Abstract
Introduction
Antimicrobial resistance remains a serious and growing threat to public health, both globally and in the UK, leading to diminishing effectiveness of antimicrobials. Despite a clear need for new antimicrobials, the clinical pipeline is insufficient, driven by high research and development costs and limited expected returns on investment. To counteract this, National Institute for Health and Care Excellence (NICE) and National Health Service (NHS) England have launched a reimbursement mechanism, de-linked from volume of sales, that aims to reduce economic risk by recognising the broader population-level value of antimicrobials. The objective of this study was to quantify the value of ceftazidime–avibactam for treating gram-negative infections in the UK considering some of these broader value elements unique to antimicrobials.
Methods
A previously developed dynamic disease transmission and cost-effectiveness model was applied to assess the value of introducing ceftazidime-avibactam to UK treatment practice in the management of gram-negative hospital-acquired infections in line with the licenced indications for ceftazidime–avibactam. Model inputs were parameterised using sources aligned to the UK perspective.
Results
The introduction of ceftazidime–avibactam into a two-line treatment sequence saved over 2300 lives, leading to a gain of 27,600 life years and 22,000 quality-adjusted life years (QALY) at an additional cost of £17 million, over a ten-year transmission period. Ceftazidime–avibactam was associated with a net monetary benefit of £642 million at willingness to pay threshold of £30,000 per QALY; even at a lower threshold of £20,000 per QALY, the net monetary benefit is £422 million.
Discussion
Increasing the diversity of antimicrobial treatments through the introduction of an additional antimicrobial, in this instance ceftazidime–avibactam, was associated with substantial clinical and economic benefits, when considering broader population-level value. Despite revealing considerable benefits, the value of ceftazidime–avibactam is only partially reflected in this analysis. Further efforts are required to fully operationalise the spectrum, transmission, enablement, diversity and insurance (STEDI) value framework and accurately reflect the population-level value of antimicrobials.
Similar content being viewed by others
By considering broader population level value attributes in health technology assessment (HTA) methods, the clinical and economic value of antimicrobials can be more accurately reflected. |
This research can support policy makers and HTA bodies in implementing novel reimbursement models to incentivise antimicrobial research and development efforts. |
1 Introduction
The global increase in antimicrobial resistance (AMR) is an urgent public health concern and is a significant threat to health. The development of AMR is mainly a result of antimicrobial misuse and suboptimal infection control practices [1]. Furthermore, treatment options for resistant bacterial infections are decreasing and resistance to antimicrobials traditionally considered to be last line is growing, with few alternative options. This is of particular concern as resistant infections result in longer hospital stays, higher medical costs and increased mortality. Forecasts from the World Bank indicate that, without action, by 2050, drug resistant infections could cause 10 million deaths worldwide and cost up to $100 trillion in hospital costs and productivity losses [2]. Consistent with these global trends, the rise in AMR is an important health concern for the UK as well. The Organisation for Economic Co-operation and Development (OECD) estimated that, in the period 2015–2050, the UK will have 90,000 additional deaths associated with drug-resistant bacteria [3, 4], with financial losses in excess of £8.7–£34.6 billion per annum [5].
Several measures have been suggested to combat AMR, including limiting antimicrobial use in agriculture and the environment, implementing stewardship programmes in hospitals, vaccination, and infection prevention and control measures. Furthermore, the struggle against AMR also requires innovative antimicrobial agents and improved diagnostics to ensure optimal and targeted use of antimicrobial agents. Although there is a clear need for new antimicrobials that are safe, efficacious and that diversify current treatment strategies, the current clinical development pipeline is not sufficient [1, 6, 7]. This is primarily due to high research and development costs and limited expected returns on investment; these pressures have resulted in bankruptcy for some companies and have led others to abandon the antimicrobials market, leading to market failure [8].
One important way of stimulating antimicrobial development is by developing frameworks to assess antimicrobial value such that economic risks and returns achieve a more sustainable balance for manufacturers [9]. Novel reimbursement frameworks, such as the innovative health technology evaluation process and new payment model developed by National Health Service England (NHSE) and The National Institute for Health and Care Excellence (NICE), [10] aim to better recognise the population-level value of antimicrobials and incentivise the development of new antimicrobials. The NHSE and NICE reimbursement model pays a fixed annual fee; up to £10 million as part of a 3-year contract with the option to extend up to 10 years, de-linked from the volume of sales. Thus, reducing financial uncertainty for manufacturers. These new economic models propose to incorporate the population-level value elements that antimicrobials bring to patients, healthcare system and society, when answering a crucial question: “What is the price worth paying for a novel antimicrobial that can treat drug resistant infections?” [11]. Alternative approaches to value assessment could allow enhanced reimbursement mechanisms which stimulate investment in research and development of new antimicrobials, enabling new products to be brought to market. In this context, the investigation of suitable alternative health technology assessment (HTA) methods may play a crucial role in the value quantification of antimicrobial treatments [12].
Traditional HTA methodologies have been developed primarily for the economic evaluation of technologies for noncommunicable diseases focussing on benefits to an individual patient. However, this misses the broader population benefits of antimicrobials, particularly with respect to the population level impact on AMR. In other words, for antimicrobials, economic evaluation methodology needs to consider broad/population value elements and the communicable/dynamic aspects of infectious diseases [11, 13]. These elements of value have been described extensively in the literature with a high degree of consensus, including with payers and HTA bodies, and are often referred as “STEDI”, comprising spectrum value (the benefits of replacing broad-spectrum antimicrobials with narrow-spectrum ones that reduce collateral damage to the microbiome and minimize opportunities for resistant organisms to establish a niche), transmission value (the benefits of avoiding spread of resistant organisms), enablement value (the benefits associated with prophylactic use of antimicrobials allowing surgical or medical procedures to occur safely), diversity value (the benefits of having multiple antimicrobials available that may be used within treatment strategies to reduce selection pressure and minimise resistance development), and insurance value (the benefits of having a treatment available as an insurance against future AMR increases and outbreaks) [13, 14].
Although evaluation frameworks including the above value elements for antimicrobials have been previously proposed [Office of Health Economics (OHE) [13], Policy Research Unit in Economic Evaluation of Health and Care Interventions (EEPRU) [14]], in practice economic evaluations of antimicrobials do not routinely incorporate these added elements of value. This has been recognised in a recent study which indicates that, for HTA to be part of the solution in the struggle against AMR, it will require an expansion in the technical tool kit and a change in philosophy: “HTA risks becoming part of the problem, destroying value and destroying incentives to bring life-saving new technologies to future populations” [12].
The objective of this study is to apply a previously developed approach [15] that expands on the technical toolkit of the standard HTA methodology to quantify the value of ceftazidime–avibactam (CAZ/AVI) for treating severe aerobic gram-negative bacterial infections in the UK. Following the completion of the NHSE/NICE pilot, which evaluated CAZ/AVI, this study aims to assess the value of CAZ/AVI to patients and populations, accounting for transmission of infection, development of resistance, and antimicrobial availability, efficacy and patterns of use.
2 Methods
2.1 Population
The economic analysis considered hospital-acquired infection with three gram-negative bacterial species (Escherichia coli, Klebsiella spp. or Pseudomonas aeruginosa) across three indications of serious infections managed within the hospital setting [complicated urinary tract infections (cUTI), complicated intra-abdominal infections (cIAI) and hospital-acquired pneumonia/ventilator-associated pneumonia (HAP/VAP)], which represent some of the most common infections in the UK [16]. These indications were chosen in line with the licenced indications for CAZ/AVI [17] but do not include all indications and pathogens in which CAZ/AVI is used. A pooled analysis was performed, combining results from all three pathogens for each indication.
2.2 Intervention and comparators
CAZ/AVI is a fixed drug combination of the cephalosporin ceftazidime, and the novel non-β-lactam β-lactamase inhibitor avibactam. There are no guidance on cIAI or cUTI published by NICE; however, acute pyelonephritis guidance is recommended for cUTIs where penicillin in combination with β‑lactamase inhibitors, second or third generation cephalosporin, fluoroquinolones or aminoglycosides are recommended as first-choice intravenous treatment [18]. A NICE summary of evidence reports broadly the same treatments are recommended for cIAI in guidance from the Surgical Infection Society and the Infectious Diseases Society of America and the World Society of Emergency Surgery [19]. NICE guidance for hospital-acquired pneumonia recommends prompt treatment using broad-spectrum antibiotics, including penicillin with β-lactamase inhibitors, carbapenems, second or third generation cephalosporins, tetracyclines or glycopeptides as first-choice intravenous treatment with limited differences demonstrated in efficacy [20].
In the context of the model, CAZ/AVI was assessed considering the antimicrobial stewardship (AMS) benefit of increasing the diversity of antimicrobial use: that, having multiple antimicrobials available may be used within treatment strategies to reduce selection pressure and minimise resistance development. Comparators were simplified to reflect the most clinically relevant therapies in the empirical (i.e. the resistance status of infection to the treatment is not known) and confirmed/suspected resistance settings; this aimed to maximise clinical relevance across England in a situation where only a finite number of scenarios can be reasonably modelled given the variability of local resistance patterns. Considering the modelled population included serious infections and are likely to be systemically unwell, piperacillin/tazobactam (cIAI and cUTI) or colistin (HAP/VAP) then meropenem (all modelled indications) were considered the most appropriate comparators as informed by clinical experts. This treatment sequence represented the comparator arm. The intervention arm included the addition of CAZ/AVI; the sequences are described further in the base case and scenario analysis.
2.3 Model structure
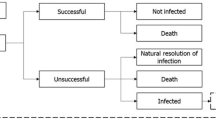
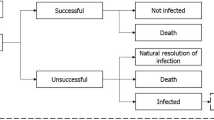
This analysis utilised a previously developed and validated model to support the evaluation of antimicrobial treatment and resistance which combined a multi-state disease transmission and decision-tree treatment pathway component (Fig. 1) [15]. Further details of the model are provided in Gordon et al. [15], but are briefly described here.
Overview of the model structure. A Disease transmission flow diagram and B treatment decision pathway, sourced from Gordon et al. [15]. Upon becoming infected (A), patients enter the treatment pathway (shown in B). In part A, patients may move between discrete health states representing: colonised [X]: patients colonised with the pathogen of interest (XW colonisation with a sensitive pathogen (i.e. no resistance to modelled treatments); XR colonisation with a resistant pathogen (i.e. resistance to either one, two, or all three modelled treatments). Infected [I]: patients infected with pathogen of interest (IW infected with a sensitive pathogen (i.e. no resistance to modelled treatments); IR infected with a resistant pathogen (i.e. resistance to either one, two, or all three modelled treatments). Susceptible [S]: patients not colonised or infected with a pathogen of interest. Death [D] (absorbing state). Note: the model schematic shows a representation of the transmission dynamics for a single pathogen and treatment, the model considers these dynamics for all pathogen and treatment combinations simultaneously. Details of transitions [a–o] between health states are outlined in Online Resource 1. [a] transition between health states: susceptible and infected with resistant pathogen, [b] transition between health states: susceptible and infected with sensitive pathogen, [c] transition between health states: susceptible and colonised with sensitive pathogen, [d] transition between health states: susceptible and colonised with resistant pathogen, [e] transition between health states: colonised with sensitive and resistant pathogen, [f] transition between health states: colonised with a sensitive pathogen and infected with a sensitive pathogen, [g] transition from colonised with a sensitive pathogen to infected with a resistant pathogen, [h] transition between health states: colonised with a resistant pathogen and infected with a sensitive pathogen, [i] transition between health states: colonised with a resistant pathogen and infected with a resistant pathogen. Transitions to death are [j] (from infected with a sensitive pathogen), [k] (from infected with a resistant pathogen), [l] (from colonised with sensitive pathogen), [m] (from susceptible), [n] (from colonised with resistant pathogen), [o] transitions for admissions and discharges from the infectious environment.
2.3.1 Transmission component
The dynamic transmission element of the model accounts for the transmissible nature of infectious diseases and explicitly models population and AMR dynamics over the modelled time horizon, enabling the assessment of the transmission and diversity value associated with the introduction of an additional antimicrobial into the treatment environment.
Dynamic transmission modelling is the process of mathematically representing the population’s transition through “infected”, “colonised”, “susceptible” or “dead” health states [21], and is conceptually similar to the Susceptible Exposed Infected Recovered (SEIR) approach regularly used in infectious disease modelling, including in coronavirus disease 2019 (COVID-19) [22]. Colonised and infected states contain further sub-states split by pathogen type and pathogen resistance status to a given antimicrobial. This allows exploration of the diversity value through a reduction in AMR by introducing an additional antimicrobial alongside, rather than as a replacement for current antimicrobials, aligning with recent recommendations on a framework for the value assessment of new antimicrobials [10, 14].
Both the development and transmission of resistance are captured in this dynamic copoint of the model and then propagated throughout the infectious environment. De novo development of resistance is primarily a function of treatment exposure. Each day a patient receives a given antimicrobial the exposed pathogen has a probability of developing resistance to that treatment. The development of resistance is therefore time updated as a function of the number of patients requiring treatment, the antimicrobials used and treatment efficacy. Pathogens may develop resistance to multiple treatments. De novo resistance gain occurs in the infected states and is shown as transitions [h] and [i] (multi-drug resistance) in Fig. 1. Treatment-resistant infection or colonisation may also arise through transmission, i.e. as a result of patients becoming infected with an already resistant pathogen (transitions [d] and [e] in Fig. 1). The model also includes two mechanisms by which resistance is lost: following successful treatment and due to “fitness cost”. Following successful treatment, a proportion of patients can enter the susceptible health state, at which point they are no longer colonised or infected with the pathogen of interest, including resistant pathogens. The second mechanism, “fitness cost” is a concept whereby mutations conferring antimicrobial resistance can make the physiological processes in bacterial cells less effective, impacting the “fitness” of the microorganism, i.e. its ability to survive and replicate in a given environment [23]. When selection pressure from the antimicrobial is removed (e.g. antimicrobials are switched as part of a cycling treatment strategy), resistant pathogens lose their competitive advantage and, as potentially less “fit”, may be outcompeted by susceptible organisms. Further information on resistance gain and loss can be found in the Online Resource 1.
2.3.2 Treatment pathway component
Patients in the infected health state (resistant or sensitive) enter the treatment pathway component. Individuals are treated according to pre-determined antimicrobial regimens until they are either cured (via successful treatment or an infection resolving spontaneously) or die from infection. As a simplifying assumption, the model assumes that patients can receive up to three lines of treatment; subsequent lines of treatment are only received if prior treatment is unsuccessful. Treatment success is determined by pathogen-specific clinical effectiveness data and pathogen resistance status. Treatment exposure is captured in this component of the model and is used to inform the probability of resistance gain within the disease transmission component.
2.4 Model inputs and data sources
Model inputs were derived from published sources or based on assumptions verified by external experts. A list of model assumptions and bases for their adoption, as informed by expert opinion and validated in a modified Delphi panel, is available in Table 1.
2.4.1 Clinical and epidemiological inputs
The baseline distribution of patients across the susceptible, colonised and infected health states by pathogen and indication was calculated on the basis of published infection incidence data and is summarised in Table 2 [16, 24]. Transitions between health states were controlled by the incidence and prevalence of bacterial infection and colonisation within the modelled environment and were derived from data reported by UK Health Security Agency (UKHSA), formerly Public Health England (PHE) [24] and the Health Protection Agency [16]. Where evidence was unavailable, model inputs describing infection transmission dynamics and resistance development were derived empirically through calibration, such that the model reproduced observed data from PHE describing infection incidence and resistance development over time to ensure the model aligns to real-world resistance development and infection incidence. Baseline pathogen resistance levels were sourced primarily from the 2019 English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report [25]; where required data were not available, the 2018 report was used as supplementary evidence [26]. Data from the 2020 and 2021 ESPAUR was not used due to the impact the COVID-19 pandemic had on observed data, likely due to changes in healthcare delivery and capacity for testing and reporting. ATLAS surveillance data was used for the CAZ/AVI baseline resistance in the UK [27]. Please refer to Online Resource 1 for further information.
In the base case analysis, it was assumed for all three indications that 80% of modelled patients initially received empirical treatment, the remaining 20% received directed treatment where it was assumed that the resistance status was known from the outset. Patients who proved to be infected with a pathogen resistant to the empirical therapy moved to the subsequent line of treatment that was effective against the infectious pathogen if one was available. The duration of unsuccessful treatment, due to either resistance or clinical ineffectiveness, in the model was assumed to be 2 days for all pathogens and indications as it was assumed that unsuccessful treatment will become apparent before the full course of antimicrobial treatment is complete. This was a simplifying assumption and was validated by a panel of clinical and microbiology experts during model development [15].
The efficacy of modelled therapies against susceptible pathogens and indication-specific treatment durations were sourced from the literature and are listed in Online Resource 1. The efficacy of treatment against resistant pathogens was assumed to be 0%, and a 3% probability of the infection resolving spontaneously was assumed.
Infected patients experience a risk of death associated with bacterial infection, described using an indication-specific daily probability of mortality. Patients whose treatment was unsuccessful had a higher daily probability of death compared with successfully treated patients. Patients who were successfully treated were subject to an age-matched daily mortality rate, derived from the Office for National Statistics (ONS) data [28], consistent with the general population. The model did not consider mortality from other causes. Please refer to Online Resource 1 for further mortality information.
2.4.2 Cost and resource use
Costs and resource use were applied to patients in the infected health state, Table 3. Three cost categories were considered: costs associated with existing antimicrobial treatment options, hospitalisation, and Clostridioides difficile infection (CDI). The unit cost of CAZ/AVI was excluded as the analysis aimed to capture the expected clinical and economic impact relevant to investment decisions regarding CAZ/AVI in the UK setting. The daily cost of hospital stay (LOS) in a general ward were sourced from the National Cost Collection [29]. The cost associated with CDI included the cost of a 10-day course of oral vancomycin, at 125 mg administered every 6 hours, and associated costs of LOS for the duration of treatment. All costs were accrued over the modelled time horizon (10 years in the base case) and discounted at 3.5% annually.
2.4.3 Health‑related quality of life
Published indication-specific utility values were applied to infected patients (Table 3). For non-infected patients, age-matched general population utility estimates were used, with the average age of patients experiencing cUTI, cIAI, and HAP/VAP based on NHS hospital activity data [30]. Patients who died due to infection were assumed to lose quality-adjusted life years (QALYs) corresponding with the quality-adjusted life expectancy of the general population.
A specific disutility associated with CDI was not identified and a disutility for diarrhoea was used as a proxy [31] and was applied for any additional LOS associated with CDI. All benefits were discounted at 3.5% annually.
2.5 Model setting
The economic model considered a single hospital with a constant infectious environment of 1000 patients parameterised with national-level data over a 10-year transmission horizon with a monthly cycle length, where life years (LYs) and QALYs were assessed over a patient’s lifetime. The model cycle length and time horizon were informed by a previously published antimicrobial cost-effectiveness evaluation [32] and expert opinion elicited during model development [15]. Model outcomes were then scaled to reflect the population-level hospital environment in England, assumed to be 100,000 [33]. As a simplifying assumption, health states were assumed to be homogeneous; consequently, each infection is independent and once a patient is discharged from the infectious environment they are not readmitted.
2.5.1 Model outcomes
Outcomes included number of infections avoided, deaths and net monetary benefit (NMB), as well as LYs and QALYs lost due to infection. The NMB was calculated by multiplying the incremental gain in QALYs by the cost per willingness-to-pay (WTP) threshold of £30,000 per QALY and subtracting the incremental costs (or adding incremental cost savings).
2.5.2 Base case scenario
In the base case, the introduction of an additional antimicrobial, CAZ/AVI, was assessed at the first line within the all-lines treatment diversification AMS strategy compared with a two-line treatment scenario without CAZ/AVI (Fig. 2). The all-line treatment diversification strategy evenly split the treated patients across all three treatments as their first-line treatment (i.e. 33% patients received CAZ/AVI, 33% received piperacillin/tazobactam and 33% received meropenem as their first-line treatment). Following unsuccessful treatment, patients move to the next available treatment defined by the treatment sequence, regardless of which treatment they started with, until all available treatments are exhausted. This approach is in line with the stewardship recommendations for the UK, as reflected in, for example, the NICE guideline for antimicrobial prescribing in hospital acquired pneumonia [34].
Visualisation of treatment strategies. Base case—CAZ/AVI first line (all lines diversified); scenario 1: CAZ/AVI first line (first two lines diversified); scenario 2: CAZ/AVI first line (no diversification); scenario 3: CAZ/AVI third line (no diversification). *for the HAP/VAP indication colistin is used instead of PIP/TAZ. PIP/TAZ, piperacillin/tazobactam; MERO, meropenem; CAZ/AVI, ceftazidime with avibactam
2.5.3 Scenario and sensitivity analyses
Three alternative scenarios explored the impact of different utilisation settings of CAZ/AVI outlined in Fig. 2. In scenario 1 CAZ/AVI was introduced at first line where the first two lines were diversified, scenario 2 CAZ/AVI was introduced at first line with no diversification strategy, and scenario 3 CAZ/AVI was introduced at third line with no diversification.
Two deterministic sensitivity analysis frameworks were used to assess the impact of uncertainty: (1) scenario analyses were conducted considering different utilisation settings of CAZ/AVI (Fig. 2), and (2) one way sensitivity analysis was conducted to assess the impact of variation in specific parameters on base case model results. Scenario and one-way sensitivity analysis details are listed in the Online Resource 1. Probabilistic sensitivity analysis (PSA) was conducted to assess uncertainty in all parameters of the model. Random sampling of parameters was conducted using appropriate distributions for each parameter (costs parameters: gamma, utility parameters: triangular, calibrated inputs: triangular, probability parameters: beta). The means of the parameters were pooled and their standard errors propagated through the calculation of incremental costs and incremental QALYs and NMB for each combination of pathogen and indication.
2.6 Validation
Validation compared the accuracy of the calibration model, measuring estimated outputs for resistance trajectories to observed data from the 2018 and 2019 ESPAUR reports [25, 26]. The model was run over a 5-year period, utilising default settings (i.e. availability of piperacillin/tazobactam and meropenem only), to replicate resistance levels by pathogen to piperacillin/tazobactam in England between 2013 and 2018 and compared with those observed in the 2018 and 2019 ESPAUR reports.
3 Results
3.1 Base case scenario
Results highlight the benefit associated with the availability of an additional antimicrobial and the increased diversity of available treatments.
The addition of CAZ/AVI into a treatment sequence as part of an all-lines diversification strategy resulted in 2338 fewer deaths resulting in a gain of 27,637 LYs (21,978 QALYs) over a 10-year time horizon, combined for all three indications: cUTI, cIAI and HAP/VAP (Fig. 3). Furthermore, the introduction of CAZ/AVI was associated with £17,092,162 in additional costs (£309,023,296 with two lines of treatment and £326,115,458 with three lines of treatment with CAZ/AVI). Despite fewer deaths occurring, which led to a reduced cost of death, costs were driven by an increased length of stay as the availability of an additional treatment for patients who have exhausted all available options meant patients remained in hospital for longer.
The NMB of introducing CAZ/AVI was estimated at £642 million, at a WTP of £30,000 per QALY. Even when a reduced WTP of £20,000 per QALY was considered, the NMB was £422 million (Table 4).
The greatest clinical benefits of CAZ/AVI were observed in the HAP/VAP indication, which resulted in avoiding 1172 deaths and leading to a saving of 14,015 LYs and 10,930 QALYs. These benefits in HAP/VAP population accounted for 50% of the overall NMB (£322 million).
3.2 Scenarios
Scenario analyses were conducted to explore different utilisation settings of CAZ/AVI. The differences between alternative treatment strategies that include CAZ/AVI, were not substantial. Total deaths ranged from 1765 to 1802, with LYs and QALYs lost due to infection ranging between 20,543–21,060 and 16,229–16,632, respectively (Table 4). Total costs ranged from £309 million to £326 million and NMB in the alternative treatment scenarios ranged from £633 million to £656 million. These results highlight that there is substantial diversity value induced by the availability of the additional antimicrobial; however, we did not observe large differences among various treatment diversification strategies.
3.3 Resistance development
When considering resistance patterns, when CAZ/AVI is used at first line, overall resistance to other antimicrobials decreases (from 7.2 to 6.8%) while resistance to CAZ/AVI increases (from 2.2 to 7.6%) as a result of changes in selection pressure (Fig. 4). Where CAZ/AVI is used last in the treatment sequence, resistance to CAZ/AVI remains low, in 10 years it increases by 2.8 percentage points from 2.2 to 5.0%. However, resistance to other antimicrobials rises further than in a two-line only strategy due to increased survival of patients, meaning they remain in the infectious environment for longer and there is a greater chance of resistance developing (from 7.2 to 10.6%). Strategies that include two-line or all-line diversification strategies have resistance gains that lie between the extremes of using a third antimicrobial first or last line only. There is therefore a trade-off to consider when using additional antimicrobials. Additional resistance outcomes are shown in Online Resource 2 Figs. S1–3.
3.4 Sensitivity analyses
A one-way sensitivity analysis was conducted to assess the impact of variation in specific parameters on NMB. Results were most sensitive to the variation in treatment efficacy (all treatments) and baseline resistance; adjusting inputs for treatment efficacy had the greatest influence on NMB outcomes, varying from £173.6 million to £1.3 billion. When an “at risk” population was considered, representing vulnerable patients with reduced utility and life expectancy inputs (−20%), NMB was still nearly £430 million (Online Resource 2 Fig. S4).
The range of outcomes of the PSA for incremental costs, incremental QALYs and NMB include the modelled estimate for each scenario, giving confidence that the modelled results do not suffer from non-linearities or compounding errors due to the uncertainties with input parameters. The PSA results for the base-case scenario (CAZ/AVI all-lines diversification) for the incremental costs, incremental QALYs and NMB were £16.9 million [95% confidence interval (CI) £14.4 million; £19.4 million], 22,236 (95% CI 21,685; 22,786) and £650 million (95% CI £633 million; £667 million), respectively (Table 5).
3.5 Validation
The accuracy of the model calibration in relation to 5-year resistance projects was subject to a validation presented in Online Resource 2 (Fig. S5). An ordinary least squares linear regression line (OLS LRL) was fitted with an intercept of zero where the value of the slope was determined to be 0.9851, indicating model predictions of resistance are accurate. The coefficient of determination (R2) was 0.9986, which indicates a high degree of linear correlation.
4 Discussion
This study aimed to estimate the value of introducing CAZ/AVI into UK clinical practice, accounting for transmission of infection and development of resistance, as well as antimicrobial availability, efficacy and patterns of use. Applying the modelling approach developed by Gordon et al. [15], compared to a standard HTA assessment, we estimated the broader value of CAZ/AVI.
Our results show that considerable clinical and economic value can be recognised by adding CAZ/AVI in the treatment pathway in the UK. Over a 10-year period, the introduction of CAZ/AVI saves 2338 lives, equating to approximately 10% of all deaths from hospital-acquired infections in the NHS (2016/17) [35], this translates into 21,978 QALYs gained. When WTP is valued at £30,000 per QALY, introducing CAZ/AVI is associated with a NMB of £642 million, over the 10-year period. This analysis does not include the cost of CAZ/AVI as the outcomes are used to inform a value-based payment for CAZ/AVI and therefore, reporting an incremental cost-effectiveness ration (ICER)(total incremental costs divided by total incremental QALYs) is not appropriate. Two exploratory analyses were conducted to investigate the ICER using two payment strategies from the NHSE perspective: (1) including a unit cost for CAZ/AVI (£257.19 per day) and (2) the NHSE and NICE maximum contract value (£10 million per/year). The ICERs for the base case scenario was estimated to be £2940 and £4694, for each payment model respectively, demonstrating the maximum NHSE and NICE contract is both cost-effective and a provides fairer compensation value for CAZ/AVI, compared with conventional reimbursement policies that pay per unit used.
While there are clear benefits in terms of outcomes of introducing an additional antimicrobial, our results were broadly similar when considering how that antimicrobial is used in the system. However, there is a trade-off when considering resistance patterns, with increasing resistance to the new antimicrobial associated with increasing usage. Using the new antimicrobial as last line treatment to limit resistance development and therefore preserve efficacy, may result in increased resistance gain in the existing antimicrobials. This model captures the impact of this trade-off by changes in clinical outcomes; the difference in outcomes was not significant in the scenarios considered. However, in areas with high resistance rates, the need for an effective treatment against infections may outweigh the need to minimise resistance development in the new antimicrobial. It is therefore important to consider local and clinical driven infection prevention and AMS programs to optimise clinical outcomes while minimising consequences of antimicrobial use. When accounting for model and parameter uncertainty in PSA, NMB ranged from £631 million to £650 million; even when the most modest estimate of NMB from sensitivity analyses is considered (£174 million), the value of CAZ/AVI to the NHS exceeds the £100 million contract limit set out by NHSE and NICE, over 10 years. The contract value is suitably large to act as an effective pull incentive; however, similar incentives need to be implemented globally [36]. The UK has led the way as the first country to introduce a de-linked subscription style reimbursement model; since the NICE/NHSE pilot, the USA, with the Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) act, and other G7 members (EU, Canada and Japan) have started discussions to implement similar innovative policies [37]. Whilst developing such policies, it is important that contract caps are carefully considered, with international coordination, to represent a countries fair share of an effective “global pull incentive”. Whilst contract values will be set to ensure sufficient return on investment, there should also be flexibility to reward novel products providing the highest value. Furthermore, it is important that eligibility criteria for products included under the policy are communicated to drug developers early, to allow planning during lengthy clinical development cycles. Policies should also work to ensure AMS and access to new antimicrobials for low- and middle-income countries who experience the greatest burden, but with limited resources to develop similar policies [38]. Before the combined value of “global pull incentives” is large enough there will still be a need for push incentives to encourage pre-clinical investment [39].
This study has some noteworthy strengths. First, it captures some of the unique value aspects associated with new antimicrobials, including diversity value and transmission value. However, not all the aspects of value associated with antimicrobials were able to be captured within the present analysis. The analyses only partially reflect insurance and enablement value. For example, the insurance value associated with avoiding outbreaks and other major catastrophic health consequences cannot be captured and outcomes relevant to enablement value, such as prophylactic antimicrobial use, gains in hospital capacity and the enablement of procedures that would otherwise be foregone, were not included. Given this limitation, the approach should be considered to underestimate the population wide value of CAZ/AVI availability. Whilst there is alignment between industry and payer stakeholders on the STEI value, this framework is the first example to apply these in the assessment of an antimicrobial [12, 14, 40, 41]. Despite intentions to reflect these elements in NICE’s new HTA model for antimicrobials, the final economic evaluation failed to capture these [40]. The modelling approach described in this study differs from NICE’s economic evaluation of CAZ/AVI in a number of key areas [40]. Most significantly, the NICE appraisal utilised a decision-analytic model; this approach fails to adequately capture the importance of avoiding the spread of an infection (transmission) and limiting the development of resistance of existing antimicrobials (diversity). In our analysis utilising a disease transmission model and introducing CAZ/AVI as an additional antimicrobial alongside, rather than replacing a comparator, enabled an estimate for transmission and diversity value from the STEDI framework. The NICE economic evaluation only considered a selective population described as high-value clinical scenarios, which underestimates the number of patients currently receiving CAZ/AVI in the UK [40], whereas our model aligns more closely to the licenced indication of CAZ/AVI and real-world usage. This choice of population also led to efficacy data for CAZ/AVI being derived from in vitro susceptibility data in the absence of clinical trial data in these populations, which may lead to further uncertainty in the NICE evaluation as susceptibility does not equate to treatment success. Neither the NICE evaluation nor our model was able to fully quantify spectrum, enablement or insurance value; however, as a broad-spectrum antimicrobial, the spectrum value for CAZ/AVI may be limited. Furthermore, insurance and diversity value may be conflicting value elements—to maximise diversity value the new antimicrobial needs to be used; however, the new antimicrobial should be preserved to maximise insurance value. A particular challenge preventing the conceptualisation of spectrum value is a paucity of evidence informing the relationship between reducing collateral damage of commensal pathogens in the microbiome, from replacing broad-spectrum antimicrobials with narrow-spectrum alternatives, and reducing resistance gain in these non-infection causing pathogens. Further research efforts are required to develop a framework capable of quantifying the remaining STEDI value attributes; these research efforts should also include enhanced data collection to reduce uncertainty in modelling these outcomes.
Second, this study shows how an HTA evaluation can help unlock the value of adding a new antimicrobial which is very relevant from public health perspective. Several of the CAZ/AVI clinical trials used carbapenems as comparators [40,41,42,43], supporting a role for CAZ/AVI as a potential alternative to carbapenems, which are considered the last line of therapy for severe infection management in critically ill patients. This is extremely important considering a recent increase in carbapenem prescribing in some countries has been observed during the COVID-19 pandemic which may be driven by challenges operating effective AMS strategies in secondary care [24, 46]. This increase in prescribing comes at a time of growing carbapenem resistance, posing a potential risk. While still relatively rare, detection of carbapenem-resistant bacteria has increased by approximately 1000-fold in England in recent years (2010–2018) [47]. CAZ/AVI therefore provides a carbapenem-sparing treatment option, which is an important advantage where carbapenem resistance has been confirmed, or its development is a concern, and could reduce selection pressure from the use of carbapenems and other existing antimicrobials.
4.1 Limitations
This study has several limitations. First, model inputs describing infection transmission dynamics and resistance development were derived empirically through calibration, in line with recommendations from the EEPRU evaluation framework [14]. As a result of this approach, no estimates of uncertainty or covariance are produced that would enable robust PSA; however, we have tried to characterise this uncertainty by conducting a PSA using triangular distributions around the calibrated inputs. Scenario analyses were therefore conducted to analyse the impact of variation in specific parameters. Second, the outcomes of HAP/VAP are analysed together, and the model does not permit the two indications to be explored separately. This is an important limitation because, while VAP prevention remains an important goal in NHS hospitals, the burden of non-VAP HAP is also substantial. Thirdly, whilst the modelling approach used in this study may not follow a traditional infectious disease modelling structure, the current approach represents a standardised approach that can be used across numerous applications, appropriate for use in HTA. Fourth, the model was validated against 5-year resistance data; therefore, there is uncertainty around the accuracy of projections over longer time horizons; sensitivity and scenario analyses were implemented to attempt to characterise this uncertainty. The ability to robustly validate model outputs is limited by a paucity of published evidence reporting suitably granular data; therefore, the ESPAUR reports were considered the best available data source to validate against [25, 26]. Finally, the treatment pathway incorporated in the model is restricted to a maximum of three antimicrobials, whereas in clinical practice many more antimicrobial agents could be and are used within a hospital setting. However, there is an inherent difficulty in determining which comparator antimicrobials should be modelled, as antimicrobial use is heterogeneous and dependent on local stewardship guidelines, risk factors for resistance and known susceptibility results. The modelled comparator selection therefore captures only part of the antimicrobial treatment landscape within NHSE. The current analysis aims to estimate the value of CAZ/AVI under the current AMR burden; therefore, it does not account for changes in population size or implement updates to the empiric treatment strategies in response to changing resistance patterns. With likely increasing populations and using optimised treatment sequences, net health benefits could be higher than those estimated in this study. This simplified landscape reflects the pragmatism required to develop a robust model including the most clinically relevant therapies in the empirical and confirmed/suspected resistance settings.
5 Conclusions
Inaction against AMR is likely to result in significant socioeconomic risk. The success of novel reimbursement models to incentivise research and development efforts depend upon accurately recognising the value of antimicrobials in totality to the healthcare system. Despite value being underestimated, the presented analyses demonstrate that the incorporation of additional antimicrobials has significant value to the NHS and represents an important first step in quantifying the wider value of antimicrobials to the population. However, further work is required to capture and quantify additional antimicrobial value elements that are not traditionally captured in economic evaluations.
References
World Health Organisation. 2020 antibacterial agents in clinical and preclinical development: an overview and analysis.
Jonas OB, Irwin A, Berthe F, Jean C, Le Gall FG, Marquez PV. Drug-resistant infections: a threat to our economic future (vol. 2): final report (English). HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, DC: World Bank Group 2016. http://documents.worldbank.org/curated/en/323311493396993758/final-report.
The Organisation for Economic Co-operation and Development (OECD). Stemming the superbug tide: just a few dollars more. 2018.
Office for National Statistics. Gross Domestic Product: United Kingdom March 2021. https://www.ons.gov.uk/economy/grossdomesticproductgdp/timeseries/abmi/ukea. Cited 1 June 2021.
Smith RD, Yago M, Millar M, Coast J. Assessing the macroeconomic impact of a healthcare problem: the application of computable general equilibrium analysis to antimicrobial resistance. J Health Econ. 2005;24(6):1055–75.
World Health Organisation. 2019 antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline.
Beyer P, Paulin S. Priority pathogens and the antibiotic pipeline: an update. Bull World Health Organ. 2020;98(3):151.
Plackett B. Why big pharma has abandoned antibiotics. Nature. 2020;586(7830):S50–S.
AMR Industry Alliance. 2020 progress report.
National Institute for Health and Care Excellence. Models for the evaluation and purchase of antimicrobials.
Morton A, Colson A, Leporowski A, Trett A, Bhatti T, Laxminarayan R. How should the value attributes of novel antibiotics be considered in reimbursement decision making? MDM Policy Prac. 2019;4(2):2381468319892237.
Colson AR, Morton A, Årdal C, Chalkidou K, Davies SC, Garrison LP, et al. Antimicrobial resistance: is health technology assessment part of the solution or part of the problem? Value Health. 2021;24(12):1828–34.
Karlsberg Schaffer S, West P, Towse A, Henshall C, Mestre-Ferrandiz J, Masterton R, et al. Assessing the Value of New Antibiotics: Additional elements of value for health technology assessment decisions. 2017.
Rothery C, Woods B, Schmitt L, Claxton K, Palmer S, Sculpher M. Framework for value assessment of new antimicrobials: implications of alternative funding arrangements for NICE Appraisal. Policy Research Unit in Economic Evaluation of Health and Care Interventions. Universities of Sheffield and York. EEPRU Research Report 059. 2018.
Gordon J, Darlington O, McEwan P, Lumley M, Taie A, Hicks M, et al. Estimating the value of new antimicrobials in the context of antimicrobial resistance: development and application of a dynamic disease transmission model. Pharmacoeconomics. 2020;38(8):857–69.
Health Protection Agency. English national point prevalence survey on healthcare-associated infections and antimicrobial use.
Pfizer. Zavicefta—summary of product characteristics. 2021.
National Institute for Health and Care Excellence. Pyelonephritis (acute): antimicrobial prescribing [NG111]. 2018. https://www.nice.org.uk/guidance/ng111.
National Institute for Health and Care Excellence. Complicated intra-abdominal infections: ceftolozane/tazobactam [ESNM75]. 2016. https://www.nice.org.uk/advice/esnm75/chapter/Key-points-from-the-evidence.
National Institute for Health and Care Excellence. Pneumonia (hospital-acquired): antimicrobial prescribing [NG139]. 2019. https://www.nice.org.uk/guidance/ng139.
Ud-Din R, Shah K, Ahmad I, Abdeljawad T. Study of transmission dynamics of novel COVID-19 by using mathematical model. Adv Differ Equ. 2020;2020(1):323.
He S, Peng Y, Sun K. SEIR modeling of the COVID-19 and its dynamics. Nonlinear Dyn. 2020;101(3):1667–80.
Vogwill T, MacLean RC. The genetic basis of the fitness costs of antimicrobial resistance: a meta-analysis approach. Evol Appl. 2015;8(3):284–95.
Public Health England. AMR local indicators—fingertips profile. https://fingertips.phe.org.uk/profile/amr-local-indicators. Cited 26 Jan 2021.
Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2018—2019. In: Department of Public Health., editor. UK: © Crown copyright [2019]; 2019.
Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2018. In: Department of Public Health., editor. UK: © Crown copyright [2018]; 2018.
Pfizer. Antimicrobial testing leadership and surveillance. https://atlas-surveillance.com/#/database/mic-distribution. Cited 30 Apr 2021.
Office for National Statistics. National life tables: United Kingdom 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables. Cited 26 Jan 2021.
NHS. National Cost Collection for the NHS. https://www.england.nhs.uk/national-cost-collection/#ncc1819. Cited 10 Mar 2021.
NHS Digital. Hospital admitted patient care activity 2018–19.
Mathers CD, Bernard C, Iburg KM, Inoue M, Ma Fat D, Shibuya K, et al. Global burden of disease in 2002: data sources, methods and results. Geneva: World Health Organization; 2003. p. 54.
Jansen JP, Kumar R, Carmeli Y. Cost-effectiveness evaluation of ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections accounting for antibiotic resistance. Value Health. 2009;12(2):234–44.
NHS England. Average daily number of available and occupied beds open overnight by sector: October to December 2019.
National Institute for Health and Care Excellence. Pneumonia (hospital-acquired): antimicrobial prescribing [NG139]. 2019.
Guest JF, Keating T, Gould D, Wigglesworth N. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ Open. 2020;10(1): e033367.
Outterson K. Estimating the appropriate size of global pull incentives for antibacterial medicines. Health Aff. 2021;40(11):1758–65.
Outterson K, Rex JH. Global pull incentives for better antibacterials: the UK leads the way. App Health Econ Health Policy. 2023;21(3):361–4.
Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55.
Cama J, Leszczynski R, Tang PK, Khalid A, Lok V, Dowson CG, et al. To push or to pull? In a post-covid world, supporting and incentivizing antimicrobial drug development must become a governmental priority. ACS Infect Dis. 2021;7(8):2029–42.
National Institute for Health and Care Excellence. Final guidance: Ceftazidime–avibactam for treating severe drug-resistant gram-negative bacterial infections. 2022. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials/ceftazidime-with-avibactam#committee_discussion.
The Association of the British Pharmaceutical Industry. ABPI response to guidance on the value of new antibiotics. 2022. https://www.abpi.org.uk/media/news/2022/april/abpi-response-to-guidance-on-the-value-of-new-antibiotics/. 14 July 2023.
Torres A, Zhong N, Pachl J, Timsit J-F, Kollef M, Chen Z, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–95.
Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62(11):1380–9.
Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, Chen Q, et al. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017;49(5):579–88.
Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Inf Dis. 2016;63(6):754–62.
Tiri B, Sensi E, Marsiliani V, Cantarini M, Priante G, Vernelli C, et al. Antimicrobial stewardship program, COVID-19, and infection control: spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J Clin Med. 2020;9(9):2744.
Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2020 report. In: Department of Public Health., editor. UK: © Crown copyright [2020]; 2020.
Tlaskalová-Hogenová H, Štěpánková R, Hudcovic T, Tučková L, Cukrowska B, Lodinová-Žádnı́ková R, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93(2):97–108.
Office for National Statistics. Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland. Cited 26 Jan 2021.
Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D.The University of York Centre for Health Economics. 1999. https://www.york.ac.uk/che/pdf/DP172.pdf. Cited 3 Mar 2021.
Ernst EJ, Ernst ME, Hoehns JD, Bergus GR. Women's quality of life is decreased by acute cystitis and antibiotic adverse effects associated with treatment. Health Qual Life Outcomes. 2005;3:45.
Brasel KJ, Borgstrom DC, Weigelt JA. Cost-utility analysis of contaminated appendectomy wounds. J Am Coll Surg. 1997;184(1):23–30.
Beusterien KM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O’Toole A, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50.
Department of Health and Social Care. Drugs and pharmaceutical electronic market information tool (eMIT). 2021.
National Institute for Health and Care Excellence. British National Formulary.
Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379(18):1732–44.
Acknowledgements
The authors thank Prof. Alec Morton for providing their invaluable contribution to research analysis and interpretation. The authors would also like to thank Cale Harrison and Dr. Daniel Sugrue for providing advice and support in manuscript preparation, which was funded by Pfizer in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Pfizer Ltd. who provided support for analysis and medical writing for this study.
Conflict of interest
A.T. and M.G. are employees of Pfizer Ltd. J.G., R.M. and J.D. are employees of Health Economics and Outcomes Research Ltd. Health Economics and Outcomes Research Ltd. received fees from Pfizer in relation to this study. S.G. has received consulting fees from Enterobiotx, Shinogi and Tilotts Pharma, and speakers fees from Tilotts Pharma.
Author contributors
J.G., A.T. and M.G. conceptualised and designed the study. R.M. was responsible for data analysis. All authors contributed to interpretation of the results, and review of the manuscript, and approval of the final manuscript for publication.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gordon, J., Gheorghe, M., Goldenberg, S. et al. Capturing Value Attributes in the Economic Evaluation of Ceftazidime with Avibactam for Treating Severe Aerobic Gram-Negative Bacterial Infections in the United Kingdom. PharmacoEconomics 41, 1657–1673 (2023). https://doi.org/10.1007/s40273-023-01310-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01310-6