Abstract
Although diabetes mellitus (DM) is one of the risk factors associated with increased breast cancer (BC) mortality, the effects of glycaemic control on the prognosis of BC have not been thoroughly evaluated. This retrospective study aimed to evaluate the relationship between glycaemic control and BC prognosis and to determine an optimal target of glycaemic control for BC patients with diabetes. We included 2812 stage 0–3 BC women, of whom 145 were diabetic and were 2667 non-diabetic. In those with diabetes, a mean haemoglobin A1C (HbA1C) < 7% (n = 77) was defined as well-controlled diabetes, while a mean HbA1C > 9% (n = 16) was defined as poorly controlled diabetes. All of the BC populations were followed from the date on which BC was diagnosed until 31 December 2015. Cox regression analysis was performed to estimate the adjusted hazards for all-cause mortality and BC-specific mortality. After controlling for the baseline and BC-related confounders, the adjusted hazard ratio (HR) for all-cause mortality and the HR for BC-specific mortality were 3.65 (95% confidence interval [95% CI] 1.13–11.82) and 8.37 (95% CI 1.90–36.91), respectively, for poorly controlled diabetic women and non-DM women. However, for the diabetic women with good glycaemic control, the HRs of all-cause mortality and BC-specific mortality were not significantly different (HR 0.91, 95% CI 0.42–1.01; HR 0.77, 95% CI 0.18–3.32, respectively) from those for both mortalities in non-DM patients. For moderate controlled diabetic women, the HRs for all-cause mortality and BC-specific mortality were 1.95 (95% CI 0.89–4.27) and 3.55 (95% CI 1.369–9.30), respectively. This pilot and retrospective cohort study reveals a relationship between glycaemic control and BC prognosis in diabetic women. In addition, well-controlled HbA1C, with maintained mean HbA1C values under 7%, may be associated with a better progression outcome of BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) has become the most common malignancy among women [1, 2]. Given recent advances in medical care, the prognosis of BC has improved, with current 5-year relative survival rates of approximately 86–89% in Taiwan and the USA [3, 4]. Due to the decreased mortality rate of stage 0–3 BC and longer survival periods, the impact of comorbidity on BC prognosis cannot be ignored.
Diabetes mellitus (DM) represents an increasing global public health concern [5,6,7]. As a result, DM and BC are fairly prevalent chronic diseases among women, and approximately 16% of BC patients suffer from type 2 DM [8]. The effect of DM on the prognosis of BC patients has been extensively investigated in recent years [9,10,11,12,13,14], with findings showing that DM is an independent risk factor for BC.
As a stable measurement of glycaemic control, haemoglobin A1C (HbA1C) has been used to show that good glycaemic control can reduce the risk of long-term micro-vascular complications in patients with type 2 DM [15, 16].
To date, few studies have examined the relationship between HbA1C levels and BC prognosis [17,18,19]. It is also not known how the impact of glycaemic control, reflected by the levels of HbA1c, could affect the prognosis of BC.
In this retrospective cohort study, we analysed the follow-up data of diabetic women with BC and adjusted prognostic factors that might affect the long-term survival of BC patients [20]. We also attempted to identify the ideal glycaemic level for better BC outcomes.
Methods
Data source
We obtained delinked data from the electronic medical record database of Taichung Veterans General Hospital (TCVGH), a medical centre in central Taiwan, and combined them with data from the Cancer Registry database. We included body mass index (BMI), medical diagnoses for the Charlson comorbidity index (CCI) calculation, drug prescriptions, and laboratory data during the follow-up period. The age at initial diagnosis of primary BC, lifestyle habits (including cigarette and alcohol use), BC pathology stage at diagnosis classified according to the American Joint Committee on Cancer (AJCC) stage and tumour–node–metastasis (TNM) staging system [21, 22], hormone status (triple-negative BC or not), and subsequent surgery/radiation/chemo/hormone/bone marrow transplant therapy/target therapy were recorded.
All of the HbA1C values that were measured were recorded. To account for individual changes in the HbA1C value over time, we used the post-index mean HbA1C values instead of baseline HbA1C values to express the glycaemic control, which was calculated as the mean of all of the observations recorded between the index date (first diagnosed with BC) and the outcome event (death) or censoring point (the last recorded database observation).
Patient population
The eligible patients included females ≥ 20 years of age with newly diagnosed stage 0–3 BC between 2004 and 2014. The definition of DM was based on any of the 3 following conditions: (1) inpatients: at least 1 diagnosis of DM or a prescription for anti-diabetic medications; (2) ambulatory care: at least 2 diagnoses of DM or at least 1 diagnosis of DM with a prescription for anti-diabetic medications; or (3) a mean HbA1C ≥ 6.5% or random blood sugar greater than 200 mg/dL before the BC diagnosis. Patients were excluded if their follow-up time was < 3 months, if they had a non-analytic case in the TCVGH Cancer Registry database, or if they were in the DM group and were without HbA1C data. Because almost half of the DM participants were lost to follow-up for the HbA1C test in our hospital, those DM patients included were compared with participants who had DM and were without HbA1C data to ensure the similarity between these two groups. The follow-up began on the date of the first cancer diagnosis and ended on the date of death or on the date of the end of follow-up. The patients were followed until 31 December 2015. The study was approved by the Institutional Review Board at Taichung Veterans General Hospital in Taichung, Taiwan. Figure 1 shows the flow chart of the patient selection methods.
Outcomes
The primary outcomes were all-cause mortality and BC-specific mortality. All-cause mortality was defined as death from any cause among women with BC, whereas BC-specific mortality was defined as death attributed to BC among women with BC. Breast cancer-specific mortality was the cause of death classified by International Classification of Diseases, Ninth Revision (ICD-9) codes, which is death due to primary malignant BC. Patients who were still alive were censored from analysis at the date of the last follow-up.
Statistical analysis
The data are expressed as the mean ± SD, as the median and interquartile range (IQR) for continuous variables, or as counts and proportions for categorical variables. The Kruskal–Wallis test, Mann–Whitney test, Chi-square test, and Fisher’s exact test were used for continuous and categorical variables to compare the characteristics between the BC patients with mean HbA1C values of < 7, 7–9, and > 9% and those without DM or to compare the differences between the DM groups of patients with and without HbA1C values. The analyses for both the continuous and categorical variable HbA1C indicators were performed using univariate and multivariate Cox regression models to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality and BC-specific mortality. In all of the multivariate models, the analysis was adjusted for potential confounders, which are listed in Table 1. Statistical significance was defined as a p value < 0.05. All of the analyses were performed using SPSS 22.0 (IBM, New York, USA).
Results
Our study cohorts included 2812 patients with BC who were either non-DM patients (n = 2667) or DM patients who had a post-index HbA1C (n = 145) measurement taken during the study period. The characteristics of the study population are summarized in Table 1. Compared to women without DM, patients with DM who had HbA1C monitoring tended to be older, obese (BMI > 30), and with stage 3 BC.
Of the patients with pre-existing diabetes (n = 283), 138 (49%) did not have HbA1C levels and were thus excluded from this study. The rest of the patients (n = 145) had a series of HbA1C levels recorded during follow-up and were eligible for the HbA1C analysis (Fig. 1). As shown in Table 2, the participants (with HbA1C data) in this study were not significantly different from those without HbA1C data, except for that the participants had lower CCI scores.
Compared to those of BC patients without DM, the adjusted HRs for continuous HbA1C (per one-unit increase) of all-cause mortality and BC-specific mortality were 1.56 (95% CI 1.11–2.20) and 3.07 (95% CI 1.45–6.48), respectively (Table 3).
The adjusted HRs for the mortality rates with categorical HbA1C levels are shown in Table 4. In adjusted model 2, the risk of all-cause mortality in women with an HbA1C value < 7% was not statistically significant compared to that in the non-diabetes group. However, a mean HbA1C > 9% in BC women was associated with a 3.65-fold (95% CI 1.13–11.82) higher risk of all-cause mortality. For those BC women in the suboptimal glycaemic control group (HbA1c between 7 and 9%), only the BC-specific mortality showed a statistically significant difference in comparison with the non-DM group after controlling for confounders.
Discussion
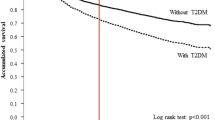
Despite a significant number of studies showing that DM patients show a BC prognosis with a worse outcome [10, 14], the novel findings from the present study demonstrate that glycaemic control, as reflected by the HbA1c levels, also influences the prognosis in diabetic women with BC. In clinical practice, patients with concurrent diabetes and cancer are very common. Thus, the current study evaluated the association between glycaemic control and mortality in BC patients. To our knowledge, this is the first study to investigate the relationship of glycaemic control and BC by setting a cut-off point of the HbA1C level in stage 0–3 breast cancer patients. Poorly controlled diabetes (a mean HbA1C > 9%) was associated with an increased risk of all-cause and BC-specific mortalities among women with BC. These associations persisted after adjusting for potential confounders, including BC stages. However, when patients presented with a mean HbA1C under 7% (defined as well-controlled diabetes), the survivals of the participants with DM and those without DM appeared to show no significant difference.
In patients with suboptimal glycaemic control (a mean HbA1C between 7 and 9%), model 2 showed no statistical significance in all-cause mortality compared to the non-DM group. However, the risk of BC-specific mortality was increased. It suggested that DM patients with BC should maintain good glycaemic control in order to reduce the risk of BC-related mortality. To our knowledge, no other study has yet revealed this association. Nevertheless, the poor glycaemic control group did show a poor prognosis in both all-cause and BC-specific mortalities. Although the causal relationship cannot be established by the current findings, we strongly suggest that those patients with DM, after being diagnosed with BC, should be periodically measured for HbA1C values and that the mean HbA1C value should be kept below 7%.
Among the limited number of studies evaluating the effect of HbA1C glycaemic control on cancer outcomes, the Women’s Healthy Eating and Living (WHEL) study was the first to indicate the association between the HbA1C level and BC prognosis in DM patients. In this study, the HbA1C level and BC prognosis were obtained using a health status questionnaire [17]. The risk of all-cause mortality was twice as high in women with an HbA1C ≥ 7.0% than in women who had a lower HbA1C level (HbA1C < 6.5%) but was not significantly different from those with an HbA1C between 6.5 and 6.9% after adjusting for confounders, which demonstrated that good glycaemic control might lead to a better BC prognosis. In the WHEL study, the HbA1C values were not followed up throughout the whole study period; instead, these data were only collected once at the beginning of the study, which did not reflect the level of glycaemic control during course of disease. In contrast, our study used a longitudinal dataset of HbA1C values from diabetes patients, which reflects the real-world situation in the clinical setting. Thus, these longitudinal HbA1c values make our study results more reliable. We also used a mean HbA1C ≤ 7% as a criterion for good glycaemic control, since this target has been shown to reduce micro-vascular complications and macro-vascular disease. [23, 24] Moreover, this cut-off point could be directly applied in clinical practice.
Another cohort study used the glycaemic control status to generate two groups of patients (HbA1C < 6.5% and HbA1C ≥ 6.5%) and examined the associations between the HbA1C value and mortality in women with BC. The results showed that the higher HbA1C group had a higher but not significantly different mortality rate (HR = 2.6) than the lower HbA1C group [18]. However, due to the limitation of the study database, adjusting for prognostic factors and an analysis of cancer-specific survival data was not able to be performed. In addition, how these authors dichotomously classified the patients into low-HbA1C and high-HbA1C groups was not specified.
To further evaluate the associations between the HbA1C and mortality, our study showed that when participants had a 1 unit increase in the HbA1C level, the risks for all-cause and BC-specific mortalities were significantly increased. However, a retrospective cohort study that used a single HbA1C measurement as a continuous variable at the time of diagnosis indicated that there was no association between the HbA1C level and all-cause mortality in BC patients of all stages [19]. Since cancer patients of all stages were included in this study, it is possible that the low survival rate in stage 4 BC patients may have compromised the effect of glycaemic control.
Data with missing values are common when using a restricted database and in long-term observational studies. It could be expected that those excluded patients without HbA1C values might compromise our study results. However, we observed no significant differences in the baseline and BC characteristics of both groups, except for the participants with HbA1C data who had a lower CCI score. In addition, we controlled for several confounders in the multivariate analysis, so we believe that these bias effects were minimized.
The mechanisms of the association between DM and increased tumour growth are not fully understood. Recent studies have suggested that hyperglycaemia can influence cancer prognosis by affecting cancer cell pathways, including cancer cell proliferation, apoptosis inhibition, migration, and invasion [25, 26]. Thus, it is reasonable to speculate that more intensive glycaemic control may benefit the prognosis of patients with BC and DM.
Several limitations of our study should be addressed. First, the cancer registry database is not immediately updated. Thus, the final date of the last contact will be underestimated. Second, we were not able to access patients’ medical records if they were seeking medical care for DM outside of our hospital. Therefore, the DM diagnosis could also be underestimated. However, the pattern of pre-DM in the BC population was similar to that of other studies, [27] and the stage of BC was also similar to that in the national data. [3] Thus, this limitation may not have had a significant effect on the results. Finally, the effects of anti-diabetic medications were not able to be studied due to the relatively small sample size of the DM group. Metformin therapy plays a protective role in BC prognosis, [28, 29] and the use of insulin glargine may lead to a higher risk of developing BC [30, 31]. Adherence to oral hypoglycaemic drugs is also known to influence glycaemic control after BC diagnosis [32]. Thus, the impact of anti-diabetic medications and adherence on the prognosis of BC cannot be ignored and need to be explored further.
Conclusion
Our pilot study focused on developing an available and reliable HbA1C target for health providers and patients to assess the effectiveness of management plans of glycaemic control. Our data suggest that patients who have DM and stage 0–3 BC should maintain a mean HbA1C value under 7%. Future large research studies are needed to verify the effect of our glycaemic control target and to adjust for other possible confounders in DM patients, such as anti-diabetic agents.
References
Chang L-Y, Yang Y-L, Shyu M-K, Hwa H-L, Hsieh F-J. Strategy for breast cancer screening in Taiwan: obstetrician-gynecologists should actively participate in breast cancer screening. J Med Ultrasound. 2012;20(1):1–7. https://doi.org/10.1016/j.jmu.2012.01.001.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ, Lai MS. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J Formos Med Assoc. 2016;115(12):1076–88. https://doi.org/10.1016/j.jfma.2015.10.011.
America Cancer Society. Breast cancer facts and figures 2013–2014. Atlanta: American Cancer Society, Inc.; 2013.
Al-Lawati JA. Diabetes mellitus: a local and global public health emergency! Oman Med J. 2017;32(3):177–9. https://doi.org/10.5001/omj.2017.34.
Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr Diab Rep. 2013;13(6):805–13. https://doi.org/10.1007/s11892-013-0425-5.
Roglic G. WHO Global report on diabetes: a summary. Int J Noncommun Dis. 2016;1(1):3–8.
Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6(2):103–11. https://doi.org/10.1016/S1470-2045(05)01736-5.
Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. https://doi.org/10.2337/dc10-0666.
Gold HT, Makarem N, Nicholson JM, Parekh N. Treatment and outcomes in diabetic breast cancer patients. Breast Cancer Res Treat. 2014;143(3):551–70. https://doi.org/10.1007/s10549-014-2833-x.
He DE, Bai JW, Liu J, Du CW, Huang WH, Zhang GJ. Clinicopathological characteristics and prognosis of breast cancer patients with type 2 diabetes mellitus. Mol Clin Oncol. 2015;3(3):607–12. https://doi.org/10.3892/mco.2015.522.
Patterson RE, Flatt SW, Saquib N, et al. Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat. 2010;122(3):859–65. https://doi.org/10.1007/s10549-010-0732-3.
Zhao XB, Ren GS. Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(49):e5602. https://doi.org/10.1097/MD.0000000000005602.
Zhou Y, Zhang X, Gu C, Xia J. Influence of diabetes mellitus on mortality in breast cancer patients. ANZ J Surg. 2015;85(12):972–8. https://doi.org/10.1111/ans.12877.
Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23(2):187–91. https://doi.org/10.2337/diacare.23.2.187.
Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. The Lancet. 2010;375(9713):481–9. https://doi.org/10.1016/s0140-6736(09)61969-3.
Erickson K, Patterson RE, Flatt SW, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29(1):54–60. https://doi.org/10.1200/JCO.2010.29.3183.
Jousheghany F, Phelps J, Crook T, Hakkak R. Relationship between level of HbA1C and breast cancer. BBA Clin. 2016;6:45–8. https://doi.org/10.1016/j.bbacli.2016.04.005.
Boursi B, Giantonio BJ, Lewis JD, Haynes K, Mamtani R, Yang Y-X. Serum glucose and hemoglobin A1C levels at cancer diagnosis and disease outcome. Eur J Cancer. 2016;59:90–8. https://doi.org/10.1016/j.ejca.2016.02.018.
Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107(3):309–30. https://doi.org/10.1007/s10549-007-9556-1.
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. New York: Springer; 2010.
Frederick LG, Page DL, Fleming ID, et al. AJCC cancer staging manual. New York: Springer; 2013.
Glycemic targets. Diabetes care. 2017;40 (Suppl 1):S48–S56. https://doi.org/10.2337/dc17-s009.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract. 2017;23(2):207–38. https://doi.org/10.4158/EP161682.CS.
Duan W, Shen X, Lei J, et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int. 2014;2014:461917. https://doi.org/10.1155/2014/461917.
Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J. 2014;38(5):330–6. https://doi.org/10.4093/dmj.2014.38.5.330.
Luo J, Hendryx M, Virnig B, et al. Pre-existing diabetes and breast cancer prognosis among elderly women. Br J Cancer. 2015;113(5):827–32. https://doi.org/10.1038/bjc.2015.249.
Xu H, Chen K, Jia X, et al. Metformin use is associated with better survival of breast cancer patients with diabetes: a meta-analysis. Oncologist. 2015;20(11):1236–44. https://doi.org/10.1634/theoncologist.2015-0096.
Kim HJ, Kwon H, Lee JW, et al. Metformin increases survival in hormone receptor-positive, HER2-positive breast cancer patients with diabetes. Breast Cancer Res BCR. 2015;17:64. https://doi.org/10.1186/s13058-015-0574-3.
Peeters PJ, Bazelier MT, Leufkens HG, et al. Insulin glargine use and breast cancer risk: associations with cumulative exposure. Acta oncologica (Stockholm, Sweden). 2016;55(7):851–858. https://doi.org/10.3109/0284186x.2016.1155736.
Bronsveld HK, ter Braak B, Karlstad O, et al. Treatment with insulin (analogues) and breast cancer risk in diabetics; a systematic review and meta-analysis of in vitro, animal and human evidence. Breast Cancer Res BCR. 2015;17:100. https://doi.org/10.1186/s13058-015-0611-2.
Calip GS, Hubbard RA, Stergachis A, Malone KE, Gralow JR, Boudreau DM. Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment. Pharmacoepidemiol Drug Saf. 2015;24(1):75–85. https://doi.org/10.1002/pds.3660.
Acknowledgements
This study was conducted and supported by the Taichung Veterans General Hospital Research Program (TCVGH-1056103B). The study was approved by the institutional review board (CG16013A) at Taichung Veterans General Hospital in Taichung, Taiwan. We express thanks to the Clinical Informatics Research and Development Centre of Taichung Veterans General Hospital for the support of the clinical data and to the Biostatistics Task Force of Taichung Veterans General Hospital for the assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chang, YL., Sheu, W.HH., Lin, SY. et al. Good glycaemic control is associated with a better prognosis in breast cancer patients with type 2 diabetes mellitus. Clin Exp Med 18, 383–390 (2018). https://doi.org/10.1007/s10238-018-0497-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-018-0497-2