Abstract
Metal deterioration inhibitors are known to be materials that have shielding power with clear manifestation that is solely focused on usage on a metallic external texture. The advancement in the utilization of metal deterioration inhibitors are now well recognised, nonetheless, majority of current inhibitors have some disadvantages. Benevolent macromolecule inhibitors with biological origins are environmentally un-harmful substances with numerous polar ends having ability to form coordination bonds. New prospects of deoxyribonucleic acid (DNA) extracted from plant species, animal species and different species of pisces used for the purpose of metal deterioration inhibition welcomes some unique research possibility for engineering applications. As a result, it is expected that DNA would have high level of effectiveness when the strains of DNA of the three species are extricated for making inhibitors due to their feasible inclusions in averting metal deterioration.

(Adapted form Ref. [12] with permission from Elsevier)

(Adapted form Ref. [12] with permission from Elsevier)

(Adapted form Ref. [12] with permission from Elsevier)

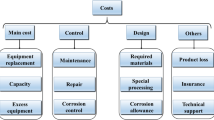
(Adapted form Ref. [62] with permission from Springer Nature)

(Adapted form Ref. [62] with permission from Springer Nature)

(Adapted form Ref. [62] with permission from Springer Nature)
Similar content being viewed by others
References
Johnson D (2018) Properties and use of steel. Sciencing. https://sciencing.com/properties-uses-steel-7271721.html. Accessed 18 Oct 2019
Maanonen M (2014) Steel pickling in challenging conditions. Bachelor of Engineering Thesis, Helsinki Metropolia University of Applied Sciences
Qian Y, Li Y, Jungwirth S, Seely N, Fang Y, Shi X (2015) The application of anti-corrosion coating for preserving the value of equipment asset in Chloride-Laden environments: a review. Int J Electrochem Sci 10:10756–10780
Uhlig HH (2011) UHLIG’S Corrosion Handbook. The electrochemical society series, 3rd edn. Wiley, New Jersey
Sastri VS (2011) Green corrosion inhibitors: theory and practise. Wiley, New Jersey
Umoren SA, Eduok UM (2016) Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr Polym 140:314–341
Gräfen H, Horn EM, Schlecker H, Schindler H (2002) Ullmann’s encyclopedia of industrial chemistry. In Corrosion. Wiley-VC, Weinheim
Peme T, Olasunkanmi LO, Bahadur I, Adekunle AS, Kabanda MM, Ebenso EE (2015) Adsorption and corrosion inhibition studies of some selected dyes as corrosion inhibitors for mild steel in acidic medium: gravimetric, electrochemical, quantum chemical studies and synergistic effect with iodide ions. Molecules 20:16004–16029
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41
Kontanis EJ, Reed FA (2006) Evaluation of real-time PCR amplification efficiencies to detect PCR Inhibitors. J Forensic Sci 51:795–804
Demeke T, Jenkins GR (2010) Influence of DNA extraction methods, PCR inhibitors and quantification methods on real-time PCR assay of biotechnology-derived traits. Anal Bioanal Chem 396:1977–1990
Ke H, Zhuang J, Ding J, Ma Z, Wang F, Zeng X (2017) Influence of biomacromolecule DNA corrosion inhibitor on carbon steel. Corros Sci 125:68–76
Krishnan M, Subramanian H, Dahms H-U, Sivanandham V, Seeni P, Gopalan S, Mahalingam A, Rathinam AJ (2018) Biogenic corrosion inhibitor on mild steel protection in concentrated HCl medium. Sci Rep 8:1–16
Ali MAA (2016) Inhibition of mild steel corrosion in cooling systems by low- and nontoxic corrosion inhibitors. Doctor of Philosophy, University of Manchester
Fink JK (2012) Petroleum engineer’s guide to oil field chemicals and fluids. Elsevier Inc, Amsterdam
Javaherdashti R (2008) Microbiologically influenced corrosion-an engineering insight. Springer, London
Taghavikish M, Dutta NK, Choudhury NR (2017) Emerging corrosion inhibitors for interfacial coating. Coatings 7(217):1–28
UL Hamid A (2017) Corrosion resistant coating composition of Ni and a phosphate corrosion inhibitor and an electrodeposition method for the manufacture thereof. Patent, Pub. No.: US 2017/0190921 A1
Dutral AC, Nunes LP (2011) Cathodic protection- Control techniques. ed., Rio de Janeiro: interciências
Bardal E (2004) Corrosion and protection. Springer, London
Abdel Rahman HH, Seleim SS, Hafez AM, Helmy AA (2015) Study of electropolishing inhibition of steel using natural products as a green inhibitor in ortho-phosphoric acid. Green Chem Lett Rev 8:88–94
Hoar TP, Holiday RD (1953) The inhibition by quinolines and thioureas of the acid dissolution of mild steel. J Appl Chem Banner 3(11):502–513
Cavallaro L, Felloni L, Trabanelli G, Pulidori F (1964) The anodic dissolution of iron and the behaviour of some corrosion inhibitors investigated by the potentiodynamic method. Electrochim Acta 9(5):485
Pillai CK, Narayan R (1983) Anodic dissolution of mild steel in HCl solutions containing thio-ureas. Corros Sci 23(2):151–166
Kondratova IL, Montes P, Bremner TW (2003) Natural marine exposure results for reinforced concrete slabs with corrosion inhibitors. Cem Concr Compos 25:483–490
Ahmed Z (2006) Principles of corrosion engineering and corrosion control. Elsevier, UK
Amini M, Aliofkhazraei M, Kashani AHN, Rouhaghdam AS (2017) Mild steel corrosion inhibition by benzotriazole in 0.5 M sulfuric acid solution on rough and smooth surfaces. Int J Electrochem Sci 12:8708–8732
Marek M (1987) Metals handbook corrosion, vol 13, 9th edn. ASM International, Cleveland
Frankel GS, Hughes AE et al (eds) (2016) Active protective coatings: New-generation coatings for metals. Springer, Dordrecht
Shipilov SA (2002) Mechanism of corrosion fatigue crack propagation. Fatigue Fract Eng Mater Struct 25(3):243–259
Saad AJ, Mohammed IF (2015) Reducing of corrosion rate in boiler tubes by using oxygen scavengers. Iraqi J Chem Pet Eng 16(4):21–29
Kalaivani R, Arasu PT, Rajendran S (2013) Inhibitive nature of carboxymethylcellulose with Zn2+ ion. Chem Sci Trans 2(4):1352–1357
Ait Chikh Z, Chebabe D, Dermaj A, Hajjaji N, Srhiri A, Montemor MF, Ferreira MGS, Bastos AC (2005) Electrochemical and analytical study of corrosion inhibition on carbon steel in HCl medium by 1,12-bis(1,2,4- triazolyl)dodecane. Corros Sci 47:447–459
Söylev TA, Richardson MG (2008) Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr Build Mater 22:609–622
Abbasov VM, Abd El-Lateef HM, Aliyeva LI, Qasimov EE, Ismayilov IT, Khalaf MM (2013) A study of the corrosion inhibition of mild steel C1018 in CO2-saturated brine using some novel surfactants based on corn oil. Egypt J Pet 22:451–470
Coin project report No:22, Corrosion inhibitors: State of the art. Project no.: 3D006151, 2010
Olusegun SJ, Oluwasina OO, Alaneme KK, Olubambi PA (2016) Corrosion inhibition of mild steel in acidic solution by cow dung extract as an eco-friendly inhibitor. J Mater Environ Sci 7(4):1086–1097
Jayaperumal D (2010) Effects of alcohol-based inhibitors on corrosion of mild steel in hydrochloric acid. Mater Chem Phys 119:478–481
Popova A, Christov M, Zwetanova A (2007) Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid. Corros Sci 49:2131–2143
Prenosil M (2001) Supplement to Materials Performance. CORTEC CORP
Ansari FA, Verma C, Siddiqui YS, Ebenso EE, Quraishi MA (2018) Volatile corrosion inhibitors for ferrous and non-ferrous metals and alloys: a review. Int J Corros Scale Inhib 7(2):126–150
Rammelt U, Koehler S, Reinhard G (2009) Use of vapour phase corrosion inhibitors in packages for protecting mild steel against corrosion. Corros Sci 51:921–925
Teixeira DA, Valente MAG Jr, Benedetti AV, Feliciano GT, da Silva SC, Fugivara CS (2015) Experimental and theoretical studies of volatile corrosion inhibitors adsorption on zinc electrode. J Braz Chem Soc 26:434–450
Diki NYS, Bohoussou KV, Kone MGR, Ouedraogo A, Trokourey A (2018) Cefadroxil drug as corrosion inhibitor for aluminum in 1 M HCl medium: experimental and theoretical studies. IOSR J Appl Chem 11(4):4–36
Bastidas DM, Cano E, Mora EM (2005) Volatile corrosion inhibitors: a review. Corros Methods Mater 52:71–77
Premkumar P, Kannan K, Natesan M (2009) Evaluation of menthol as vapor phase corrosion inhibitor for mild steel in NaCl environment. Arab J Sci Eng 34:71–79
Myles K (1995) Corrosion control: Principles and practice, 1st edn. South Africa, Myles Publication, Randburg
Nhlapo NS (2013) TGE-FTIR study of the vapours released by volatile corrosion inhibitor mode systems. PhD Thesis, University of Pretoria
Singh A, Ebenso EE, Quraishi MA (2012) Corrosion inhibition of carbon steel in HCl solution by some plant extracts. Int J Corros 1:20
Rajam K, Rajendran S, Saranya R (2013) Allium sativum (Garlic) extract as nontoxic corrosion inhibitor. J Chem 2013:1–4
Mahmoud SS, Ahmed MM (2006) Corrosion inhibition of carbon steel in HCl solution using aminopyrimidine derivatives. Portugaliae Electrochem Acta 24:37–52
Guo L, Obot IM, Zheng X, Shen X, Qiang Y, Kaya S, Kaya C (2017) Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl Surf Sci 406:301–306
Muralidhan S, Phani KLN, Pitchumani S, Iyer SVK (1995) Polyamino-benzoquinone polymers: a new class of corrosion inhibitors for mild steel. J Electrochem Soc 142:1478–1483
Rani BEA, Basu BBJ (2011) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros 1:15
Kaya S, Tüzün B, Kaya C, Obot IB (2016) Determination of corrosion inhibition effects of amino acids: quantum chemical and molecular dynamic simulation study. J Taiwan Inst Chem Eng 58:528–535
Kıcır N, Tansuğ C, Erbil M, Tüken T (2016) Investigation of ammonium (2,4-dimethylphenyl)- dithiocarbamate as a new, effective corrosion inhibitor for mild steel. Corros Sci 105:88–99
Tian H, Li W, Hou B, Wang D (2017) Insights into corrosion inhibition behavior of multi-active compounds for X65 pipeline steel in acidic oilfield formation water. Corros Sci 117:43–58
Fotouhi G (2015) Study of microtip-based extraction and purification of DNA from human samples for portable devices. PhD Dissertation, University of Washington
Lipfert J, Doniach S, Das R, Herschlag D (2014) Understanding nucleic acid-ion interaction. Annu Rev Biochem 83:813–841
Karn SK, Fang G, Duan J (2017) Bacillus sp. acting as dual role for corrosion induction and corrosion inhibition with carbon steel (CS). Front Microbiol 8:1–11
Tüzün B, Kaya C (2018) Investigation of DNA-RNA molecules for the efficiency and activity of corrosion inhibition by DFT and molecular docking. J Bio Tribo-Corros 4:69
Agboola O, Achile F, Fayomi SO, Sanni SE, Abatan O, Sadiku ER, Popoola P, Mubiayi MP, Akinlabi ET, Makhatha ME, Adedotin T, Ekere I (2019) Adsorptive performance mechanism of the DNA of calf thymus gland (CTGDNA) on 3CR12 stainless steel as corrosion inhibitor in acidic medium. J Bio Tribo-Corros 5:52
Agboola O, Adedoyin T, Sanni SE, Fayomi SO, Omonidgbehin EA, Adegboye BE, Ayoola A, Omodara O, Ayeni AO, Popoola P, Sadiku R, Alaba PA (2019) Evaluation of DNA from Manihot esculenta leaf (Cassava leaf) as corrosion inhibitor on mild steel in acidic environment. Anal Bioanal Electrochem 11(10):1304–1328
Stewart R (2011) deoxyribonucleic acid (DNA). School of Health Sciences and Purdue University. http://faculty.washington.edu/trawets/vc/theory/dna/index.html. Accessed 23 Nov 2018
Audain K (2018) The difference of the genomic DNA extraction between animal and plant. Sciencing. https://sciencing.com/difference-genomic-dna-extraction-between-animal-plant-2551.html. Assessed 26 Nov 2018
Cai B, Wang S, Huang L, Ning Y, Zhang Z, Zhang G-J (2014) Ultrasensitive label-free detection of PNA-DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano 8:2632–2638
Ahmed MU, Nahar S, Safavieh M, Zourob M (2013) Real-time electrochemical detection of pathogen DNA using electrostatic interaction of a redox probe. Analyst 138:907–915
Md Mahbubur R, Li X-B, Lopa NS, Ahn SJ, Lee J-J (2015) Electrochemical DNA hybridization sensors based on conducting polymers. Sensors 15:3801–3829
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agboola, O., Sanni, S.E., Fayomi, S.O. et al. Prospects of DNA Macromolecule for Corrosion Inhibitor Applications: A Mini Review. J Bio Tribo Corros 6, 7 (2020). https://doi.org/10.1007/s40735-019-0308-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0308-7