. Monetary benefit was estimated using a willingness-to-pay (WTP) threshold of ¥5,000,000 (US$45,556) per QALY, in line with the most conservative thresholds and discounting used in Japanese health technology assessments (HTA) plus cost savings [40]. A disease transmission horizon of 10 years was selected to appropriately reflect the benefits that continually accrue over time, whilst considering the impact of increasing uncertainty of modelling over long time horizons. Costs and benefits were discounted at an annual rate of 2%, as recommended in Japanese HTA guidelines [41]. Japanese yen were converted to US dollars using the average rate in 2021 (US$1 = ¥109.754) published by the Organization for Economic Cooperation and Development, costs were not inflated [42].
2.6 Sensitivity Analysis
A series of one-way sensitivity analyses (OWSA) were conducted to assess the impact of uncertainty around key model input parameters. Model inputs listed in Table 1 were adjusted by ±20% in each scenario and resulting hospitalization costs and QALY outcomes reported.
3 Results
Absolute and incremental outcomes for the treatment strategies within each scenario are presented at 1 and 10 years (Table 4). Outcomes for analysis over a 5-year time horizon are presented in Supplementary Table 4 (see ESM).
In the two scenarios tested, ceftazidime–avibactam was used as last-line therapy (strategy A), to reflect the current use of new antimicrobials. The alternative strategies assessed the impact of introducing ceftazidime–avibactam as a first-line option without diversification (strategy B) in scenario 1 and with diversification (strategy C) in scenario 2. Over 10 years, the burden of the infected patient population under treatment strategy A is estimated at 62,616,390 hospital bed days, equating to ¥1.65 trillion (US$15.1 billion) in hospitalization costs, and 9,532,380 life-years lost over a lifetime due to infection, corresponding to 8,107,457 QALYs lost. LYs and QALYs are estimated over the lifetime of patients based on the number of infections over a 10-year period. Over 10 years, scenario 1 where ceftazidime–avibactam is used as a first-line treatment without diversification (strategy B) is estimated to avoid 382,067 bed days, equivalent to a saving of ¥10.1 billion (US$92.1 million) in hospitalization costs, and generate a gain of 239,513 life-years, corresponding to 203,616 QALYs. Further benefits are demonstrated in scenario 2, where ceftazidime–avibactam is introduced as a first-line treatment with diversification (strategy C). Over 10 years, 798,640 bed days, equal to a saving of ¥21.0 billion (US$191.0 million) in hospitalization costs, are avoided, while 363,034 life-years, corresponding to a gain in 308,641 QALYs, are gained. The associated monetary benefit achieved over a lifetime of using ceftazidime–avibactam as first-line therapy without and with diversification (strategy B and C, respectively), instead of last-line (strategy A), ranged from ¥1.03 trillion to ¥1.56 trillion (US$9.37 billion to US$14.3 billion) in scenarios 1 and 2, respectively, based on a WTP threshold of ¥5,000,000 (US$45,556) per QALY gained.
Development of resistance to current treatments and ceftazidime–avibactam for each treatment strategy are shown in Fig. 1. When ceftazidime–avibactam was introduced as a last-line therapy without diversification, in strategy A, resistance to piperacillin/tazobactam and meropenem increased by 55.4% (from 7.9 to 12.2%) and 83.7% (from 3.9 to 7.2%), respectively. Similar proportional gains in resistance were seen for ceftazidime–avibactam in strategy A (65.2% increase, from 2.6 to 4.4%). Conversely, when ceftazidime–avibactam was used at first-line with diversification (strategy C), resistance gains for ceftazidime–avibactam were substantially greater than those observed in strategy A. Indeed, resistance to piperacillin/tazobactam and meropenem increased by 40.8% (from 7.9 to 11.1%) and 48.6% (from 3.9 to 5.8%), respectively. Resistance to ceftazidime–avibactam increased by 159.0% (from 2.6 to 6.8%); however, at 10 years, the absolute resistance to ceftazidime–avibactam was still lower than piperacillin/tazobactam and meropenem in strategy A.
3.1 Sensitivity Analysis
In the one-way sensitivity analysis (OWSA) for scenarios 1 and 2, estimates for probability of treatment success in non-resistant infections had the greatest influence on health economic outcomes. Hospitalization cost savings ranged from − ¥38.5 billion to ¥58.7 billion (− US$350.8 million to US$534.8 million) in scenario 1 and −¥27.7 billion to ¥69.6 billion (− US$252.0 million to US$633.8 million) in scenario 2. Gains in QALYs ranged from − 519,586 to 926,818 and − 414,561 to 1,031,843, in scenarios 1 and 2, respectively (Fig. 2).
4 Discussion
The analysis reported in this study demonstrated that the approach taken when introducing a new antimicrobial agent into the current treatment strategy for HAIs can have a considerable impact on clinical and economic outcomes, from the perspective of healthcare payers in Japan. Our analysis showed that the greatest benefits can be achieved when a new antimicrobial agent is introduced as a first-line treatment, with diversification. This approach is associated with a monetary benefit of ¥1.56 trillion (US$14.3 billion) over 10 years, when compared with introducing a new antimicrobial as a last resort without diversification. In this scenario, the significant clinical and economic benefits are a product of limiting exposure to existing treatments and thus slowing the development of resistance to them, through including a new antimicrobial earlier into treatment strategies. Whilst this analysis shows the greatest health economic gains can be recognized by introducing a new antimicrobial in the first line, in practice, a pragmatic approach must be taken to limit the development of resistance to both current and new antimicrobials. The monetary benefit of ¥1.56 trillion (US$14.3 billion) represents the overall clinical and economic benefits expressed in financial terms to Japanese healthcare payers according to the Japanese WTP threshold. This analysis considers population-level benefits in terms of reduced hospitalization costs and LY gains, driven by differences in treatment efficacy and resistance levels. Acquisition and administration costs of treatments and in managing adverse events were not included as we did not demonstrate cost effectiveness but monetary benefit.
The introduction of a new antimicrobial needs to take place within the framework of an AMS strategy. AMS programs are essential to preserve the efficacy of current treatment options and optimize clinical outcomes for patients [43]. The development of rapid diagnostics allows for more pathogen-specific antibiotic usage while reducing the usage of broad-spectrum antibiotics. Treating patients with the right treatment at the right time reduces overall antibiotic consumption and improves patient outcomes, through more effective AMS [44, 45]. Paradoxically, AMS programs, which aim to reduce the use of current antimicrobials and often preserve new ones, have been blamed for the commercial failure of the current antimicrobial market [46]. However, we believe that AMS will be intrinsic to the clinical adoption and commercial success of pathogen-specific antibiotics against resistant infections.
Introducing ceftazidime–avibactam led to reductions in projected AMR after 10 years in piperacillin/tazobactam and meropenem; however, these observed changes were not considerable. Reducing selection pressure on infectious pathogens through reduced antimicrobial use is only one component of AMS programs, and resistance rates are driven by a number of additional external factors [47, 48]. Our analysis showed a diversification strategy of antibiotic mixing had little impact on resistance (Fig. 1), and overall treatment sequence had a greater impact. These findings correlate with research which suggests diversification strategies alone may not be effective in reducing resistance [49, 50]. Because of a paucity of clinical data supporting the use of antibiotic mixing, it is not recommended in antibiotic stewardship guidelines [40].
Numerous studies have previously estimated the burden of resistant bacterial infections in hospitals; however, there are a number of significant differences between these studies, such as the pathogens and indications investigated and the setting and perspective considered, that limit the ability to compare outcomes with our current study [51,52,53,54,55]. In research conducted previously by ourselves, we estimated the cost of resistant infections over 10 years, to healthcare payers in Japan, across the same indications, to be ¥18.1 trillion (US$169.8 billion) [11]. To our knowledge, no studies have been published that assess the health economic impact of changing the treatment sequence of antimicrobial agents. In the context of our previous research, the value to healthcare payers in optimizing treatment sequences as presented in this current study is considerable, equivalent to nearly 10% of the burden of resistant infections, over 10 years.
As with all modelling studies, there are some limitations to our estimates that should be addressed. Firstly, this study only considers three HAI indications caused by three Gram-negative pathogens; therefore, the true impact of introducing a new antimicrobial is likely to be larger than our more conservative estimates. Hospitalization costs within the model are estimated using DPC costs; hospitals in Japan reimbursed with a DPC approach are typically acute hospitals, whilst small and middle-sized non-acute hospitals opt for fee-for-service. Secondly, transmission dynamics embedded in the regression equations were based on UK hospital data of AMR development. These dynamics were assumed to reflect Japanese dynamics. Additionally, the model considers a treatment sequence of three antimicrobial agents; this is a simplification and in practice there may be more treatments available, but this was considered by clinical experts to be reflective of the modelled indications for which limited treatments are available. Furthermore, in our previous study, analysis of MDV showed that in the modeled indications piperacillin/tazobactam and meropenem were most frequently used in Japan [11]. Finally, Japanese-specific estimates of antimicrobial resistance for piperacillin/tazobactam and meropenem were sourced from the JANIS database; however, Asia-Pacific data had to be used for ceftazidime–avibactam. This limitation was unavoidable as ceftazidime–avibactam had not been launched in Japan and JANIS data was not available. Similarly, for some parameters where local data was not available, sources relating to alternative countries or Japanese clinical experts were used. Because of limited available data, analysis assumed that uninfected patients had a utility and life expectancy aligned to the general population, when it can be expected patients with HAIs are more vulnerable patients with co-morbidities. However, these inputs were shown to not have a significant influence on hospitalization costs or QALYs when explored in sensitivity analysis.
The stalling of new antimicrobial agent development is a significant factor worldwide as the AMR problem continues to grow. It is hoped that, through novel reimbursement models and incentive strategies, research and development of new antimicrobial agents will be brought forward, leading to a renewed pipeline of novel treatments effectively delivered to global markets.
5 Conclusion
This analysis suggests that changing the treatment strategy when introducing ceftazidime–avibactam as a new antimicrobial agent in Japan, as part of AMS programs, can make an important contribution to alleviating the impact of AMR and to the generation of clinical and economic value to healthcare payers in Japan.
References
Jim ON. Tackling drug-resistant infections globally: final report and recommendations. 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf. Accessed 13 Dec 2021.
World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1. Accessed 13 Dec 2021.
Kusama Y, Tsuzuki S, Muraki Y, Koizumi R, Ishikane M, Ohmagari N. The effects of Japan’s National Action Plan on Antimicrobial Resistance on antimicrobial use. Int J Infect Dis. 2021;103:154–6.
World Health Organisation. 2021 Antibacterial agents in clinical and preclinical development: an overview and analysis. 2022. https://www.who.int/publications/i/item/9789240047655. Accessed 1 Jul 2022.
Outterson K, Orubu ESF, Rex J, Årdal C, Zaman MH. Patient access in fourteen high-income countries to new antibacterials approved by the FDA, EMA, PMDA, or Health Canada, 2010–2020. Clin Infect Dis. 2021. https://doi.org/10.2139/ssrn.3825520.
Outterson K. Estimating the appropriate size of global pull incentives for antibacterial medicines. Health Aff. 2021;40(11):1758–65.
Department of Health and Social Care; National Institute for Health Care Excellence and National Health Service. Models for the evaluation and purchase of antimicrobials. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials. 2022. Accessed 13 Aug 2022.
H. R. 3932—117th Congress: The PASTEUR Act of 2021. 2021. https://www.congress.gov/bill/117th-congress/house-bill/3932. Accessed 13 Aug 2022.
Gotham D, Moja L, van der Heijden M, Paulin S, Smith I, Beyer P. Reimbursement models to tackle market failures for antimicrobials: Approaches taken in France, Germany, Sweden, the United Kingdom, and the United States. Health Policy. 2021;125(3):296–306.
AMR Alliance Japan. AMR Alliance Japan Policy Recommendations: The Japanese Government’s Role in Promoting AMR Countermeasures. https://www.amralliancejapan.org/en/goals/. Accessed 13 Dec 2021.
Matsumoto T, Darlington O, Miller R, et al. Estimating the economic and clinical value of reducing antimicrobial resistance to three gram-negative pathogens in Japan. J Health Econ Outcomes Res. 2021;8(2):64.
Piper GL, Kaplan LJ. Antibiotic heterogeneity optimizes antimicrobial prescription and enables resistant pathogen control in the intensive care unit. Surg Infect (Larchmt). 2012;13(4):194–202.
Plüss-Suard C, Pannatier A, Kronenberg A, Mühlemann K, Zanetti G. Impact of antibiotic use on carbapenem resistance in Pseudomonas aeruginosa: is there a role for antibiotic diversity? Antimicrob Agents Chemother. 2013;57(4):1709–13.
Sandiumenge A, Diaz E, Rodriguez A, et al. Impact of diversity of antibiotic use on the development of antimicrobial resistance. J Antimicrob Chemother. 2006;57(6):1197–204.
Sandiumenge A, Lisboa T, Gomez F, Hernandez P, Canadell L, Rello J. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE Organisms. Chest. 2011;140(3):643–51.
Takesue Y, Nakajima K, Ichiki K, et al. Impact of a hospital-wide programme of heterogeneous antibiotic use on the development of antibiotic-resistant Gram-negative bacteria. J Hosp Infect. 2010;75(1):28–32.
Takesue Y, Ohge H, Sakashita M, et al. Effect of antibiotic heterogeneity on the development of infections with antibiotic-resistant gram-negative organisms in a non-intensive care unit surgical ward. World J Surg. 2006;30(7):1269–76.
Gordon J, Darlington O, McEwan P, et al. Estimating the value of new antimicrobials in the context of antimicrobial resistance: development and application of a dynamic disease transmission model. Pharmacoeconomics. 2020;38(8):857–69.
Japanese Respiratory Society. Adult Pneumonia Clinical Practice Guidelines 2017. https://www.jrs.or.jp/modules/guidelines/index.php?content_id=94. Accessed 13 Dec 2021.
Kobayashi K, Yamamoto S, Takahashi S, et al. The third national Japanese antimicrobial susceptibility pattern surveillance program: bacterial isolates from complicated urinary tract infection patients. J Infect Chemother. 2020;26(5):418–28.
Mikamo H, Takesue Y, Kusachi S, Kotaka M, Kawachi Y, Aikawa N. An open-label multicenter study of tazobactam/piperacillin in the treatment of patients with intra-abdominal infections. Jpn J Chemotherapy. 2012;60:560–72.
Clinicaltrials.gov. Study to Assess Efficacy and Safety of PF-06947386 in Japanese Adult Patients With Complicated Intra-abdominal Infection. National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/study/NCT04927312. Accessed 13 Dec 2021.
Medical Data Vision. https://en.mdv.co.jp/. Accessed 13 Dec 2021.
Ministry of Health, Labour and Welfare. Medical facility survey/hospital report (summary of results). https://www.mhlw.go.jp/toukei/list/79-1a.html. Accessed 13 Dec 2021.
Japan Nosocomial Infections Surveillance (JANIS) Clinical Laboratory Division. Annual Open Report 2019 (All Facilities). 2021. https://janis.mhlw.go.jp/english/report/open_report/2019/3/1/ken_Open_Report_Eng_201900_clsi2012.pdf. Accessed 18 Jan 2021.
Ko W-C, Stone GG. In vitro activity of ceftazidime-avibactam and comparators against Gram-negative bacterial isolates collected in the Asia-Pacific region as part of the INFORM program (2015–2017). Ann Clin Microbiol Antimicrob. 2020;19(1):14–14.
Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin Infect Dis. 2016;63(6):754–62.
Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62(11):1380–9.
Torres A, Zhong N, Pachl J, Timsit JF, Kollef M, Chen Z, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–95.
Titov I, Wunderink RG, Roquilly A, Rodriguez Gonzalez D, David-Wang A, Boucher HW, et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis. 2021;73(11):e4539–48.
Namias N, Solomkin JS, Jensen EH, Tomassini JE, Abramson MA. Randomized, multicenter, double-blind study of efficacy, safety, and tolerability of intravenous ertapenem versus piperacillin/tazobactam in treatment of complicated intra-abdominal infections in hospitalized adults. Surg Infect (Larchmt). 2007;8(1):15–28.
Kaye KS, Bhowmick T, Metallidis S, Bleasdale SC, Sagan OS, Stus V, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the Tango I randomized clinical trial. JAMA. 2018;319(8):788–99.
Ministry of Health LaW. Diagnosis Procedure Combination (DPC) Electronic Score Table. 2020. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000198757_00003.html. Accessed 13 Dec 2021.
National Institute of Population and Social Security Research. Japanese Mortality Database. 2016. https://www.ipss.go.jp/p-toukei/JMD/00/index.html. Accessed 13 Dec 2021.
NHS England. Hospital Admitted Patient Care Activity 2018-19. 2019. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2018-19. Accessed 13 Dec 2021.
Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. 2016;25(3):707–19.
Ernst EJ, Ernst ME, Hoehns JD, Bergus GR. Women’s quality of life is decreased by acute cystitis and antibiotic adverse effects associated with treatment. Health Qual Life Outcomes. 2005;3:45.
Brasel KBD, Weigelt J. Cost-utility analysis of contaminated appendectomy wounds. J Am College Surgeons. 1997;184(1):23–30.
Beusterien KM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O’Toole A, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50.
Medical Economics Division HIB, Ministry of Health, Labour and Welfare (MHLW). Full Scale Introduction of Cost-Effectiveness Evaluations in Japan. 2019. https://c2h.niph.go.jp/tools/system/overview_en.pdf. Accessed 13 Dec 2021.
Center for Outcomes Research and Economic Evaluation for Health. Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council 2019. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed 18 Jan 2021.
Organization for Economic Cooperation and Development OECD Data. Exchange rates. https://data.oecd.org/conversion/exchange-rates.htm. Accessed 25 Jan 2022.
Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the infectious diseases Society of America and the society for healthcare epidemiology of america. Clin Infect Dis. 2016;62(10):e51-77.
Majumder MAA, Rahman S, Cohall D, et al. Antimicrobial Stewardship: fighting antimicrobial resistance and protecting global public health. Infect Drug Resist. 2020;13:4713–38.
Honda H, Ohmagari N, Tokuda Y, Mattar C, Warren DK. Antimicrobial Stewardship in inpatient settings in the Asia Pacific region: a systematic review and meta-analysis. Clin Infect Dis. 2017;64(2):S119–26.
Altarac D, Gutch M, Mueller J, Ronsheim M, Tommasi R, Perros M. Challenges and opportunities in the discovery, development, and commercialization of pathogen-targeted antibiotics. Drug Discovery Today. 2021;26(9):2084–9.
Lax S, Sangwan N, Smith D, et al. Bacterial colonization and succession in a newly opened hospital. Sci Transl Med. 2017;9:391.
Dik J-WH, Poelman R, Friedrich AW, et al. An integrated stewardship model: antimicrobial, infection prevention and diagnostic (AID). Future Microbiol. 2015;11(1):93–102.
Nijssen S, Bootsma M, Bonten M. Potential confounding in evaluating infection-control interventions in hospital settings: changing antibiotic prescription. Clin Infect Dis. 2006;43(5):616–23.
Van Duijn PJ, Verbrugghe W, Jorens PG, Spöhr F, Schedler D, Deja M, et al. The effects of antibiotic cycling and mixing on antibiotic resistance in intensive care units: a cluster-randomised crossover trial. Lancet Infect Dis. 2018;18(4):401–9.
Lee BY, Singh A, David MZ, Bartsch SM, Slayton RB, Huang SS, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect. 2013;19(6):528–36.
Lee JY, Chong YP, Kim T, Hong HL, Park SJ, Lee ES, et al. Bone and joint infection as a predictor of community-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a comparative cohort study. J Antimicrob Chemother. 2014;69(7):1966–71.
Wozniak TM, Bailey EJ, Graves N. Health and economic burden of antimicrobial-resistant infections in Australian hospitals: a population-based model. Infect Control Hosp Epidemiol. 2019;40(3):320–7.
Reynolds CA, Finkelstein JA, Ray GT, et al. Attributable healthcare utilization and cost of pneumoniae due to drug-resistant Streptococcus pneumoniae: a cost analysis. Antimicrob Resist Infect Control. 2014;3:16.
Bartsch SM, McKinnell JA, Mueller LE, Miller LG, Gohil SK, Huang SS, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23(1):48.
Acknowledgements
The authors’ heartfelt appreciation goes to James Dennis of Health Economics and Outcomes Research Ltd. for providing medical writing support/editorial support, which was funded by Pfizer Japan Inc. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The MDV data was extracted and tabulated by Linghua Xu, Pfizer Japan Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Pfizer Japan Inc., who provided support for the model development/analysis and medical writing for this study.
Conflicts of interest
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TM has been on the speakers’ bureau for Pfizer Japan Inc. and MSD K.K. AY and TO are full-time employees of Pfizer Japan Inc. RM, CP, and JG are employees of Health Economics and Outcomes Research Ltd., which received funding from Pfizer Japan Inc. to undertake the research outlined in this study. AT is a full-time employee of Pfizer R&D UK Limited. AY and AT hold stocks and stock options from Pfizer Inc.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Availability of data and material
The anonymized patient data underlying this manuscript are derived from the Medical Data Vision database and the national surveillance program (Japan Nosocomial Infections Surveillance) and cannot be made available by the authors. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The economic model was submitted with the manuscript for peer review.
Code availability
Except for confidential data associated with modeling and data analysis, all data are available from the corresponding author upon reasonable request.
Author contributions
TM ensured applicability of the model and analysis to the Japanese clinical setting and provided expert guidance to that end. AY and JG conceptualized and designed the study. CP and RM were responsible for data analysis. AY and TO provided local data to inform the model. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matsumoto, T., Yuasa, A., Miller, R. et al. Estimating the Economic and Clinical Value of Introducing Ceftazidime/Avibactam into Antimicrobial Practice in Japan: A Dynamic Modelling Study. PharmacoEconomics Open 7, 65–76 (2023). https://doi.org/10.1007/s41669-022-00368-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00368-w