Abstract
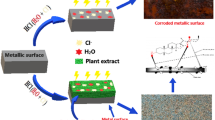
Deterioration of metals in the form of corrosion is the major problem faced in almost all industries. Annual cost of corrosion world-wide has been estimated to exceed 3% of world’s GDP. Corrosion control of metals is an activity of economic, environmental, aesthetic, and technical importance. Zinc, one of the most important non-ferrous metals, has a wide range of applications in industry as well as in domestic sectors. It is used as a coating material on steel and iron. Corrosion of zinc and galvanized articles can be prevented by using chemical inhibitors. Even though they are proven to be effective for industrial applications, environmental hazards resulted as a consequence cannot be ignored. Over the last decades, research is more focussed towards the use of environmental friendly green inhibitors. In this regard, plant extracts have become important as an environmentally acceptable, readily available, and renewable source for a wide range of inhibitors. This review consolidates and documents an overview of the application of plant products as corrosion inhibitors for the corrosion mitigation of zinc.












Similar content being viewed by others
References
Zhang XG (1996) Corrosion and electrochemistry of zinc. Springer, New York
Narayana H, Praveen BM, Prasanna BM, Venkatesha TV (2015) Anticorrosion potential of a pharmaceutical intermediate Floctafenine for zinc in 0.1 M HCl solution. Int J Ind Chem 6:221–231
Odnevall WI, Leygraf C (2017) A critical review on corrosion and runoff from zinc and zinc-based alloys in atmospheric environments. Corros 73:1060–1061
Peter M, Peter P (2011) Corrosion and corrosion protection-handbook of hot-dip galvanization. Wiley, Germany
Pandian BR, Ismail M, Seyedmojtaba G, Jahangir M, Mokthar CI, Saeid K, Afidah AR (2016) Reviews on corrosion inhibitors—a short view. Chem Eng Commun 203:1145–1156
Fiori-Bimbi MV, Alvarez PE, Vaca H, Gervasi CA (2015) Corrosion inhibition of mild steel in HCl solution by pectin. Corros Sci 92:192–199
Roy P, Karfa P, Adhikari U, Sukul D (2014) Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: effect of intramolecular synergism. Corros Sci 88:246–253
Sigircik G, Tuken T, Erbil (2016) Assessment of the inhibition efficiency of 3,4-diamino benzonitrile against the corrosion of steel. Corros Sci 102:437–445
Chigondo M, Chigondo F (2016) Recent natural corrosion inhibitors for mild steel: an overview. J Chem 2016:1–7
Sanjay KS, Ackmez M, Essam K, Gargi J (2008) Green corrosion inhibitors: an overview of recent research. Int J Corros Sci Eng 11:1–32
Znini M, Majidi L, Bouyanzer L, Paolini J, Desjobert JM, Costa J, Hammouti B (2012) Essential oil of Salvia aucheri mesatlantica as a green inhibitor for the corrosion of steel in 0.5 M H2SO4. Arab J Chem 5:467–474
Deepa P, Padmalatha R (2013) Coriandrum sativum L-A novel green inhibitor for the corrosion inhibition of aluminium in 1.0 M phosphoric acid solution. J Environ Chem Eng 1:676–683
Stefania M, Luisella V, Stefano PT (2019) Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019:24–48
Hofer R, Bigorra J (2008) Biomass-based green chemistry: sustainable solutions for modern economies. Green Chem Lett Rev 1:79–97
Ismail M, Abdulrahman AS, Hussain MS (2011) Solid waste as environmental benign corrosion inhibitors in acidic medium. Int J Eng Sci 3:1742–1748
Odewunmi NA, Umoren SA, Gasem ZM (2015) Watermelon waste products as green corrosion inhibitors for mild steel in HCl solution. J Environ Chem Eng 3:286–296
Grassino AN, Halambek J, Djakovic S, Rimac Brncic S, Dent M, Grabaric Z (2016) Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll 52:265–274
Pramudita M, Sukirno Nasikin M (2018) Rice husk extracts ability to reduce the corrosion rate of mild steel. Int J Chem Eng App 9:143–146
Amr AK, Samy S, Mohamed N, Mona R (2018) Effect of fungal glycolipids produced by a mixture of sunflower oil cake and pineapple waste as green corrosion inhibitors. J Environ Sci Technol 11:119–131
Rexin Thusnavis G, Vinod Kumar KP (2014) Green corrosion inhibitor for steel in acid medium. Application No. 6278/CHE/2014 A, 12 December 2014
Extract Corrosion Inhibitor of Sweet Potato Stems and Lettuce Flower Stalks and Preparation Method Thereof. Patent No. CN102492948B, 31 July 2013
Indian Oil Corporation Limited. Naturally derived corrosion inhibitors composition, process for preparing the same and use thereof 2008. Patent Application 10 March 2008
Ponciano Gomes JA, Cardoso Rocha J, D’Elia E (2015) Use of fruit skin extracts as corrosion inhibitors and process for producing same. U.S. Patent US8926867B2, 6 January 2015
Raja PB, Sethuraman MG (2008) Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62:113–116
Sangeetha M, Rajendran S, Muthumegala TS, Krishnaveni A (2011) Green corrosion inhibitors—an overview. Zastita Materijala 52:1–19
Hassane L, Subrahmanya BK, Rachid S, Shubhalaxmi C, Shehdeh J, Manuel A, Belkheir H, Ismat HA, Azzouz E (2017) Insights into corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution. J Mol Liq 225:271
Lgaz H, Salghi R, Jodeh S (2016) Corrosion inhibition potentiality of some benzimidazole derivatives for mild steel in hydrochloric acid: electrochemical and weight loss studies. Int J Corros Scale Inhib 5:347–359
Lgaz H, Toumiat K, Jodeh S, Salghi R (2016) Inhibition of mild steel corrosion in 1 M HCl medium by tangeretin. Appl J Environ Eng Sci 2:72–77
Selvakumar P, Balanaga KB, Thangavelu C (2013) Corrosion inhibition study of stainless steel in acidic medium—an overview. Res J Chem Sci 3:87–95
Binder L, Kordesch K (1984) Corrosion of zinc electrode mixtures in alkaline media. J Electroanal Chem Interfacial Electrochem 180:495–510
Ismail WMIM, Zulkefeli NSW, Masri MN (2016) A sight of zinc corrosion in various alkaline media. J Trop Resour Sustain Sci 4:95–97
Tuan KAH, Nam LD, Kyung EKS, Chen P (2015) Corrosion chemistry and protection of zinc & zinc alloys by polymer-containing materials for potential use in rechargeable aqueous batteries. RSC Adv 5:41677–41691
Lindström R, Svensson JE, Johansson LG (2000) The atmospheric corrosion of zinc in the presence of NaCl the influence of carbon dioxide and temperature. J Electrochem Soc 147:1751
Mouanga M, Berçot P, Rauch JY (2010) Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I: corrosion layer characterization. Corros Sci 52:3984–3992
Anjali P, Obot IB, Sanjay KS (2015) Use of natural gums as green corrosion inhibitors: an overview. Int J Ind Chem 6:153–164
Yıldırım A, Çetin M (2008) Synthesis and evaluation of new long alkyl side chain acet- amide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors. Corros Sci 50:155–165
Foad El-Sherbini E, Abdel Wahaab ESM, Deyab M (2005) Ethoxylated fatty acids as inhibitors for the corrosion of zinc in acid media. Mater Chem Phys 89:183–191
Morad MS (1999) Inhibition of phosphoric acid corrosion of zinc by organic onium compounds and their adsorption characteristics. J Appl Electrochem 29:619–626
Talati JD, Desai MN, Shah NK (2005) meta-Substituted aniline-N-salicylidenes as corrosion inhibitors of zinc in sulphuric acid. Mater Chem Phys 93:54–64
Shylesha BS, Venkatesha TV, Praveen BM (2011) Ziprasidone as a corrosion inhibitor for zinc in different acid medium. J Chem Pharm Res 3:501–507
Fouda AS, Madkour LH, Shafei AAE, Abd El-Maksoud SA (1995) Corrosion inhibitors for zinc in 2 M HCl solution. Bull Korean Chem Soc 16:454–458
Agrawal Y, Talati K, Shah JD, Desai MD, Shah NK (2004) Schiff bases of ethylenediamine as corrosion inhibitors of zinc in sulphuric acid. Corros Sci 46:633–651
Shylesha BS, Venkatesha TV, Praveen BM (2011) New electroactive compounds as corrosion inhibitors for zinc in acidic medium. Adv Appl Sci Res 2:333–341
Abdallah M (2003) Ethoxylated fatty alcohols as corrosion inhibitors for dissolution of zinc in hydrochloric acid. Corros Sci 45:2705–2716
Yadav M, Gope L, Kumari N, Yadav P (2016) Corrosion inhibition performance of pyranopyrazole derivatives for mild steel in HCl solution: gravimetric, electrochemical and DFT studies. J Mol Liq 216:78–86
Quraishi MA, Yadav DK, Ahamad I (2009) Green approach to corrosion inhibition by black pepper extract in hydrochloric acid solution. Open Corros J 2:56–60
Rani BEA, Basu BBJ (2012) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros 2012:1–15
Lahhit N, Bouyanzer A, Desjobert JM (2011) Fennel (Foeniculum vulgare) essential oil as green corrosion Inhibitor of carbon steel in hydrochloric acid solution. Port Electrochim Acta 29:127–138
Emran KM, Ali SM, Lehaibi HAA (2018) Green methods for corrosion control. Corros Inhib Princ Recent Appl 3:61–78
Ali AI, Megahed HE, Mona AE, Ismail MN (2013) Zinc corrosion in HCl in the presence of aqueous extract of Achillea fragrantissima. J Mater Environ Sci 5:923–930
Abiola OK, James AO (2010) The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros Sci 52:661–664
Sanjay K, Mudhoo A, Jain G, Jyoti S (2009) Inhibitory effects of Ocimum tenuiflorum (Tulsi) on the corrosion of zinc in sulphuric acid: a green approach. Rasayan J Chem 2:332–339
Abdel-Gaber AM (2007) Effect of immersion time and temperature on the inhibition of the acid corrosion of zinc by fenugreek seeds extract. Int J Appl Chem 3:161–174
El-Housseiny S (2017) Inhibition of the acid corrosion of zinc by Cannabis plant extract. Am J Chem 7:6–15
Dass PM, Onen AI, Maitera ON, Ushahemba G (2015) Corrosion inhibition of zinc in acid medium by Moringa oleifera and Mangifera indica leaves extracts. Int J Dev Sustain 4:940–950
Suedile F, Robert F, Roos C, Lebrini M (2014) Corrosion inhibition of zinc by Mansoa alliacea plant extract in sodium chloride media: extraction, characterization and electrochemical studies. Electrochim Acta 133:631–638
Orubite OK, Oforka NC (2004) Corrosion inhibition of zinc on HCl using Nypa fruticans Wurmb extract and 1,5 diphenyl carbazonen. J Appl Sci Environ Manag 8:56–61
Ugi B, Ekerete J, Ikeuba IA, Uwah IE (2015) Mangifera indica leave extracts as organic inhibitors on the corrosion of zinc sheet in 5 M H2SO4 solution. J Appl Sci Environ Manag 19:145–152
James AO, Akaranta O (2011) Inhibition of corrosion of zinc in hydrochloric acid solution by red onion skin acetone extract. Res J Chem Sci 1:31–37
Abd-El-Naby BA, Abdullatef OA, Abd-El-Gaber AM, Shaker MA, Esmail G (2014) Electrochemical studies on the inhibitive action of Damssisa and Halfabar on the alkaline corrosion of zinc. Int J Electrochem Sci 9:1163–1178
Abd-El-Naby BA, Abdullatef OA, Abd-El-Gaber AM, Shaker MA, Esmail G (2012) Effect of some natural extracts on the corrosion of zinc in 0.5 M NaCl. Int J Electrochem Sci 7:5864–5879
Sobhi M (2013) Marjoram extract as corrosion inhibitor for dissolution of zinc in 1.0 M HCl. Int J Corros 2013:1–7
Sanjay KS, Ackmez M, Gargi J, Essam K (2009) Corrosion inhibition of neem (Azadirachta indica) leaves extract as a green corrosion inhibitor for Zinc in H2SO4. Green Chem Lett Rev 2:47–51
Chauhan JS, Anita D, Gupta DK (2013) Corrosion inhibition of Zn in HCL by Nictanthes plant extract. Asian J Adv Basic Sci 1:58–61
Neha P, Divya L, Poonam W, Mukesh M, Suresh K, Nisha S (2015) Comparative study of natural inhibitor and Np-natural Inhibitor for the corrosion protection of Zinc in HCl. Nano Vision 5:295–304
Wang C, Zhang J, Chen XL, Xiang B, Duan JZ, Hou BR (2016) Inhibition of zinc corrosion by Fucoidan in natural sea water. Acta Metall Sin 30:594–600
Umoren SA, Eduok UM (2016) Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr Polym 140:314–341
Charitha BP, Padmalatha R (2017) Environmentally benign green inhibitor to attenuate acid corrosion of 6061Aluminum15%(v) SiC(P) composite. J Ind Eng Chem 58:357–368
Charitha BP, Padmalatha R (2017) Carbohydrate biopolymer for corrosion control of 6061 Al-alloy and 6061Aluminum-15%(v) SiC(P) composite—Green approach. Carbohydr Polym 168:337–345
Charitha BP, Padmalatha R (2018) Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: electrochemical and surface studies. Int J Biol Macromol 112:461–472
Charitha BP, Padmalatha R (2016) An ecofriendly approach for corrosion control of 6061 Al-15%(v) SiC(P) composite and its base alloy. Chin J Chem Eng 25:363–372
Charitha BP, Padmalatha R (2017) Enhancement of surface coating characteristics of epoxy resin by dextran: an electrochemical approach. Ind Eng Chem Res (ACS publications) 56:1137–1147
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pais, M., Rao, P. Biomolecules for Corrosion Mitigation of Zinc: A Short Review. J Bio Tribo Corros 5, 92 (2019). https://doi.org/10.1007/s40735-019-0286-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0286-9