Abstract
Background: Increasing evidence suggests that diabetes increases the risk of developing different types of cancer. Hyperinsulinemia, hyperglycemia and chronic inflammation, characteristic of diabetes, could represent possible mechanisms involved in cancer development in diabetic patients. At the same time, cancer increases the risk of developing new-onset diabetes, mainly caused by the use of specific anticancer therapies. Of note, diabetes has been associated with a ∼10% increase in mortality for all cancers in comparison with subjects who did not have diabetes. Diabetes is associated with a worse prognosis in patients with cancer, and more recent findings suggest a key role for poor glycemic control in this regard. Nevertheless, the association between glycemic control and cancer outcomes in oncologic patients with diabetes remains unsettled and poorly debated. Purpose: The current review seeks to summarize the available evidence on the effect of glycemic control on cancer outcomes, as well as on the possibility that timely treatment of hyperglycemia and improved glycemic control in patients with cancer and diabetes may favorably affect cancer outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
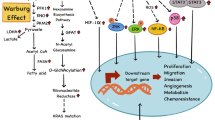
The incidence of diabetes is rapidly spreading worldwide. In 2021, 537 million adults (20–79 years) were living with diabetes (9.2% of adults), over 90% of whom with type 2 diabetes (T2D). This number is predicted to rise to 643 million by 2030 and 783 million by 2045 [1]. Diabetes is often burdened by disabling comorbidities, such as cardiovascular and renal complications, that reduce the quality of life and life expectancy of the affected individuals [2]. Of note, diabetes and its complications were responsible for 6.7 million deaths in 2021 (1 every 5 s) [1]. Interestingly, in recent years, the advances in diabetes management and increase in the life expectancy of diabetic patients have made it possible to identify less-recognized and longer-term comorbidities, defined as emerging complications of diabetes, including cancer [3]. Of note, the increase in the incidence of diabetes is paralleled by the increasing incidence of cancer [4, 5]. Patients with diabetes, particularly T2D, are characterized by an increased risk of developing different types of cancer (especially bladder, breast, colorectal, endometrial, gallbladder, liver, and pancreatic cancers) and reduced survival after cancer diagnosis [6, 7]. At the time of cancer diagnosis, ⁓18% of patients have pre-existing diabetes, and it is estimated that approximately 20% of people with cancer have or will develop diabetes [8, 9], more than double the incidence of diabetes in the global adult population. The magnitude of risk between diabetes and cancer varies across cancer sites. For hepatocellular, pancreatic, and endometrial cancers, the increased risk associated with diabetes may be up to two-fold, whereas for other cancers, such as colon and breast, the relative risk increases are closer to 20–40% [10]. On the other hand, the evidence regarding the associations of T2D with other cancers such as kidney and lung cancer remains inconclusive [6, 11, 12]. The relationship between prostate cancer and diabetes is unique, since it is the only cancer where diabetes appears to be protective [13]. The coexistence of diabetes and cancer may be related to these as widespread pathologies making the probability of their occurrence in the same patient very high. Indeed, diabetes and cancer share many risk factors, such as obesity, sedentary lifestyle, unbalanced diet, cigarette smoking, and excessive alcohol consumption, which may further increase the likelihood of co-occurrence [12, 14]. Nevertheless, growing evidence suggests that the link between diabetes and cancer may be causal with these two pathological conditions triggering each other. For instance, many cancer cells overexpress insulin receptors, especially the pro-proliferative A isoform, and therefore are more responsive to the mitogenic effects of insulin [14]. In this context, the hyperinsulinemia typical of the early stages of T2D may stimulate cancer cells proliferation [10, 14]. Insulin may also promote carcinogenesis through indirect mechanisms, via reduction in circulating levels of insulin-like growth factor (IGF)-binding proteins, leading to excess IGF-1 and IGF-2, which further promote cancer cell proliferation [10, 15]. In addition, since glucose excess is an important source of energy for cancer cells (“Warburg effect”), hyperglycemia typical of diabetes could promote tumor growth [10, 12, 14, 16]. Hyperglycemia and insulin resistance may be also responsible for further increase in insulin secretion. Chronic low-grade inflammation characteristic of diabetes may also promote neoplastic transformation, cancer cell proliferation, and tumor spreading [14]. In addition, hyperinsulinemia, hyperglycemia, and inflammation can intensify the production of reactive oxygen species, therefore promoting oxidative stress [17], which is known to be a biological event able to trigger or enhance the tumorigenic process [14], especially when it involves tumor suppressor genes [18]. Recently, it has been suggested that several miRNAs, which mainly regulate the insulin signaling pathway, may be involved in the pathogenesis of both diabetes and cancer [19]. In addition, some endocrine disruptors derived from commonly employed compounds for manufacturing and processing, particularly polybrominated diphenyl ethers (PBDEs), may interfere with both metabolic and oncogenic pathways [20]. Lastly, over the years it has been hypothesized that some anti-diabetes drugs may be responsible for the increased risk of cancer in patients with diabetes. In 2009, 4 independent studies [21,22,23,24] suggested that exogenous insulin may be associated with an increased risk of cancer, although more recent epidemiological studies seem to refute this hypothesis [25,26,27]. Similarly, incretin drugs (GLP-1 receptor agonists [GLP-1RAs] and DPP-4 inhibitors [DPP-4i]) were initially associated with an increased risk of pancreatic and medullary thyroid cancers, although this association has not been confirmed in more recent studies [28,29,30]. In 2018, an increased risk of cholangiocarcinoma was reported in patients treated with DPP-4i [31]. Although this correlation remains to be validated, it is supported by the biological evidence that high levels of GLP-1 are associated with reduced apoptosis and increased proliferation of cholangiocytes [32, 33]. In 2011, the Food and Drug Administration issued a warning regarding the use of pioglitazone [34], after studies had shown an association between its use and a higher risk of bladder cancer [35]. Since then, numerous studies have been conducted and a recent meta-analysis reported a small but statistically significant increase in the risk of bladder cancer in patients treated with pioglitazone [36]. A similar risk has also been observed in patients treated with rosiglitazone [37]. Finally, preclinical studies have suggested that the use of SGLT2 inhibitors (SGLT2i) may be associated with breast [38], adrenal, testicular and renal cancers [39]. However, safety data from clinical trials and a recent meta-analysis do not suggest an association between the use of SGLT2i and overall cancer risk [40], although a possible increased risk of bladder cancer has been reported in patients being treated with empagliflozin [40]. On the other hand, tumor cachexia is often associated with glucose intolerance, insulin resistance, and inflammation, which predispose to T2D development [41]. Cancer-related stress (especially due to acute illnesses, recurrent hospitalizations, surgeries, infections, and hemorrhages) can also induce hyperglycemia and worsen inflammation [41]. Finally, diabetes may occur when cancer affects organs involved in glycemic homeostasis, such as the pancreas and liver [4]. In addition to the increased risk of developing cancer, diabetes has been associated with a ∼10% increase in mortality for all cancers (up to 25% for several types of cancer) when compared to the absence of diabetes [42, 43]. As a consequence, while deaths from vascular diseases (which once accounted for more than 50% of the deaths in diabetic patients) have declined, in some countries, cancer has become the leading causes of mortality in people with diabetes [44, 45]. Diabetes may predict a worse prognosis in patients with cancer [42, 46], and more recent findings suggest a key role for poor glycemic control in this scenario [47]. Although only few studies have evaluated the association between glycemic control and survival in patients with both diabetes and cancer [47], several retrospective studies suggest that inadequate glycemic control during cancer follow-up could be associated with poorer tumor response to therapy and survival in patients with diabetes [48, 49]. Unfortunately, in diabetic patients with cancer, oncologists and patients are inclined to prioritize cancer treatment [50, 51] and may accept less stringent glycemic control as a justifiable adverse effect of that treatment [52]. Indeed, cancer treatment is associated with decreased diabetes medication adherence and self-management behaviors such as blood glucose monitoring [53,54,55,56]. In addition, several cancer therapies, such as corticosteroids, specific chemotherapies, immune checkpoint inhibitors, and somatostatin analogs, can directly affect glucose homeostasis, thus increasing the risk of hyperglycemia and posing significant difficulties for diabetes management [57, 58]. Despite its importance, the association between glycemic control and cancer outcomes in diabetes patients with cancer remains unsettled and poorly debated. This review article seeks to summarize the available evidence about the possibility that timely treatment of hyperglycemia and improved glycemic control in diabetic patients with cancer can favorably affect cancer outcomes, underlining the importance of careful management of hyperglycemia also in patients with cancer.
Effects of glycemic control on cancer progression in oncologic patients with diabetes
To date, most studies analyzing the correlation between glycemic control and cancer progression in diabetic patients with cancer have been retrospective in nature and have taken under consideration heterogeneous outcomes to evaluate cancer progression (Table 1). In addition, there is little consistency in how glycemic control is assessed across studies (HbA1c, fasting glucose levels [FG], or random blood glucose [RBG], with different measurement timing and cut-off points) [8]. Above all, it should be mentioned that in patients treated with anti-cancer drugs, HbA1c measurements could be misleading due to interfering non-glycemic factors such as anemia, impaired hematopoiesis, iron, vitamin B12 or folate deficiency, red blood cell transfusion and erythropoiesis-stimulating agent [59]. This makes studies very heterogeneous and difficult to be compared. Moreover, it has been shown that diabetes is associated with more advanced cancer stage and that oncologists might modify anticancer treatments in patients with cancer and diabetes because of increased rates of adverse effects and complications. Despite this, studies analyzing the association between diabetes and mortality from cancer rarely take into account stage at cancer diagnosis or cancer treatments, and this may affect the results [60, 61]. In addition, T2D patients are often obese, implying the need for adequate adjustment of the antineoplastic dosing [62]. Unfortunately, there is a lack of pharmacokinetics data on obese patients for the majority of chemotherapeutic agents, as well as for new cancer targeted therapies and immunotherapy agents [62]. Despite these flaws, most studies suggest that adequate glycemic control may be associated with more favorable neoplastic outcomes, in terms of survival, progression and cancer recurrence. In a recent meta-analysis [63] including twelve studies comprising a total of 9,872 patients with cancer, hyperglycemia was associated with worse overall survival (OS) (Hazard Ratio [HR] 2.05, 95% Confidence Interval [CI] 1.67–2.51) and disease-free survival (DFS) (HR 1.98, 95% CI 1.20–3.27), without any correlation with neoplastic progression-free survival (PFS) (Table 1). The association between hyperglycemia and OS was independent of the method of measuring blood glucose and stage of neoplastic disease. Similarly, a prospective 12-week longitudinal study [64] showed that, in 18 adult patients with T2D and a solid or hematological tumor receiving outpatient intravenous chemotherapy, a good glycemic control (HbA1c < 7.0%) at the onset of cancer therapy may contribute to less adverse events, infections and hospitalizations, and to diminish the number of cases in which a reduction in dosage or an interruption of chemotherapy was necessary (Table 1); however, this study is limited by the low number of enrolled patients. In a ten-year prospective cohort study of 1,298,385 Koreans, a linear trend in cancer-related mortality with increasing FG was observed in patients with a FG > 125 mg/dL compared with those with FG < 90 mg/dL for most cancer sites (in particular, pancreas, liver, and breast in women) [65] (Table 1). However, in this study, glucose testing was done at one time point at baseline, which may not be a reflection of persistent hyperglycemia. Likewise, in an analysis conducted on 820,900 subjects from 97 retrospective studies, it was observed that, as compared with the reference group (FG of 70 to 100 mg/dL), patients with a FG of 126 mg/dL or more exhibit an HR of 1.39 (95% CI, 1.22 to 1.59) for cancer deaths [43]. A HR for cancer death of 1.05 (95% CI 1.03–1.06) for every 1 mmol/l increase in glucose levels above 100 mg/dL was also reported [43] (Table 1). However, in this analysis, the glucose measurements were not conducted in the proximity of cancer diagnosis and thus do not reflect the levels of glycemic control at that time (it is possible that those measurements may be more relevant for assessing cancer risk rather than outcomes). In addition, this study looked at all cancer related deaths instead of death from specific types of cancer [66]. Several observational studies also suggest that inadequate glycemic control, in the pre- or postoperative of a surgical cancer treatment, significantly worsens clinical outcomes of cancer and increases the risk of cancer recurrence [50]. Furthermore, in diabetic patients with cancer, poor glycemic control could exacerbate the risk of postoperative or post-chemotherapy infections and increase the perception of pain and fatigue often experienced by oncologic patients [50]. On the contrary, in a retrospective study conducted on 7916 individuals with incident cancers and concurrent diabetes [66], higher glucose and HbA1c levels within 6 months prior to cancer diagnosis was not associated with worse OS following cancer diagnosis. Interestingly, among diabetic patients treated with insulin, increased survival with increasing serum glucose was observed, most prominent for bladder cancer (HR 0.91, 95% CI 0.84–0.99, per 1 mmol/l increase) [66] (Table 1). Similarly, in a cross-sectional study conducted on 244 diabetic or prediabetic patients with breast, gastrointestinal, gynecological or lung cancer, adequate glycemic control was not associated with the severity of tumor-related symptoms or with the patient’s quality of life [67] (Table 1). Very few studies have analyzed the importance of glycemic control in diabetic patients with terminal cancers. However, all such studies agree that, at this stage of the oncologic disease, glycemic control may play a role in symptom management and prolonging survival [8, 68]. Hypoglycemia has also been associated with a poorer prognosis in patients with diabetes and cancer [69]. In a prospective cohort analysis of 1209 participants with diagnosed diabetes from the Atherosclerosis Risk in Communities study, severe hypoglycemia was significantly associated with cancer mortality (HR 2.49, 95% CI 1.46–4.24) [70] (Table 1). Severe hypoglycemia is very likely an indicator of frailty, which is causally linked to poor cancer survival [69]. In the following sections, we will summarize the available evidence about the correlation between glycemic control and cancer outcomes in diabetic patients with cancers most linked to diabetes (bladder, breast, colon/rectum, endometrium, liver, pancreas, and prostate). To the best of our knowledge, no studies have explored such a correlation in diabetic patients with gallbladder or biliary tract cancer.
Bladder
Diabetes has been associated with higher incidence and poor prognosis of bladder cancer [71]. Furthermore, poor glycemic control results in increased oxidative stress and inflammation, which are thought to play a negative effect on bladder cancer prognosis [71]. A retrospective study conducted on 287 patients with non-muscle invasive bladder cancer (61 with DM and 266 without DM) revealed higher recurrence rate and worse recurrence-free survival (RFS) in patients with HbA1c ≥ 7% [71] (Table 2). Of note, the use of metformin or thiazolidinediones, which may influence bladder cancer outcomes, was not associated with RFS [71]. Similarly, a retrospective study on 538 patients with upper urinary tract urothelial carcinoma, has demonstrated that poor glycemic control (HbA1c ≥ 7%) is associated with increased risk of subsequent bladder cancer recurrence (HR 2.10, 95% CI 1.14–3.88) [72] (Table 2). Likewise, in a cohort of 645 patients with non-muscle invasive bladder cancer analyzed retrospectively, diabetic patients with a HbA1c ≥ 7% demonstrated a higher rate of cancer progression [73]. Kaplan–Meier analysis showed that poor baseline glycemic control and post-operative glycemic control were associated with lower PFS rate [73] (Table 2). Of note, use of metformin had no impact on the recurrence and progression of cancer [73]. Finally, in a cohort of 251 patients who underwent transurethral resection for non-muscle invasive bladder cancer analyzed retrospectively, it was observed that patients with HbA1c ≥ 7% exhibited a significantly higher rate of multiplicity and tumor grade [74] (Table 2). These results underscore the need for intensive glycemic control and close follow-up for diabetic patients with bladder cancer.
Breast
Diabetes is a known risk factor for the development of breast cancer. Approximately 10 to 20% of all postmenopausal women with breast cancer of any stage or receptor subtype have coexisting T2D [75]. Studies investigating the effects of glycemic control on breast cancer outcomes have yielded mixed results. In a recent prospective study conducted on 620 patients with breast cancer with a follow-up of approximatively 6 years, the HRs and 95% CI for mortality rate was higher in patients with inadequate glycemic control prior to cancer diagnosis compared with patients with glycemic control at target (HR 1.40, 95% CI 1.00–1.96) [47] (Table 3). In a retrospective study conducted on 243 patients with non-metastatic breast cancer with or without diabetes receiving neoadjuvant or adjuvant cytotoxic chemotherapy, higher utilization of emergency departments and higher frequency of unplanned inpatient admissions were detected in patients with HbA1c > 7% compared to those with HbA1c ≤ 7% [76]. In addition, patients with HbA1c > 7% showed a shorter time until the first emergency department visit and experienced more adverse events compared to those with HbA1c ≤ 7% [76]. Moreover, the percentage of documented infections was higher among oncologic patients with HbA1c > 7% compared to those without diabetes [76] (Table 3). Chang YL et al. have retrospectively analyzed 2812 women with early breast cancer (145 with and 2667 without diabetes), demonstrating the existence of a relationship between glycemic control and breast cancer prognosis in women with diabetes: specifically, a mean HbA1c > 9% in breast cancer women was associated with a 3.65-fold (95% CI 1.13–11.82) higher risk of all-cause mortality, including cancer-specific mortality, while patients with well-controlled diabetes (HbA1c < 7%) had comparable survival to individuals without diabetes [77]. In addition, lower HbA1c (< 7%) may be associated with more favorable breast cancer progression outcomes [77] (Table 3). Similarly, a substudy of the Women’s Healthy Eating and Living (WHEL) study found that hyperglycemia (HbA1c ≥ 7%) was statistically significantly associated with reduced OS but not with DFS (HR 1.26, 95% CI 0.78–2.02) in 3,003 individuals with early breast cancer [78]. In addition, the risk of all-cause mortality was twice as high in individuals with a HbA1c ≥ 7%, suggesting that good glycemic control may be associated with better breast cancer prognosis [78] (Table 3). In contrast, Cheung YMMM et al. retrospectively compared 244 patients with diagnosis of metastatic breast cancer with diabetes to 244 patients with diagnosis of metastatic breast cancer without diabetes [79]. OS was found not to differ among patients with good glycemic control (RBG ≤ 180 mg/dL or HbA1c ≤ 7%) compared to those with poor control [79]. However, poor glycemic control was associated with greater mortality in longer-term cancer survivors [79] (Table 3). Interestingly, at 5 years, there was a trend toward a better OS among patients who received metformin monotherapy compared to those who received metformin in addition to other glucose-lowering agents, as well as those who did not received metformin [79]. Moreover, a retrospective cohort study including 82 patients with breast cancer found that OS was not statistically different among participants with HbA1c < 6.5% and ≥ 6.5% [80] (Table 3). It should be noted that several of these studies did not adjust for confounders such as receptor subtype, cancer stage, or medication regimen and usually relied on a single HbA1c measurement to define glycemic control [79].
Colon-rectum
Diabetes is also a known risk factor for the development of colorectal cancer. Several studies have investigated the effects of glycemic control on colorectal cancer outcomes. In a recent prospective study conducted on 774 patients with colorectal cancer with a follow-up of approximatively 6 years, the HRs and 95% CI for mortality was higher in patients with glycemic control not at target prior to cancer diagnosis compared with patients at target (HR 1.45, 95% CI 1.12–1.88) [47] (Table 4). Similarly, in 741 patients with colon cancer analyzed retrospectively, the concomitant presence of uncontrolled diabetes (HbA1c ≥ 8%) resulted in significantly shorter OS and higher mortality compared to well-controlled diabetic patients [81] (Table 4). Similar results have been obtained in a case–control study involving 224 patients with colorectal cancer and 112 controls [82]. Elevated HbA1c levels showed a negative prognostic value both in terms of PFS (HR = 1.24) and OS (HR = 1.36) after adjustment for major confounders [82] (Table 4). Likewise, Siddiqui AA et al. have shown that, in 155 patients with T2D and colorectal cancer compared to 114 control patients who had colorectal cancer without T2D, poor glycemic control (HbA1c ≥ 7.5%) was associated with a more clinically aggressive cancer course (advanced cancer stage, younger age of cancer presentation, and poorer 5-year survival) [83] (Table 4). To the best of our knowledge, only one study has shown conflicting data [84]. It is a retrospective cohort study conducted on 210 patients with advanced colorectal cancer and concomitant T2D, which demonstrated that the OS of patients with a baseline FG ≤ 126 mg/dL was not significantly prolonged compared to patients with a baseline FG > 126 mg/dL [84] (Table 4). These discordant results could be attributed the fact that in this study, unlike the others, patients at an advanced stage of colorectal cancer were enrolled, in whom the OS may have been already compromised.
Endometrium
Several studies have demonstrated that patients with diabetes have an increased risk of endometrial cancer, and retrospective studies have shown that patients with endometrial cancer and coexisting diabetes have worse survival than those without [85]. In a recent retrospective study conducted on 96 women with endometrial cancer (48 with, 48 without diabetes), no statistical difference in OS was found for patients with diabetes who achieved glycemic control (mean FG value < 126 mg/dL during the year after cancer diagnosis) versus those who did not [85] (Table 5). Interestingly, Raffone A et al. [86] have reviewed and meta-analyzed the role of glycemic control in the progression of endometrial hyperplasia to endometrial cancer, demonstrating that adequate glycemic control may be required in women with endometrial hyperplasia in order to reduce the risk of imminent progression in endometrial cancer (Table 5). Finally, Stevensen EE et al. [87], analyzing 82 patients with endometrial cancer who underwent surgical staging and had HbA1c drawn within 3 months before surgery, have demonstrated that high preoperative HbA1c had a trend toward a higher stage of endometrial cancer at the time of diagnosis (Table 5).
Liver
Although there is ample evidence that diabetes is associated with increased risk of liver cancer [6, 7, 10, 88,89,90], to the best of our knowledge only one study has analyzed the role of glycemic control on liver cancer outcomes. In this study, 100 patients who underwent curative resection for solitary hepatitis C virus-related hepatocellular carcinoma (26 with diabetes and 74 without) were analyzed [91]. DFS rate was 66 and 27% at 3 years in patients with normal postoperative HbA1c level (< 6.5%) and elevated postoperative HbA1c level (≥ 6.5%), respectively [91]. In addition, multivariate analysis showed that poor glycemic control (HbA1c ≥ 6.5%) was associated with postoperative tumor recurrence in patients with diabetes [91].
Pancreas
As for liver cancer, evidence suggests that diabetes is associated with increased risk of pancreatic cancer [6, 7, 10, 92, 93]. However, in the case of pancreatic cancer it is difficult to distinguish whether it is the glycemic control that influences the cancer outcomes or vice versa, as pancreatic cancer and its treatment (pharmacological or surgical) may induce hyperglycemia [94]. Several studies have analyzed the association between glycemic control and pancreatic cancer outcomes (Table 6). Alpertunga I et al. [95] have studied 73 patients with advanced pancreatic ductal adenocarcinoma receiving chemotherapy. They found that a 3-month average RBG ≤ 120 mg/dL predicted for improved OS compared to RBG > 120 mg/dL (19 vs. 9 months; HR = 0.37) in both patients with and without diabetes [95] (Table 6). There were no differences in OS between metformin or insulin users and non-users [95]. In another retrospective study conducted on 417 patients (88 with diabetes) with pancreatic neuroendocrine neoplasms undergoing surgical resection, patients with dysglycemia (FG ≥ 140 mg/dL or HbA1c ≥ 6.5%) had greater rates of metastasis [96]. In addition, preoperative dysglycemia was associated with impaired OS (HS 1.57, 95% CI 1.01–2.46) and RFS (HR 1.78, 95% CI 1.01–3.12], regardless of the presence of diabetes [96] (Table 6). Similarly, elevated preoperative HbA1c has been associated with failure to complete anti-cancer therapy or surgery and a trend for increased risk of metastatic progression in 123 patients with localized pancreatic cancer [97] (Table 6). Finally, in a retrospective study of 52 patients with pancreatic tumors who underwent total pancreatectomy, elevated postoperative FG levels were significantly associated with complications after surgery [98]. In addition, postoperative HbA1c levels over 7% were identified as one of the independent risk factors for tumor recurrence (HR 2.655, 95% CI 1.299–5.425). Patients with postoperative HbA1c levels over 7% had poorer OS than those with HbA1c levels less than 7% (HR 3.212, 95% CI 1.147–8.999) [98] (Table 6).
Prostate
As stated above, the relationship between prostate cancer and diabetes is unique, since it is the only cancer where diabetes appears to be protective [13]. The underlying cause of this protective role is not fully understood; however, some mechanisms have been proposed [99]. Specifically, elevated circulating levels of androgen have been suggested as risk factor for prostate cancer and could work as tumor growth factors. As a consequence, the reduced levels of androgen that occur in diabetes may represent a protective factor against prostate cancer [99]. Similarly, type 1 diabetes and long-lasting type 2 diabetes with prevalent secretory dysfunction are associated with insulin depletion and decreased IGF-1 signaling which could further explain the protective role of diabetes on prostate cancer [99]. Despite the protective role if diabetes on the risk of prostate cancer, several studies suggest that prostate cancer patients with diabetes and poor glycemic control may have increased risk of biologically aggressive cancer (Table 7). In a recent prospective study conducted on 438 patients with prostate cancer with a follow-up of approximatively 6 years, the HRs and 95% CI for mortality rate was higher in patients with inadequate glycemic control prior to cancer diagnosis compared with patients with adequate glycemic control (HR 1.39, 95% CI 0.98–1.98) [47] (Table 7). A retrospective study conducted on 831 patients with prostate cancer with or without preexisting diabetes showed that mean HbA1c levels ≥ 9% had significantly increased risk for all-cause and non-prostate cancer mortality (HR 3.09, 95% CIs 1.15–8.32 and HR 5.49, 95% CIs 1.66–18.16, respectively), but not for prostate cancer-specific mortality (HR 1.03, 95% CIs 0.13–8.44) compared with the non-diabetes group [100] (Table 7). These results were confirmed also after adjusting for metformin use [100]. Nik-Ahd F et al. [49] have retrospectively reviewed data regarding 1,409 men with prostate cancer undergoing radical prostatectomy (710 with diabetes) with a median follow-up of 6.8 years. They found that a higher HbA1c value was associated with metastasis (HR 1.21, 95% CI 1.02–1.44) and castration-resistant prostate cancer (HR 1.27, 95% CI 1.03–1.56) [49]. Although not statistically significant, there were trends between higher HbA1c and risk of prostate cancer-specific mortality and all-cause mortality [49] (Table 7). In addition, Lee H et al. [101] demonstrated that poorer glycemic control (HbA1c levels ≥ 6.5% within the 6 months preceding radical prostatectomy) was significantly related with high cancer aggressiveness and biochemical recurrence-free survival in 746 prostate cancer patients with (n = 209) or without (n = 537) diabetes (Table 7). Meanwhile, metformin use was not associated with biochemical recurrence-free survival [101]. Likewise, in a retrospective study conducted on 731 men with prostate cancer (338 with a history of diabetes) poor glycemic control was associated with a higher risk of high-grade prostate cancer detection [102] (Table 7). Similar results have been demonstrated by Kim HS et al. [103], showing that men with higher HbA1c levels presented with more biologically aggressive prostate cancer at radical prostatectomy, although HbA1c levels were not significantly related to risk of biochemical recurrence [103] (Table 7). In addition, in patients with prostate cancer, average glycemia during chemotherapy was significantly associated with overall severe toxicity [104] (Table 7). Finally, Hong SK et al. [105] have demonstrated that higher HbA1c levels (≥ 6.5%) were associated with a significantly higher rate of extraprostatic extension of tumor and higher cancer aggressiveness comparted to HbA1c levels < 6.5% (Table 7). In contrast with these studies, Joentausta RM et al. [106] found that glycemic control after radical prostatectomy was not associated with cancer recurrence and short-term mortality in 1,314 men who underwent radical prostatectomy (Table 7). Importantly, duration, and dose of anti-diabetes medication use had no effect on cancer survival [106].
Conclusions
Growing evidence suggests that patients with diabetes are characterized by an increased risk of developing different types of cancer and reduced survival after cancer diagnosis [6, 7]. In particular, diabetes increases the risk of developing bladder, breast, colorectal, endometrial, gallbladder, liver, and pancreatic cancers [6, 7], while reducing the risk of developing prostate cancer [13]. While diabetes and cancer share several common risk factors, and therefore the probability of their occurrence in the same patient is high [12, 14], growing evidence suggests that diabetes and cancer could cause each other with distinct mechanisms. Indeed, hyperinsulinemia, hyperglycemia, and chronic low-grade inflammation may represent the main pathophysiological factors underlying this correlation. Research has also shown that diabetes may predict a worse prognosis in patients with cancer [42, 46], with more recent findings suggesting an important role for poor glycemic control [47]. Nevertheless, only few studies have evaluated the association between glycemic control and survival in patients with both cancer and diabetes, yielding mixed results [47]. Most, but not all, studies analyzed in this review suggest that a good glycemic control may favorably influence cancer outcomes (in terms of survival, progression, recurrence, aggressiveness, and response to therapy). However, few other studies show no effect of glycemic control on cancer outcomes, while no studies suggest that a good glycemic control could have negative effects (except, perhaps, in terms of quality of life). Altogether, these results endorse the importance of multidisciplinary diabetes management in oncologic patients. Indeed, there is a growing need of interdisciplinary competence and coordination between diabetologists and oncologists to better manage patients with both diabetes and cancer, since the coexistence of the two diseases poses significant challenges for patients and health care providers [107]. It should be highlighted that most of the studies analyzing the correlation between glycemic control and cancer progression face several methodological concerns. Most of them are retrospective in nature, while prospective studies could provide better-quality evidence and the possibility of adjusting the results for more confounding factors. In addition, these studies take under consideration heterogeneous outcomes to evaluate cancer progression (OS, PFS, RFS, and others), and there is little consistency in how glycemic control is measured across studies (HbA1c, FG, or RBG, with different measurement timing and cut-off points) [8]. Moreover, most of these studies do not take into account several important factors that may affect cancer outcomes, such as population ethnicity, age and stage at cancer diagnosis, nutritional status, or cancer treatments [60, 61, 94]. Studying the association between diabetes, glycemic control, cancer risk, and cancer outcomes is further complicated by evidence that anti-diabetes drugs themselves may influence the risk of cancer development and progression (reviewed in [10]). In particular metformin, for its preventive effect on a fair number of cancers [108], and pioglitazone or empagliflozin, for their possible association with a higher risk of bladder cancer [35, 36, 40, 109, 110]. Nevertheless, in only a few studies among those analyzed in this review, data are adjusted for anti-diabetes therapy or the use of metformin. Information on anti-diabetes therapy is often lacking. Ultimately, despite its importance, the association between glycemic control and cancer outcomes in diabetic patients with cancer remains unsettled and poorly debated. Although there are good reasons to believe that a good glycemic control may favorably influence cancer outcomes, further prospective studies, including larger patients’ cohorts and addressing all relevant methodological issues, are needed.
Data availability
Not applicable.
References
IDF Diabetes atlas. https://diabetesatlas.org/. Accessed 11 Jan 2024
Biondi G, Marrano N, Borrelli A et al (2023) The p66Shc redox protein and the emerging complications of diabetes. Int J Mol Sci 25:108. https://doi.org/10.3390/IJMS25010108
Gregg EW, Sattar N, Ali MK (2016) The changing face of diabetes complications. Lancet Diabetes Endocrinol 4:537–547. https://doi.org/10.1016/S2213-8587(16)30010-9
Natalicchio A, Faggiano A, Zatelli MC et al (2022) Metabolic disorders and gastroenteropancreatic-neuroendocrine tumors (GEP-NETs): how do they influence each other? an Italian Association of Medical Oncology (AIOM)/ Italian Association of Medical Diabetologists (AMD)/ Italian Society of Endocrinology (SIE)/ Italian Society of Pharmacology (SIF) multidisciplinary consensus position paper. Crit Rev Oncol Hematol 169:103572. https://doi.org/10.1016/J.CRITREVONC.2021.103572
Cancer today. https://gco.iarc.fr/today/en/fact-sheets-populations#groups. Accessed 28 May 2024
Ling S, Brown K, Miksza JK et al (2020) Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care 43:2313–2322. https://doi.org/10.2337/DC20-0204
Pearson-Stuttard J, Papadimitriou N, Markozannes G et al (2021) Type 2 diabetes and cancer: an umbrella review of observational and mendelian randomization studies. Cancer Epidemiol Biomark Prev 30:1218–1228. https://doi.org/10.1158/1055-9965.EPI-20-1245
Hershey D (2017) Importance of glycemic control in cancer patients with diabetes: treatment through end of life. Asia Pac J Oncol Nurs 4:313. https://doi.org/10.4103/apjon.apjon_40_17
Joharatnam-Hogan N, Chambers P, Dhatariya K, Board R (2022) A guideline for the outpatient management of glycaemic control in people with cancer. Diabet Med 39:e14636. https://doi.org/10.1111/DME.14636
Lega IC, Lipscombe LL (2020) Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev 41:bnz014. https://doi.org/10.1210/ENDREV/BNZ014
Hu Y, Zhang X, Ma Y et al (2021) Incident type 2 diabetes duration and cancer risk: a prospective study in two US cohorts. J Natl Cancer Inst 113:381–389. https://doi.org/10.1093/JNCI/DJAA141
Giovannucci E, Harlan DM, Archer MC et al (2010) Diabetes and cancer: a consensus report. Diabetes Care 33:1674–1685. https://doi.org/10.2337/dc10-0666
Bansal D, Bhansali A, Kapil G et al (2013) Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis 16:151–158. https://doi.org/10.1038/PCAN.2012.40
Cignarelli A, Genchi VA, Caruso I et al (2018) Diabetes and cancer: pathophysiological fundamentals of a ‘dangerous affair.’ Diabetes Res Clin Pract 143:378–388. https://doi.org/10.1016/j.diabres.2018.04.002
Key TJ, Appleby PN, Reeves GK et al (2010) Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol 11:530–542. https://doi.org/10.1016/S1470-2045(10)70095-4
Heiden MGV, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324:1029–1033. https://doi.org/10.1126/SCIENCE.1160809
Matsuda M, Shimomura I (2013) Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 7:e330–e341. https://doi.org/10.1016/J.ORCP.2013.05.004
Lee SC, Chan JCN (2015) Evidence for DNA damage as a biological link between diabetes and cancer. Chin Med J (Engl) 128:1543–1548. https://doi.org/10.4103/0366-6999.157693
Natalicchio A, Montagnani M, Gallo M et al (2023) MiRNA dysregulation underlying common pathways in type 2 diabetes and cancer development: an Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Diabetology (SID)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) multidisciplinary critical view. ESMO open 8:101573. https://doi.org/10.1016/J.ESMOOP.2023.101573
Renzelli V, Gallo M, Morviducci L et al (2023) Polybrominated diphenyl ethers (PBDEs) and human health: effects on metabolism, diabetes and cancer. Cancers (Basel) 15:4237. https://doi.org/10.3390/CANCERS15174237
Colhoun HM (2009) Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish diabetes research network epidemiology group. Diabetologia 52:1755–1765. https://doi.org/10.1007/S00125-009-1453-1
Currie CJ, Poole CD, Gale EAM (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52:1766–1777. https://doi.org/10.1007/S00125-009-1440-6
Jonasson JM, Ljung R, Talbäck M et al (2009) Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia 52:1745–1754. https://doi.org/10.1007/S00125-009-1444-2
Hemkens LG, Grouven U, Bender R et al (2009) Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 52:1732–1744. https://doi.org/10.1007/S00125-009-1418-4
Sciacca L, Vella V, Frittitta L et al (2018) Long-acting insulin analogs and cancer. Nutr Metab Cardiovasc Dis 28:436–443. https://doi.org/10.1016/J.NUMECD.2018.02.010
Wu JW, Filion KB, Azoulay L et al (2016) Effect of long-acting insulin analogs on the risk of cancer: a systematic review of observational studies. Diabetes Care 39:486–494. https://doi.org/10.2337/DC15-1816
Bordeleau L, Yakubovich N, Dagenais GR et al (2014) The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 37:1360–1366. https://doi.org/10.2337/DC13-1468
Gokhale M, Buse JB, Gray CL et al (2014) Dipeptidyl-peptidase-4 inhibitors and pancreatic cancer: a cohort study. Diabetes Obes Metab 16:1247–1256. https://doi.org/10.1111/DOM.12379
R. Drab S, (2016) Glucagon-like peptide-1 receptor agonists for type 2 diabetes: a clinical update of safety and efficacy. Curr Diabetes Rev 12:403–413. https://doi.org/10.2174/1573399812666151223093841
Egan AG, Blind E, Dunder K et al (2014) Pancreatic safety of incretin-based drugs–FDA and EMA assessment. N Engl J Med 370:794–797. https://doi.org/10.1056/NEJMP1314078
Abrahami D, Douros A, Yin H et al (2018) Incretin based drugs and risk of cholangiocarcinoma among patients with type 2 diabetes: population based cohort study. BMJ 363:k4880. https://doi.org/10.1136/BMJ.K4880
Marzioni M, Alpini G, Saccomanno S et al (2009) Exendin-4, a glucagon-like peptide 1 receptor agonist, protects cholangiocytes from apoptosis. Gut 58:990–997. https://doi.org/10.1136/GUT.2008.150870
Marzioni M, Alpini G, Saccomanno S et al (2007) Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology 133:244–255. https://doi.org/10.1053/J.GASTRO.2007.04.007
FDA Drug Safety Communication: Update to ongoing safety review of actos (pioglitazone) and increased risk of bladder cancer FDA. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-update-ongoing-safety-review-actos-pioglitazone-and-increased-risk. Accessed 23 Apr 2024
Lewis JD, Ferrara A, Peng T et al (2011) Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 34:916–922. https://doi.org/10.2337/DC10-1068
Mehtälä J, Khanfir H, Bennett D et al (2018) Pioglitazone use and risk of bladder cancer: a systematic literature review and meta-analysis of observational studies. Diabetol Int 10:24–36. https://doi.org/10.1007/S13340-018-0360-4
Hsiao FY, Hsieh PH, Huang WF et al (2013) Risk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case–control study. Drug Saf 36:643–649. https://doi.org/10.1007/S40264-013-0080-4
Reilly TP, Graziano MJ, Janovitz EB et al (2014) Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus. Diabetes Ther 5:73–96. https://doi.org/10.1007/S13300-014-0053-3
De Jonghe S, Proctor J, Vinken P et al (2014) Carcinogenicity in rats of the SGLT2 inhibitor canagliflozin. Chem Biol Interact 224:1–12. https://doi.org/10.1016/J.CBI.2014.09.018
Tang H, Dai Q, Shi W et al (2017) SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia 60:1862–1872. https://doi.org/10.1007/S00125-017-4370-8
Hwangbo Y, Kang D, Kang M et al (2018) Incidence of diabetes after cancer development a Korean national cohort study. JAMA Oncol 4:1099–1105. https://doi.org/10.1001/jamaoncol.2018.1684
Currie CJ, Poole CD, Jenkins-Jones S et al (2012) Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 35:299–304. https://doi.org/10.2337/dc11-1313
Seshasai SRK, Kaptoge S, Thompson A et al (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364:829–841. https://doi.org/10.1056/NEJMOA1008862
Pearson-Stuttard J, Bennett J, Cheng YJ et al (2021) Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 9:165–173. https://doi.org/10.1016/S2213-8587(20)30431-9
Pearson-Stuttard J, Buckley J, Cicek M, Gregg EW (2021) The changing nature of mortality and morbidity in patients with diabetes. Endocrinol Metab Clin North Am 50:357–368. https://doi.org/10.1016/J.ECL.2021.05.001
Ranc K, Jørgensen ME, Friis S, Carstensen B (2014) Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia 57:927–934. https://doi.org/10.1007/S00125-014-3186-Z
de Haan-Du J, Groenier KH, Wauben-Spaetgens B et al (2023) The value of glycemic control prior to cancer diagnosis on all-cause mortality among patients with type 2 diabetes in Dutch primary care. Cancer Epidemiol Biomark Prev 32:252–259. https://doi.org/10.1158/1055-9965.EPI-22-0766
Li J, Ning N, ya, Rao Q xian, et al (2017) Pretreatment glycemic control status is an independent prognostic factor for cervical cancer patients receiving neoadjuvant chemotherapy for locally advanced disease. BMC Cancer 17:517. https://doi.org/10.1186/s12885-017-3510-3
Nik-Ahd F, Howard LE, Eisenberg AT et al (2019) Poorly controlled diabetes increases the risk of metastases and castration-resistant prostate cancer in men undergoing radical prostatectomy: results from the SEARCH database. Cancer 125:2861–2867. https://doi.org/10.1002/cncr.32141
Chowdhury TA, Jacob P (2019) Challenges in the management of people with diabetes and cancer. Diabet Med 36:795–802. https://doi.org/10.1111/dme.13919
Hershey DS, Tipton J, Given B, Davis E (2012) Perceived impact of cancer treatment on diabetes self-management. Diabetes Educ 38:779–790. https://doi.org/10.1177/0145721712458835
Gallo M, Muscogiuri G, Felicetti F et al (2018) Adverse glycaemic effects of cancer therapy: indications for a rational approach to cancer patients with diabetes. Metabolism 78:141–154. https://doi.org/10.1016/J.METABOL.2017.09.013
Ashley L, Kassim S, Kellar I et al (2022) Identifying ways to improve diabetes management during cancer treatments (INDICATE): protocol for a qualitative interview study with patients and clinicians. BMJ Open 12:e060402. https://doi.org/10.1136/BMJOPEN-2021-060402
Hershey DS, Given B, Given C et al (2014) Predictors of diabetes self-management in older adults receiving chemotherapy. Cancer Nurs 37:97–105. https://doi.org/10.1097/NCC.0B013E3182888B14
Zanders MMJ, Haak HR, van Herk-Sukel MPP et al (2015) Impact of cancer on adherence to glucose-lowering drug treatment in individuals with diabetes. Diabetologia 58:951–960. https://doi.org/10.1007/S00125-015-3497-8
Pinheiro LC, Soroka O, Kern LM et al (2020) Diabetes care management patterns before and after a cancer diagnosis: a SEER-medicare matched cohort study. Cancer 126:1727–1735. https://doi.org/10.1002/CNCR.32728
Shariff AI, Syed S, Shelby RA et al (2019) Novel cancer therapies and their association with diabetes. J Mol Endocrinol 62:R187–R199. https://doi.org/10.1530/JME-18-0002
Silvestris N, Argentiero A, Beretta G et al (2020) Management of metabolic adverse events of targeted therapies and immune checkpoint inhibitors in cancer patients: an Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. Crit Rev Oncol Hematol 154:103066. https://doi.org/10.1016/J.CRITREVONC.2020.103066
Ragni A, Retta F, Arvat E, Gallo M (2021) Diabetes in cancer patients: risks, goals and management. Front Horm Res 54:103–114. https://doi.org/10.1159/000513807
Tsilidis KK, Kasimis JC, Lopez DS et al (2015) Type 2 diabetes and cancer: Umbrella review of meta-analyses of observationlal studies. BMJ 350:g7607. https://doi.org/10.1136/bmj.g7607
Renehan AG, Yeh HC, Johnson JA et al (2012) Diabetes and cancer (2): evaluating the impact of diabetes on mortality In patients with cancer. Diabetologia 55:1619–1632. https://doi.org/10.1007/s00125-012-2526-0
Silvestris N, Argentiero A, Natalicchio A et al (2021) Antineoplastic dosing in overweight and obese cancer patients: an Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Endocrinologia (SIE)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. ESMO open 6:100153. https://doi.org/10.1016/J.ESMOOP.2021.100153
Barua R, Templeton AJ, Seruga B et al (2018) Hyperglycaemia and survival in solid tumours: a systematic review and meta-analysis. Clin Oncol 30:215–224. https://doi.org/10.1016/j.clon.2018.01.003
Hershey D, Hession S (2017) Chemotherapy and glycemic control in patients with type 2 diabetes and cancer: a comparative case analysis. Asia Pac J Oncol Nurs 4:224. https://doi.org/10.4103/apjon.apjon_22_17
Sun HJ, Ohrr H, Sull JW et al (2005) Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293:194–202. https://doi.org/10.1001/JAMA.293.2.194
Boursi B, Giantonio BJ, Lewis JD et al (2016) Serum glucose and hemoglobin A1C levels at cancer diagnosis and disease outcome. Eur J Cancer 59:90–98. https://doi.org/10.1016/j.ejca.2016.02.018
Hammer MJ, Aouizerat BE, Schmidt BL et al (2015) Glycosylated hemoglobin A1c and lack of association with symptom severity in patients undergoing chemotherapy for solid tumors. Oncol Nurs Forum 42:581–590. https://doi.org/10.1188/15.ONF.581-590
Ferrari P, Giardini A, Negri EM et al (2018) Managing people with diabetes during the cancer palliation in the era of simultaneous care. Diabetes Res Clin Pract 143:443–453. https://doi.org/10.1016/J.DIABRES.2017.12.010
Legris P, Bouillet B, Pâris J et al (2023) Glycemic control in people with diabetes treated with cancer chemotherapy: contribution of continuous glucose monitoring. Acta Diabetol 60:545–552. https://doi.org/10.1007/S00592-023-02032-Z
Lee AK, Warren B, Lee CJ et al (2018) The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 41:104–111. https://doi.org/10.2337/DC17-1669
Huang WL, Huang KH, Huang CY et al (2020) Effect of diabetes mellitus and glycemic control on the prognosis of non-muscle invasive bladder cancer: a retrospective study. BMC Urol 20:117. https://doi.org/10.1186/S12894-020-00684-5
ai YS, Chen CH, Huang CY, et al (2015) Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab Res Rev 31:307–314. https://doi.org/10.1002/DMRR.2614
Ahn JH, Il JS, Yim SU et al (2016) Impact of glycemic control and metformin use on the recurrence and progression of non-muscle invasive bladder cancer in patients with diabetes mellitus. J Korean Med Sci 31:1464–1471. https://doi.org/10.3346/JKMS.2016.31.9.1464
Hwang EC, Kim YJ, Hwang IS et al (2011) Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: a retrospective cohort study. Int J Urol 18:769–776. https://doi.org/10.1111/J.1442-2042.2011.02845.X
Eketunde AO (2020) Diabetes as a risk factor for breast cancer. Cureus 12:e8010. https://doi.org/10.7759/CUREUS.8010
Phillips AL, Reeves DJ, Storey S (2023) Impact of diabetes (type 2) and glycemic control on health-related outcomes of patients receiving chemotherapy for non-metastatic breast cancer: a retrospective analysis. Support Care Cancer 31:114. https://doi.org/10.1007/S00520-022-07563-9
Chang YL, Sheu WHH, Lin SY, Liou WS (2018) Good glycaemic control is associated with a better prognosis in breast cancer patients with type 2 diabetes mellitus. Clin Exp Med 18:383–390. https://doi.org/10.1007/s10238-018-0497-2
Erickson K, Patterson RE, Flatt SW et al (2011) Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 29:54–60. https://doi.org/10.1200/JCO.2010.29.3183
Cheung YMM, Hughes M, Harrod J et al (2022) The effects of diabetes and glycemic control on cancer outcomes in individuals with metastatic breast cancer. J Clin Endocrinol Metab 107:2511–2521. https://doi.org/10.1210/CLINEM/DGAC375
Jousheghany F, Phelps J, Crook T, Hakkak R (2016) Relationship between level of HbA1C and breast cancer. BBA Clin 6:45–48. https://doi.org/10.1016/J.BBACLI.2016.04.005
Lee SJ, Kim JH, Park SJ et al (2017) Optimal glycemic target level for colon cancer patients with diabetes. Diabetes Res Clin Pract 124:66–71. https://doi.org/10.1016/j.diabres.2016.12.009
Ferroni P, Formica V, Della-Morte D et al (2016) Prognostic value of glycated hemoglobin in colorectal cancer. World J Gastroenterol 22:9984–9993. https://doi.org/10.3748/wjg.v22.i45.9984
Siddiqui AA, Spechler SJ, Huerta S et al (2008) Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Dig Dis Sci 53:2486–2494. https://doi.org/10.1007/s10620-008-0264-4
Meng Q, Yu Y, Wang K et al (2022) The prognostic role of fasting plasma glucose levels on survival in advanced colorectal cancer patients with type II diabetes mellitus: a retrospective cohort study. J Gastrointest Oncol 13:3080–3089. https://doi.org/10.21037/JGO-22-1124/COIF
Kusne YN, Kosiorek HE, Buras MR et al (2020) Mortality and glycemic control among patients with diabetes mellitus and uterine or ovarian cancer. Futur Sci OA 7:FSO670. https://doi.org/10.2144/FSOA-2020-0158
Raffone A, Travaglino A, Saccone G et al (2019) Diabetes mellitus is associated with occult cancer in endometrial hyperplasia. Pathol Oncol Res 26:1377–1384. https://doi.org/10.1007/s12253-019-00684-3
Stevens EE, Yu S, Van Sise M et al (2012) Hemoglobin A1c and the relationship to stage and grade of endometrial cancer. Arch Gynecol Obstet 286:1507–1512. https://doi.org/10.1007/S00404-012-2455-7
Campbell PT, Newton CC, Freedman ND et al (2016) Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. adults. Cancer Res 76:6076–6083. https://doi.org/10.1158/0008-5472.CAN-16-0787
Cheuk-Fung Yip T, Wai-Sun Wong V, Lik-Yuen Chan H et al (2018) Effects of diabetes and glycemic control on risk of hepatocellular carcinoma after seroclearance of hepatitis B surface antigen. Clin Gastroenterol Hepatol 16:765-773.e2. https://doi.org/10.1016/J.CGH.2017.12.009
Li CI, Chen HJ, Lai HC et al (2015) Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes–national cohort of Taiwan diabetes study. Int J cancer 136:2668–2679. https://doi.org/10.1002/IJC.29321
Kaneda K, Uenishi T, Takemura S et al (2012) The influence of postoperative glycemic control on recurrence after curative resection in diabetics with hepatitis C virus-related hepatocellular carcinoma. J Surg Oncol 105:606–611. https://doi.org/10.1002/JSO.22137
Campbell PT, Newton CC, Jacobs EJ et al (2022) Prospective associations of hemoglobin A1c and c-peptide with risk of diabetes-related cancers in the cancer prevention study-II nutrition cohort. Cancer Res Commun 2:653–662. https://doi.org/10.1158/2767-9764.CRC-22-0082
Er KC, Hsu CY, Lee YK et al (2016) Effect of glycemic control on the risk of pancreatic cancer: a nationwide cohort study. Medicine (Baltimore) 95:e3921. https://doi.org/10.1097/MD.0000000000003921
Dankner R, Boker LK, Boffetta P et al (2018) A historical cohort study on glycemic-control and cancer-risk among patients with diabetes. Cancer Epidemiol 57:104–109. https://doi.org/10.1016/J.CANEP.2018.10.010
Alpertunga I, Sadiq R, Pandya D et al (2021) Glycemic control as an early prognostic marker in advanced pancreatic cancer. Front Oncol 11:571855. https://doi.org/10.3389/FONC.2021.571855
Sandini M, Strobel O, Hank T et al (2020) Pre-operative dysglycemia is associated with decreased survival in patients with pancreatic neuroendocrine neoplasms. Surg (U.S.) 167:575–580. https://doi.org/10.1016/j.surg.2019.11.007
Rajamanickam ESP, Christians KK, Aldakkak M et al (2017) Poor glycemic control is associated with failure to complete neoadjuvant therapy and surgery in patients with localized pancreatic cancer. J Gastrointest Surg 21:496–505. https://doi.org/10.1007/s11605-016-3319-4
Shi HJ, Jin C, Fu DL (2017) Impact of postoperative glycemic control and nutritional status on clinical outcomes after total pancreatectomy. World J Gastroenterol 23:265–274. https://doi.org/10.3748/wjg.v23.i2.265
Rastmanesh R, Hejazi J, Marotta F, Hara N (2014) Type 2 diabetes: a protective factor for prostate cancer? an overview of proposed mechanisms. Clin Genitourin Cancer 12:143–148. https://doi.org/10.1016/J.CLGC.2014.01.001
Lin CC, Wu MF, Chang YL et al (2022) Glycemic control was associated with nonprostate cancer and overall mortalities in diabetic patients with prostate cancer. J Chin Med Assoc 85:331–340. https://doi.org/10.1097/JCMA.0000000000000623
Lee H, Kuk H, Byun SS et al (2015) Preoperative glycemic control status as a significant predictor of biochemical recurrence in prostate cancer patients after radical prostatectomy. PLoS ONE 10:e0124761. https://doi.org/10.1371/journal.pone.0124761
Park J, Cho SY, Lee YJ et al (2014) Poor glycemic control of diabetes mellitus is associated with higher risk of prostate cancer detection in a biopsy population. PLoS ONE 9:e104789. https://doi.org/10.1371/JOURNAL.PONE.0104789
Kim HS, Presti JC, Aronson WJ et al (2010) Glycemic control and prostate cancer progression: results from the SEARCH database. Prostate 70:1540–1546. https://doi.org/10.1002/pros.21189
Brunello A, Kapoor R, Extermann M (2011) Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol 34:292–296. https://doi.org/10.1097/COC.0b013e3181e1d0c0
Sung KH, Seung TL, Sung SK et al (2009) Significance of preoperative HbA1c level in patients with diabetes mellitus and clinically localized prostate cancer. Prostate 69:820–826. https://doi.org/10.1002/PROS.20932
Joentausta RM, Kujala PM, Visakorpi T et al (2016) Tumor features and survival after radical prostatectomy among antidiabetic drug users. Prostate Cancer Prostatic Dis 19:367–373. https://doi.org/10.1038/pcan.2016.32
Silvestris N, Franchina T, Gallo M et al (2023) Diabetes management in cancer patients. An Italian Association of Medical Oncology, Italian Association of Medical Diabetologists, Italian Society of Diabetology, Italian Society of Endocrinology and Italian Society of Pharmacology multidisciplinary consensus position paper. ESMO Open 8:102062. https://doi.org/10.1016/J.ESMOOP.2023.102062
Najafi F, Rajati F, Sarokhani D et al (2023) The relationship between metformin consumption and cancer risk: an updated umbrella review of systematic reviews and meta-analyses. Int J Prev Med 14:90. https://doi.org/10.4103/IJPVM.IJPVM_62_21
Copur S, Yildiz AB, Covic A, Kanbay M (2023) Is there any robust evidence showing that SGLT2 inhibitor predisposes to cancer? Eur J Clin Invest 54:e14131. https://doi.org/10.1111/ECI.14131
Dicembrini I, Nreu B, Mannucci E, Monami M (2019) Sodium-glucose co-transporter-2 (SGLT-2) inhibitors and cancer: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 21:1871–1877. https://doi.org/10.1111/DOM.13745
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the conception and design of the manuscript. FG, AN, and NM conceived the document. All authors reviewed published literature, drafted the article, revised the manuscript critically, and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
M.G. has received honoraria for speaker fees and/or travel grants for scientific meetings from AAA, AstraZeneca, Boehringer-Ingelheim, Bruno Farm, Eli-Lilly, IBSA, Lifescan, Mundipharma, Novo Nordisk and Sanofi, and served on scientific advisory panels for Boehringer-Ingelheim, Merck Sharp & Dohme and Novo Nordisk. S.F. serves on the scientific advisory board of, has a consulting relationship with and reports receiving support for travel expenses from Novartis, Teva, Roche, BMS, Lilly and Ipsen. S.G. serves on the scientific advisory board of, has a consulting relationship with and reports receiving support for travel expenses from Novartis, Teva, Roche, BMS, Lilly and Ipsen. A.R. has received a travel grant from Movi and IBSA. M.M. reports direct competing interests with Sanofi. V.R. has received a travel grant from Androlabs. A.C. received grants for consultancies/advisory boards from BMS, MSD, OncoC4, IQVIA, Roche, GSK, AstraZeneca, Access Infinity, Ardelis Health and REGENERON; he also received speaker fees from AstraZeneca, EISAI, MSD, SANOFI/REGENERON and Pierre-Fabre. M.D.M reports honoraria from AstraZeneca, Janssen, Merck Sharp & Dohme (MSD), Novartis, Pfizer, Roche, GlaxoSmithKline, Amgen, Merck, Takeda, Ipsen for consultancy or participation to advisory boards; direct research funding from Tesaro/GlaxoSmithKline; institutional funding for work in clinical trials/contracted research from Beigene, Exelixis, MSD, Pfizer and Roche. R.C. reports honoraria for advisory board from Novo-Nordisk, Sanofi; consulting feed from Abbott, Sanofi, Eli Lilly, Bayer, Novo Nordisk, MSD, Menarini Diagnostics; payment for lectures, presentation, speaker from Eli Lilly, Boehringer Ingelheim, Novi Nordisk, Roche Diabetes Care, Sanofi. F.P. declares institutional grants or contracts from Roche, Bayer, AstraZeneca, Pfizer, Incyte, Tesaro/GSK, Merck; consulting fees from Bayer, Pierre Fabre, Astra Zeneca, Incyte, Ipsen, Clovis, Astellas, Sanofi, Roche, Pfizer; leadership in scientific society: President of AIOM 2023–2025. G.A. received grants for advisory board from Sanofi, NN, Eli-lilly. N.S. received fees for consulting from from Bristol, Roche, Eisai, Servier. F.G. has served as an advisor for AstraZeneca, Eli Lilly and Novo Nordisk; has served as a research investigator for Eli Lilly and Roche Diabetes Care; has served as a speaker for AstraZeneca and Eli Lilly; has served as a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Roche Diabetes Care and Sanofi; and has received grants from Eli Lilly, Lifescan and Roche Diabetes Care. All other authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
No Informed Consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Natalicchio, A., Marrano, N., Montagnani, M. et al. Glycemic control and cancer outcomes in oncologic patients with diabetes: an Italian Association of Medical Oncology (AIOM), Italian Association of Medical Diabetologists (AMD), Italian Society of Diabetology (SID), Italian Society of Endocrinology (SIE), Italian Society of Pharmacology (SIF) multidisciplinary critical view. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02417-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02417-z