Abstract
Elements that are deliberately added to aluminum alloys or are incorporated into the alloy later depending on the production process affect the final product properties. In addition, liquid metal cleaning is important in minimizing undesirable elements. Considering the production process, one of the most harmful impurities that is likely to pass into the alloy via diffusion for aluminum is the element, Fe. It is known that this is due to the fact that although Fe is highly soluble in liquid aluminum and its alloys, it has very little solubility in solids. Depending on the Fe content, mechanical properties, porosity and fluidity properties are affected in aluminum alloys. In this study, stainless and carbon steel rods were dipped into the melt at 700 °C and 750 °C for 1, 2 and 5 h. Castings were performed before and after degassing. Four-channel fluidity mold with different section thickness was used in the trials. Additionally, microstructure characterization was performed under varying casting conditions. Fluidity Index was proposed which is a single value measured from all fluidity values in different sections. When the results were examined, it was determined that the diffusion material, holding time, casting temperature and liquid metal cleanliness had an effect on the fluidity. Due to the increase in diffusion time, a decrease in fluidity was observed in both carbon steel and stainless steel. It was found that fluidity was significantly reduced when using stainless steel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Casting is known as a manufacturing process performed by pouring liquid metals into the mold cavity. With this method, complex shaped parts can be easily produced from all kinds of materials that can be melted.1 Due to its strength and lightness, thin-walled castings are produced in applications such as such as aviation, defense and automotive industry. In these castings, the ability of the casting material to fill the mold is important. Studies on design and casting conditions are constantly carried out to meet the requirements of producing products with good mechanical properties. Aluminum alloys and composites are widely used in these studies due to their material properties.2 However, in thin-walled castings of aluminum, it may cause production problems related to mold filling. Rapid cooling of some parts of the casting reduces the fluidity of the molten metal. This can cause the molten metal to solidify before completely filling the mold cavity. Therefore, one of the main factors to be considered in foundry applications of thin-section castings is the measurement of fluidity, and attention should be paid to various factors affecting fluidity.3
Fluidity is not just a property of the liquid, such as viscosity and density, but its complex behavior under certain conditions during solidification in the mold. Fluidity is used to indicate how far a molten metal can flow before solidifying. Fluidity represents an important property for casting alloys.4,5 Fluidity is a length usually measured in millimeters or meters. There are test methods that use molds such as spiral mold, u-shaped sample, rod-shaped sample for fluidity measurement.6,7,8,9 Many factors affect the fluidity of liquid metal. The factors that determine the fluidity can be listed as the composition of the alloy, alloy properties, mold properties, casting temperature and melt cleanliness level.9,10,11,12,13,14 Additionally, solidification range and phase composition have an impact on fluidity. The fluidity of aluminum alloys is also affected by the presence of inclusions such as aluminum oxide, which can reduce fluidity. In addition, casting conditions such as solidification mode, metal composition and pouring temperature are critical factors affecting the fluidity of aluminum alloys.15,16
The most important factor in the use of aluminum alloys and the production of quality parts is the quality of the molten metal and the minimization of undesirable elements. Elements affecting impurities in aluminum casting are usually listed as Fe, Zn, Cu, Mg and Mn.17,18,19 Iron is one of the elements that has the most negative impact on the impurities of aluminium casting alloys. It supports the formation of intermetallic phases during the solidification of aluminium alloys, which can affect the properties of the final product.20,21,22,23 These intermetallic phases can block the interdendritic flow channels, resulting in decreased fluidity, and may also affect the overall properties of the alloy.24 These intermetallic compounds are known to have adverse effects on mechanical properties, porosity, castability, corrosion resistance and surface treatments25 due to their complex shape and geometries. Although iron is highly soluble in liquid aluminium and its alloys, it has very little solubility in the solid and therefore tends to form compounds with other elements to form various types of intermetallic phase particles.26,27 Fe element forms phases such as Al3Fe, Al6Fe, Al8Fe2Si, Al5FeSi, Al8FeMg3Si6 in the alloy. It was observed that Fe in the melt decreases fluidity due to increased intermetallic formation and increased sludge formation.28
Procedures such as liquid metal cleaning, addition of neutralizing alloying elements, use of filters, etc. are applied to eliminate the harmful effects of impurity elements in the alloy. The most widely used procedure in this context is liquid metal cleaning such as rotary degassing. In the studies, it has been observed that the mechanical properties of alloys subjected to liquid metal cleaning increase, with a decrease in porosity, and they contribute positively to fluidity.29,30,31 There are studies showing that the fluidity of aluminum alloys can be improved by adding rare earth elements.32,33 Consequently, iron plays a crucial role in influencing the fluidity of aluminum alloys through its interaction with other elements, the formation of intermetallic compounds, and its influence on solidification processes. Understanding the effects of iron on aluminum alloys is crucial to optimizing their properties and performance in various applications.
In this study, the effect of iron diffusion on the fluidity of A356 cast aluminum alloy was investigated. A 4-channel mold was used for the fluidity tests and castings were carried out at two different temperatures with different Fe contents.
Material Method
A356 aluminum alloy was used in the experiments. In the study, in order to increase the Fe content of the alloy, two rods were dipped into the melt and held for 1, 2 and 5 h. Diffusion time was determined considering the foundry conditions. One of the rods was carbon steel (St1020) and the other was stainless steel (SS430). The application temperatures were 700 °C and 750 °C. Experimental parameters are given in Table 1.
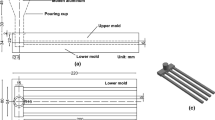
Samples were collected before and after degassing. Rotary degassing was carried out with nitrogen at 300 rpm, 5 l/min flow rate and 5 minutes in 8 kg capacity SiC crucible. In the study, a total of 28 castings were made in accordance with the test parameters, along with 4 reference castings. In the casting experiments, the filling of the liquid metal into the mold was carried out at the same speed. Filling continued until the liquid metal advance stopped and the mold was no longer filled. Figure 1 shows a schematic view of the experimental application.
The fluidity tests were carried out in a 4-channel permanent with thickness ranging from 2 to 8 (as seen in Figure 2, dimensions are in mm). The die was coated with BN and preheated to 250 °C.
The mold design was selected in an aim to understand the fluidity length of the alloy in different section thickness in one casting. Therefore, the lengths at different thickness were compared. In such analysis, the interpretation of the pouring becomes complicated. Therefore, a fluidity index was proposed in this work when such different section thickness was used as fluidity test. The length of fluidity was divided to the thickness and the sum of each channel value was used as a single number as an indication of fluidity length which was named as “Fluidity Index” (Equation 1):
Samples were taken from a 6 mm thick section for microstructural examinations. The samples were subjected to grinding, polishing and etching for metallographic preparation. Grinding was done with 180, 400, 800, 1000, 1200 and 2000 grit SiC papers. The polishing process was carried out with diamond suspension, colloidal silica and water. Etching was done with Keller reagent solution. Microscopic examination of the samples was made with a Nikon Eclipse LV 150N model optical microscope.
Experimental Work
After castings were completed, the specimens were removed from the mold and examined. Chemical analysis results of the samples obtained from experiments carried out to detect changes in chemical composition due to the diffusion effect are given in Table 2.
When the values given in Table 2 were examined, it was determined that the amount of Fe% element increased with the increase in diffusion time and casting temperature. Additionally, an increase in the amount of Cr% and Ni% was detected in experiments where stainless steel diffusion was applied. Images of the reference samples without diffusion are shown in Figure 3, with carbon steel dipped samples in Figure 4, and with stainless steel dipped ones in Figure 5.
The liquid metal flow distances of the cast samples in each channel were measured, and the results and fluidity index values are given in Table 3. The graphical representation of the results are given in Figure 6.
Figure 6 summarizes the findings of fluidity test results. It can be seen that as expected, as the casting temperature increases, fluidity increases regardless of the condition of the melt. This can also be generalized for section thickness as well: as the section thickness increases, the fluidity increases regardless of the casting conditions. As a general observation, the increased Fe content of the melt resulted in decreased fluidity. In the case of stainless-steel dipped melt, the fluidity decreases even further compared to carbon steel dipped melt.
The values obtained when the experimental parameters are analyzed in the 4-channel fluidity pattern; it was observed that the liquid metal advances were between 1 mm and 33 mm with a 2 mm section thickness, between 103 mm and 300 mm with a 8 mm section thickness, between 31 mm and 298 mm with a 4 mm section thickness, and between 14 mm and 290 mm with a 6 mm section thickness. When the fluidity index values were calculated, the values were found to be between 28.21 and 133.83. It was observed that the lowest fluidity index value was in the samples where liquid metal cleaning was not performed using 700 °C stainless steel, and the highest fluidity index value was in the samples where liquid metal cleaning was applied at 750 °C without diffusion effect. If the test parameters are examined separately, the effect of casting temperature and liquid metal cleaning process on fluidity will be evaluated first.
The fluidity index was measured as 49.99 at 700 °C without cleaning and 80.87 when cleaning was performed. The fluidity index of the samples that were not cleaned at the alloy casting temperature of 750 °C was measured as 69.08 and when the liquid metal cleaning was done, it was measured as 101.63. Studies on the subject also emphasize that temperature changes in aluminium casting alloys affect the fluidity of the alloy. The fluidity of aluminium alloys is a multifaceted phenomenon affected by various factors. Adding various elements to the A356 aluminium alloy increases the recrystallization temperature and affects the fluidity of the alloy.34 When the alloy temperature was increased at certain intervals, an increase in the fluidity of the alloy was observed and it was observed that the fluidity of the alloy increased at the highest casting temperatures.35 It has also been stated that increasing the melting temperature when melting aluminium alloys leads to a proportional increase in the fluidity length of aluminium alloys.36 In a different study, fluidity increases not only by increasing the casting temperature but also by increasing the mold temperatures.37 Studies generally show that temperature plays a very important role in affecting the fluidity of aluminium casting alloys and that different alloys exhibit different responses to temperature changes.
The average fluidity index obtained in castings made without diffusion is 129.33; it was measured as 81.69 at 1-h diffusion application time, 64.16 at 2-h diffusion application time and 53.35 at 5-h diffusion application period. In Figure 6, the results obtained from the experiments on the effect of liquid metal cleaning on fluidity at varying temperatures after the diffusion of carbon steel and stainless-steel materials, which are used to examine the diffusion effect by material effect, are given graphically.
In the experiments using stainless steel, the fluidity index was measured as 39.10 at 700 °C without cleaning, and 64.21 when cleaning was performed. The average fluidity index of samples that were not cleaned at the alloy casting temperature of 750 °C was measured as 56.42, and when liquid metal cleaning was performed, it was measured as 89.14. In castings made by placing a carbon steel rod into the liquid metal, the fluidity index was measured as 40.33 at 700 °C without cleaning process, and 79.88 when cleaning was done. The average fluidity index of samples that were not cleaned at the alloy casting temperature of 750 °C was measured as 62.74, and when liquid metal cleaning was performed, it was measured as 99.4. In casting processes, the relationship between temperature and section thickness plays a critical role in completely filling the mold. Figure 6 shows the results obtained at varying cross-sections and temperatures under all experimental conditions graphically.
Liquid metal flow distances at 700 °C; it was calculated as 8.71 mm with 2 mm section thickness, 70.36 mm with 4 mm section thickness, 105.93 mm with 6 mm section thickness and 206.64 mm with 8 mm section thickness. Under the same conditions, the liquid metal advance distance was calculated as 13.79 mm with a 2 mm section thickness, 87.57 mm with a 4 mm section thickness, 154.36 mm with a 6 mm section thickness and 246.71 mm with an 8 mm section thickness at 750 °C. When the section thicknesses were examined due to the temperature increase, the temperature value did not differ much in the 2 and 4 mm sections, but it was clearly seen in the 6 and 8 mm thick sections. It is thought that the similar progress in 2 and 4 mm section thickness despite the temperature increase is due to very rapid heat loss due to the thin section thickness in contact with the movement of the liquid and mold and the pressure effect depending on the section. In general, as the section thickness and casting temperature increase, the liquid metal advance distance also increases. Similar results have been obtained in studies conducted in the literature on the subject.38,39,40,41
As a result of the evaluation of the test samples made by applying the test parameters of diffusion time, liquid metal cleanliness and casting temperature in the A356 aluminum alloy, it was determined that Fe diffusion had negative effects on the fluidity by placing both carbon steel and stainless-steel rods. When the liquid metal advances due to Fe diffusion were examined, it was seen that the liquid metal advance distances decreased more in experiments using stainless steel. As a result of the examinations, it was determined that the fluidity properties of the alloy decreased in castings made by iron diffusion. This situation is thought to be due to the compounds that may emerge in the structure due to iron diffusion affecting the fluidity. In studies conducted in this context, similar results can be found in the literature.
Fe is an impurity element found in aluminum-silicon casting alloys and contributes to the formation of intermetallic compounds.42 Different compounds contained in aluminum alloys tend to form brittle intermetallic compounds, which emphasize the reactivity of iron and aluminum.43 In addition, the presence of Mg in the aluminum alloy causes an increase in the diffusion of Fe into aluminum and promotes the formation of the Fe2Al5 intermetallic compound.44 The solid solubility of the Fe element in aluminum alloys is quite low. The low solid solubility of the Fe element increases the formation of intermetallic compounds.45 The formation of intermetallic compounds is affected by the metals present in the alloy and the size of the interaction zones of the connections of the compounds.46 In aluminium based alloys, iron leads to the formation of intermetallic compounds that show the reactivity of iron and aluminium at contact interfaces within the alloy.47 It is also known that the diffusion of the iron element into liquid aluminium causes the formation of intermetallic Fe2Al5 phases in different connections within the alloy in aluminium and steel materials.42 Iron dissolved in aluminium alloys has a strong tendency to decompose due to being solid particles and therefore increases the formation of iron-containing intermetallic compounds.36 The iron element forms various intermetallic phases when combined with aluminium and manganese elements, clearly demonstrating the reactivity of iron and aluminium in the alloy.48 In addition, aluminium-rich compounds tend to form aluminium-rich intermetallic compounds such as Fe2Al5 or FeAl3.49 Based on the cooling curve analysis and Fe phase formation sequences,50,51,52,53,54,55,56,57 after α-Al dendrite formation, around 574 °C, eutectic Si starts to form.
When all the fluidity data is subjected to regression analysis using MiniTab, Equation 2 and 3 were obtained. It was found that p value is less than 0.001 with 90.66 % R2 value.
Before degassing:
After degassing:
Where X1 is temperature, X2 is duration and X3 is thickness. Based on Equation 1, the data of 4-channel fluidity tests results were evaluated with the new proposed fluidity index and the results are presented in Figure 7. It can be seen that as temperature is increased, fluidity index increased. The fluidity index is higher when carbon steel is dipped into the melt, however the index is lower when stainless steel is dipped into the melt. As duration of the dipped steels are increased (i.e. increased Fe content), fluidity index decreases exponentially. If the melt is not treated (no degassing), the fluidity index low but it increases almost double when melt is treated, and oxides are removed. When all the fluidity data is subjected to regression analysis using MiniTab, Equation 4 and 5 were obtained for fluidity index. It was found that p value is less than 0.001 with 90.66 % R2 value.
Before degassing:
After degassing:
where X1 is temperature, X2 is duration.
Equation 4 and 5 show the equations of fluidity index by Fe content, casting temperature and treatment of the melt with and R2 of 94.1 %. It can be seen that duration of dipping of steel rods has 60 % effect on the fluidity index which indicates that Fe content has significant effect on the fluidity whereas treatment of the melt has 25 % effect on the fluidity index. Representative microstructure images of the casting samples taken at different magnifications under varying conditions are given in Figure 8.
In the base alloy, there was no observation of Fe-intermetallic phases in the microstructure. This is due to the low Fe content of the alloy which was around 0.12 wt%. As the duration of dipping the steel rods in molten aluminum, the number and distribution of Fe phases starts to increase. In 1 h, when carbon steel was dipped, approximately 50 μm length needle-type beta-Fe intermetallics were observed. In 2 h, the segregated and accumulated beta-Fe phases in the size of 100 μm were observed in the microstructure. On the other hand, when stainless steel was used, after 1 h of dipping, alpha type chunky Fe-intermetallics were observed in the microstructure. The sizes of these alpha phases were almost not changed by duration being in the range of 200 μm however their numbers were increased with duration. It is well-known and reported in the literature that cooling rates higher than 20 °C/s can suppress the platelet-like morphology and
provide a less harmful α-phase shape.58,59,60,61,62 In this work, permanent die moulds were used for the fluidity tests, therefore the observation of alpha phases was considered to be due to high cooling rate.
Based on the chemical composition data in Table 2, sludge factor was measured for all testing parameters.58,59,63,64 Figures 9 and 10 were plotted where the effect of sludge factor, temperature and fluidity index can be seen. In castings at 750°C, the decrease in fluidity index with increased sludge factor is much higher than the castings at 700°C. This could mainly be due to the fact that dissolution of Fe, Cr and Ni is much faster and higher at high temperatures. The increase of these elements in molten aluminum will result in formation of more Fe-based intermetallic phases.
Considering the relationship between sludge factor, casting temperature and fluidity index, Figure 10 reveals that higher the casting temperature and lower the sludge factor will result in the highest fluidity index. On the other, it is important to note that degassing increases fluidity as well. In the presence of oxides in the unclean melt, the nucleation of Fe-phases are enhanced which was shown by Cao65,66,67,68,69,70 in their work. Bas71 had also reported similar findings where the density of distribution of Fe-phases were directly related with the oxide content of the melt.
Conclusion
It is possible to summarize the results obtained from the experiments carried out within the scope of the study as follows;
-
It has been observed that increasing the casting temperature of the liquid metal increases the fluidity values of A356 alloy regardless of Fe content.
-
It has been found that melt cleanliness has a significant effect on the fluidity. The fluidity length is almost doubled after degassing.
-
When the effect of Fe diffusion was examined, it was determined that Fe diffusion had negative effects on fluidity by dipping carbon and stainless steel rods.
-
It has been determined that the fluidity properties decrease with increasing diffusion time (i.e. increased Fe content).
-
In experiments using stainless steel in liquid A356, due to increased Fe and Cr content in the alloy, it was observed that the fluidity was decreased significantly lower than carbon steel dipped melt.
-
As the section thickness increases, the liquid metal advance distance also increases. While there is not much difference between the liquid metal advancement conditions in 2 and 4 mm cross-section channels, the differences become evident as the temperature increases in 6 mm and 8 mm cross-section channels.
-
The fluidity length at different thickness channels gives more indicative information compared to standard spiral test moulds. The newly proposed fluidity index based on the 4 channel mould provides a single value that includes fluidity distance in different thickness which can be effectively used as an indication of fluidity length of cast aluminum alloys.
References
J. Campbell, Complete casting handbook: metal casting processes, metallurgy, techniques and design, Butterworth-Heinemann, (2015).
W. Harrigan, S. Ochiai, Handbook of metallic composites, (1994).
K. Ravi, R. Pillai, K. Amaranathan, B. Pai, M. Chakraborty, Fluidity of aluminum alloys and composites: a review. J. Alloys Compd. 456, 201–210 (2008)
R. Vignesh, M.S. Gandhi, A. Vignesh, P. Rajarajan, Effect of squeeze cast process parameters on fluidity of aluminium LM6 alloy. Int. J. Adv. Technol. 7, 157 (2016)
M. Di Sabatino, L. Arnberg, D. Apelian, Progress on the understanding of fluidity of aluminium foundry alloys. Inter. Metalcast. 2, 17–27 (2008). https://doi.org/10.1007/BF03355430
M. Di Sabatino, L. Arnberg, A review on the fluidity of Al based alloys, Metall. Sci. Tecnol. 22 (2004).
M. Colak, R. Kayikci, D. Dispinar, Influence of different cross sections on fluidity characteristics of A356. Trans. Indian Inst. Met. 68, 275–281 (2015)
E. Erzi, Ç. Yüksel, Ö. Gürsoy, M. Çolak, D. Dispinar, Characterisation of aluminum alloys by octopus fluidity test. J. Polytech. 27(1), 21–25 (2024). https://doi.org/10.2339/politeknik.746862
H. Mirzadeh, B. Niroumand, Fluidity of Al–Si semisolid slurries during rheocasting by a novel process. J. Mater. Process. Technol. 209(11), 4977–4982 (2009)
F.R. Mollard, M.C. Flemings, E.F. Niyama, Aluminum fluidity in casting. JOM 39, 34–34 (1987)
G. Zou, Y. Chai, Q. Shen et al., Analysis of the fluidity and hot tearing susceptibility of AlSi3.5Mg0.5Cu0.4 and A356 aluminum alloys. Inter. Metalcast. 16, 909–923 (2022). https://doi.org/10.1007/s40962-021-00649-w
A. Dahle, P. Tøndel, C. Paradies, L. Arnberg, Effect of grain refinement on the fluidity of two commercial Al–Si foundry alloys. Metall. Mater. Trans. A 27, 2305–2313 (1996)
M. Di Sabatino, L. Arnberg, Effect of grain refinement and dissolved hydrogen on the fluidity of A356 alloy. Int. J. Cast Met. Res. 18, 181–186 (2005)
Y.E. Asan, M. Colak, Modeling the effect of pour height, casting temperature and die preheating temperature on the fluidity of different section thicknesses in permanent mold casting of Al12Si alloys, Erzincan Univ. J. Sci. Technol. 15, 14–27 (2022)
G. Zhang, Z. Wang, J. Niu, H. Xu, X. Ren, Enhanced fluidity of ZL205A alloy with the combined addition of Al–Ti–C and La. Materials 14(20), 6169 (2021)
T. Haga, S. Imamura, H. Fuse, Fluidity investigation of pure Al and Al-Si alloys. Materials 14(18), 5372 (2021)
Q. Han, S. Viswanathan, Analysis of the mechanism of die soldering in aluminum die casting: physical metallurgical and materials science, metall. Mater. Trans. 34A, 139–146 (2003)
J. Zheng, Y. Pang, H. Shi et al., Effect of Mn and B addition on the microstructure and properties of Al–Si–Cu–Mg cast alloy. Inter. Metalcast. (2023). https://doi.org/10.1007/s40962-023-01175-7
A.M.A. Mohamed, M.F. Ibrahim, E. Samuel et al., Assessment of the effect of mg addition on the solidification behavior, tensile and impact properties of Al–Si–Cu cast alloys. Int. J. Metalcast. 17(1), 82–108 (2022). https://doi.org/10.1007/s40962-022-00786-w
C. Rathinasuriyan, A. Bharath, K. Sridhar, Reducing iron content from aluminium molten bath through filter bag, centrifugal separation and flux refining method. Mater. Today Proc. 62, 1026–1032 (2022)
V.E. Bazhenov, A.V. Petrova, A.V. Sannikov et al., Relationship between critical solid fraction and dendrite coherency point in Al–Si alloys. Int. J. Metalcast. 17(1), 1–13 (2022). https://doi.org/10.1007/s40962-022-00772-2
G.K. Sigworth, Fundamentals of solidification in aluminum castings. Int. J. Metalcast. Lead. Transf. Res. Technol. Glob. Metalcast. Ind. 8(1), 7–20 (2014). https://doi.org/10.1007/BF03355567
H.L. de Moraes, J.R. de Oliveira, D.C.R. Espinosa, J.A.S. Tenório, Removal of iron from molten recycled aluminum through intermediate phase filtration. Mater. Trans. 47(7), 1731–1736 (2006)
Y.C. Tzeng, J.K. Nieh, H.Y. Bor, S.L. Lee, Effect of trace Be and Sc additions on the mechanical properties of A357 alloys. Metals 8(3), 194 (2018)
J.G. Kaufman, E.L. Rooy, Aluminum alloy castings: properties, processes, and applications, Asm International, (2004).
J.A. Taylor, Iron-containing intermetallic phases in Al–Si based casting alloys, Procedia. Mater. Sci. 1, 19–33 (2012)
L.F. Mondolfo, Aluminum alloys: structure and properties, Elsevier, (2013).
E. Taghaddos, M. Hejazi, R. Taghiabadi, S. Shabestari, Effect of iron-intermetallics on the fluidity of 413 aluminum alloy. J. Alloys Compd. 468, 539–545 (2009)
E. Erzi et al., Determination of acceptable quality limit for casting of A356 aluminium alloy: supplier’s quality index (SQI). Metals 9(9), 957 (2019)
M. Çolak, Modification of eutectic Al–Si alloys by Sr and CuSn5. Materials Research Express 6(10), 10652 (2019)
M. Çolak, R. Kayikci, D. Dispinar, Melt cleanliness comparison of chlorine fluxing and Ar degassing of secondary Al-4Cu. Metall. Mater. Trans. B. 47, 2705–2709 (2016)
K.C. Dizdar, H. Sahin, F. Tezer, D. Dispinar (2022). Characterization of casting properties of rare-earth modified A356. aluminium alloys: design and development of innovative alloys. Manuf. Process. Appl. 71
G. Niu, Y. Wang, L. Zhu, J. Ye, J. Mao, Fluidity of casting Al–Si series alloys for automotive light-weighting: a systematic review. Mater. Sci. Technol. 38(13), 902–911 (2022)
W. Prukkanon, N. Srisukhumbowornchai, C. Limmaneevichitr, Influence of Sc modification on the fluidity of an A356 aluminum alloy. J. Alloys Compd. 487, 453–457 (2009)
G. Timelli, F. Bonollo, Fluidity of aluminium die castings alloy. Int. J. Cast Met. Res. 20, 304–311 (2007)
Y. Liu, M. Liu, L. Luo, J. Wang, C. Liu, The solidification behavior of AA2618 aluminum alloy and the influence of cooling rate. Materials 7, 7875–7890 (2014)
H. Huang, Y. Wang, P. Fu, L. Peng, H. Jiang, W. Xu, Fluidity of AZ91D and Mg–3Nd–0 2Zn–Zr (wt-%) magnesium alloys: response to pouring and mould temperature. Int. J. Cast Met. Res. 26, 213–219 (2013)
V.E. Bazhenov, A.V. Petrova, A.A. Rizhsky et al., Simulation and experimental validation of A356 and AZ91 alloy fluidity in a graphite mold. Inter. Metalcast. 15, 319–325 (2021). https://doi.org/10.1007/s40962-020-00468-5
A.W. Abboud, M.V. Glazoff, D. Weiss et al., Methodology for thermodynamic analysis coupled with computational fluid dynamics modeling for casting a novel aluminum-cerium alloy. Inter. Metalcast. (2023). https://doi.org/10.1007/s40962-023-01134-2
M. Çolak, D. Dispinar, The influence of metallostatic pressure, grain refiner, and modification on the critical solid fraction (CSF) of cast A380 alloy. J. Engg. Res. 9(4B), 269–280 (2021)
S. Köse, M. Çolak, E.E. Şüküroğlu, Investigation of the effects of filling speed, casting temperature and metallurgical quality on fluidity of lamellar graphite cast iron at different section thicknesses. Inter. Metalcast. (2024). https://doi.org/10.1007/s40962-024-01301-z
L. Shao, Y. Shi, J. Huang, S. Wu, Effect of joining parameters on microstructure of dissimilar metal joints between aluminum and galvanized steel. Mater. Des. 66, 453–458 (2015)
Z.W. Chen, M.Z. Jahedi, The effect of temperature on soldering and the sequence of formation of the soldered layer during high pressure die casting of Al-11Si-3Cu alloy. Int. J. Cast Met. Res. 11, 129–138 (1998). https://doi.org/10.1080/13640461.1998.11819267
T. Watanabe, H. Sakuyama, A. Yanagisawa, Ultrasonic welding between mild steel sheet and Al–Mg alloy sheet. J. Mater. Process. Technol. 209, 5475–5480 (2009)
W. Zhang, D. Sun, L. Han, W. Gao, X. Qiu, Characterization of intermetallic compounds in dissimilar material resistance spot welded joint of high strength steel and aluminum alloy. Isij Int. 51, 1870–1877 (2011)
D.G. Mohan, J. Tomków, S.S. Karganroudi, Laser welding of UNS S33207 hyper-duplex stainless steel to 6061 aluminum alloy using high entropy alloy as a filler material. Appl. Sci. 12, 2849 (2022)
I. Goto, K. Shirai, R. Ohyama, K. Kurosawa, Dissolution mechanism of intermetallic layer by iron erosion in aluminum-based molten binary alloys. Mater. Trans. 63, 730–739 (2022)
C. Scharf, A. Ditze, Iron pickup of AZ91 and AS31 magnesium melts in steel crucibles. Adv. Eng. Mater. 9, 566–571 (2007)
T. Sasaki, T. Yakou, K. Mochiduki, K. Ichinose, Effects of carbon contents in steels on alloy layer growth during hot-dip aluminum coating. ISIJ Int. 45, 1887–1892 (2005)
J. Mathew, G. Remy, M.A. Williams, F. Tang, P. Srirangam, Effect of Fe intermetallics on microstructure and properties of Al–7Si alloys. JOM 71, 4362–4369 (2019). https://doi.org/10.1007/s11837-019-03444-5
B. Liu, Q. Yang, Y. Wang, Intereaction and intermetallic phase formation between aluminum and stainless steel. Results Phys. 12, 514–524 (2019). https://doi.org/10.1016/j.rinp.2018.11.076
J. Wang, P.D. Lee, R.W. Hamilton, M. Li, J. Allison, The kinetics of Fe-rich intermetallic formation in aluminium alloys: In situ observation. Scr. Mater. 60, 516–519 (2009). https://doi.org/10.1016/j.scriptamat.2008.11.048
W. Khalifa, F. Samuel, J. Gruzleski, Iron intermetallic phases in the Al corner of the Al–Si–Fe system. Metall. Mater. Trans. A 34, 807–825 (2003)
L.A. Narayanan, F.H. Samuel, J.E. Gruzleski, Crystallization behavior of iron-containing intermetallic compounds in 319 aluminum alloy. Metall. Mater. Trans. A 25, 1761–1773 (1994). https://doi.org/10.1007/BF02668540
H. Becker, T. Bergh, P.E. Vullum, A. Leineweber, Y. Li, Effect of Mn and cooling rates on α-, β- and δ-Al–Fe–Si intermetallic phase formation in a secondary Al–Si alloy. Materialia 5, 100198 (2019). https://doi.org/10.1016/j.mtla.2018.100198
J. Santos, A.E.W. Jarfors, A.K. Dahle, Formation of iron-rich intermetallic phases in Al-7Si–Mg: influence of cooling rate and strontium modification. Metall. Mater. Trans. A 50, 4148–4165 (2019). https://doi.org/10.1007/s11661-019-05343-5
J.P. Schoß, E. Schramm, P. Schönherr, N.M. Mrowka, H. Schumann, H. Becker, A. Keßler, M. Szucki, G. Wolf, Investigation of the formation of iron-rich intermetallic phases in Al–Si alloys via thermal analysis cooling curves, including a real-time detection for filtration process. Adv. Eng. Mater. 25, 2201576 (2023)
S. Ferraro, A. Fabrizi, G. Timelli, Evolution of sludge particles in secondary die-cast aluminum alloys as function of Fe, Mn and Cr contents. Mater. Chem. Phys. 153, 168–179 (2015)
S. Ferraro, G. Timelli, Influence of sludge particles on the tensile properties of die-cast secondary aluminum alloys. Metall. Mater. Trans. B. 46, 1022–1034 (2015)
E. Granhed, A.D. Mori, E. Battaglia, L. Arnberg, G. Lindwall, A CALPHAD-based investigation of the sludge factor. Int. J. Metalcast. 18(1), 343–351 (2024)
S. Ferraro, A. Bjurenstedt, S. Seifeddine, On the formation of sludge intermetallic particles in secondary aluminum alloys. Metall. Mater. Trans. A. 46, 3713–3722 (2015)
T. Tunçay, S. Bayoğlu, The effect of iron content on microstructure and mechanical properties of A356 cast alloy. Metall. Mater. Trans. B. 48, 794–804 (2017)
S. Shankar, D. Apelian, Mechanism and preventive measures for die soldering during Al casting in a ferrous mold. Jom 54, 47–54 (2002)
S. Shankar, D. Apelian, Die soldering: effect of process parameters and alloy characteristics on soldering in the pressure die casting process. Int. J. Cast Met. Res. 15(2), 103–116 (2002)
X. Cao, J. Campbell, The nucleation of Fe-rich phases on oxide films in Al-11.5 Si-0.4 Mg cast alloys. Metall. Mater. Trans. A. 34, 1409–1420 (2003)
X. Cao, J. Campbell, The solidification characteristics of Fe-rich intermetallics in Al-11.5 Si-0.4 Mg cast alloys. Metall. Mater. Trans. A. 35, 1425–1435 (2004)
X. Cao, J. Campbell, Morphology of β-Al5FeSi phase in Al–Si cast alloys. Mater. Trans. 47(5), 1303–1312 (2006)
X. Cao, J. Campbell, Effect of Sr on primary α-Fe phase in liquid Al-11.5 Si-0.4 Mg cast alloy. Mater. Sci. Technol. 20(4), 514–520 (2004)
X. Cao, J. Campbell, Effect of precipitation and sedimentation of primary α-Fe phase on liquid metal quality of cast Al–11.1 Si–0.4 Mg alloy. Int. J. Cast Metals Res. 17(1), 1–11 (2004)
X. Cao, J. Campbell, Effect of melt superheating on convection-free precipitation and sedimentation of primary α-Fe phase in liquid Al-11.5 Si-0.4 Mg alloy. Int. J. Cast Metals Res. 15(6), 595–608 (2003)
E.N. Bas, S. Alper, T. Tuncay, D. Dispinar, S. Kirtay, Influence of melt quality on the formation of Fe intermetallic in A360 alloy. Arch. Foundry Eng. 22(3), 53 (2022)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/f.
About this article
Cite this article
Durmuş, M., Dispinar, D., Gavgali, M. et al. Evaluation of Fe Content on the Fluidity of A356 Aluminum Alloy by New Fluidity Index. Inter Metalcast (2024). https://doi.org/10.1007/s40962-024-01396-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40962-024-01396-4