Abstract
Background and Objectives
Abaloparatide (ABL) significantly increases bone mineral density in men with osteoporosis similar to what was reported in postmenopausal women with osteoporosis. The cost effectiveness of sequential treatment with ABL followed by alendronate (ALN) in men at high fracture risk was compared to relevant alternative treatments.
Methods
A Markov-based microsimulation model based on a lifetime US healthcare decision maker perspective was developed to evaluate the cost (expressed in US$2021) per quality-adjusted life-years (QALYs) gained of sequential ABL/ALN. Comparators were sequential treatment unbranded teriparatide (TPTD)/ALN, generic ALN monotherapy, and no treatment. Discount rates of 3% were used. Consistent with practice guidelines, patients received 18 months of ABL or TPTD followed by ALN for 5 years, or 5 years of ALN monotherapy. Analyses were conducted in high-risk men aged over 50 years defined as having a bone mineral density T-score ≤−2.5 and a recent fracture. Time-specific risk of subsequent fracture after a recent fracture, incremental costs up to 5 years following fractures, real-world medication adherence, and mostly US men-specific data were included in the model. One-way and probabilistic sensitivity analyses were conducted to assess the robustness of results.
Results
Over the full age range, sequential ABL/ALN led to more QALYs for lower costs than sequential unbranded TPTD/ALN, while no treatment was dominated (more QALYs, lower costs) by ALN monotherapy. The costs per QALY gained of sequential ABL/ALN were lower than the US threshold of US$150,000 versus generic ALN monotherapy. The probabilities that sequential ABL/ALN was cost effective compared to ALN monotherapy were estimated at 51% in men aged 50 years and between 88 and 90% in those aged ≥ 60 years.
Conclusions
Sequential therapy using ABL/ALN may be cost effective compared with generic ALN monotherapy in US men aged ≥ 50 years at high fracture risk, especially in those aged ≥ 60 years. Unbranded TPTD/ALN and no treatment were dominated interventions (less QALY, more costs) compared with ABL/ALN or ALN monotherapy.
Similar content being viewed by others
Sequential treatment abaloparatide/alendronate improves quality-adjusted life-years for less costs compared with a similar sequence using unbranded teriparatide first. |
Sequential treatment abaloparatide/alendronate may be cost effective compared with oral alendronate monotherapy in men at high risk of fractures aged ≥ 50 years in real-world settings, especially in those aged ≥ 60 years. |
This study shows the comparability of the cost effectiveness of abaloparatide in men to published data on cost effectiveness in women. |
1 Introduction
Osteoporosis is perceived as a disease largely in women; however, it also affects men substantially. In the USA, men aged ≥ 50 years sustain approximately 600,000 incident osteoporosis-related fractures annually, accounting for about 30% of all fractures and costing more than US$4 billion [1]. In the 27 countries of the European Union plus the UK and Switzerland, the number of new fragility fractures in 2019 was estimated at 1,400,000 in men aged ≥ 50 years [2]. Recently, the ATOM clinical trial showed that treatment with abaloparatide (ABL) resulted in a significant increase in bone mineral density (BMD) in men with osteoporosis, suggesting similar clinical effects in men and women [3]. Based on these data, ABL was granted US Food and Drug Administration approval on 19 December, 2022 for men with osteoporosis and numerous payers have therefore extended the ABL label to men with osteoporosis with a pre-authorization as is commonly done for this class of medications.
Previous health economic analyses suggested that sequential treatment beginning with ABL followed by oral alendronate (ALN) led to more quality-adjusted life-years (QALYs) for lower costs than a similar sequence using teriparatide (TPTD) first in postmenopausal women with osteoporosis [4, 5], and was further cost effective versus monotherapy with ALN in those women aged ≥ 60 years [6]. A recent systematic review has revealed a limited number of cost-effectiveness analyses in men with osteoporosis of which only two studies were published in the last 5 years [7]. This review further suggested that anti-osteoporosis medication, nutrition supplements, screening, and post-fracture care programs have the potential to be cost effective in men with osteoporosis. However, as compared to studies in women, no study has yet been conducted to estimate the cost effectiveness of sequential treatment and/or of ABL in men with osteoporosis. To elucidate the impact of any potential differences in the cost effectiveness between men and women, including differences in fracture risk and consequences thereof (e.g., fracture costs, excess mortality), this study aims to estimate the cost effectiveness of sequential ABL/ALN in US men at high fracture risk from the healthcare decision-maker perspective.
2 Methods
2.1 Study Context
The study followed the ESCEO-IOF guideline for economic evaluations in the field of osteoporosis [8], the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 statement [9], the US PHS Panel recommendations [10], and the 2020 Academy of Managed Care Pharmacy format for economic information submission [11]. A health economic analysis plan including data, assumptions, and analyses was developed and approved by the research team including US clinicians and experts in economic modeling in osteoporosis. US men-specific data were used to populate the economic model when available.
A description of the model, population, fracture costs and utilities, treatment data, analyses, and sensitivity analyses is provided below. Table 1 includes the key model data. Additional details regarding the model could be found in Sect. 1 of the Electronic Supplementary Material (ESM) and in previous publications [4, 12]. Section 2 of the EMS includes the completed CHEERS 2022 checklist, the osteoporosis-specific checklist for reporting cost-effectiveness studies, and the ESCEO-IOF checklist for designing and conducting cost-effectiveness studies in osteoporosis.
2.2 Economic Model
A lifetime Markov-based microsimulation model based on a previously published model [4, 6, 12] evaluated the cost effectiveness of sequential ABL/ALN in US men from the healthcare decision-maker perspective. A microsimulation technique was employed to track fracture events and patient characteristics, to avoid unnecessary restrictions between health states [8, 13]. The model also incorporated a new feature reflecting the imminent risk of fractures [14], to mitigate the risk of a subsequent fracture according to the time since the fracture event. In addition, the model included, for the first time ever, time-dependent incremental costs of fractures (per type) over a 5-year post-fracture follow-up period [15].
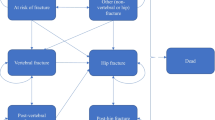
The five model health states included ‘no fracture,’ ‘hip fracture,’ ‘vertebral fracture,’ ‘nonhip nonvertebral fractures (NHNV),’ and death (see Fig. 1). As data (including fracture costs, excess mortality, and increased risk of subsequent fractures) were available per 6-month period, and as ABL and TPTD were given for an 18-month period, a 6-month cycle length was selected in line with previous studies and recommendations [8], and the model included a lifetime horizon to capture the long-term consequences of fractures. Multiple fractures may be experienced by patients at either the same site or several different sites. We followed the US PHS Panel recommendations and used discount rates of 3% for both costs and QALYs [10]. TreeAge Pro 2022 R1.2 (TreeAge Pro Inc., Williamston, MA, USA) software was used to build up the model.
2.3 Population and Fracture Risk
The target population were US men aged ≥ 50 years at high (and imminent) risk of fracture defined as having a recent fracture and a BMD T-score ≤−2.5. The fracture risk in the model was based on four elements: fracture risk of the general male population, increased fracture risk associated with densitometric osteoporosis (BMD T-score ≤−2.5), increased fracture risk due to a previous fracture, and the possible fracture risk reduction from treatment. Fracture risk was updated after each fracture and any time the patient age changed.
Hip and vertebral fractures incidence in the US male general population were extracted from Ettinger et al. [16], similarly to what was done to derive the current version of the US FRAX® Tool and in line with recent cost-effectiveness analyses with US women with osteoporosis [17, 18]. Ettinger et al. used 2006 hospital discharge data from 38 states from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample. Although previous studies reported a decline in hip fracture rates in the USA, more recent trends have suggested that hip fracture rates are no longer declining and even increasing [19], leading to no time adjustment in fracture incidence rates in our model. As the study of Ettinger et al. [16] did not include the incidence of NHNV, combined incidence data for wrist, pelvis, and other fractures (including clavicle, humerus, legs, and hands/fingers) were derived using 2001 Healthcare Cost and Utilization Project Nationwide Inpatient Sample [1]. The increased risk due to osteoporosis (BMD T-score ≤−2.5) was based on a previously validated method [20]. In line with clinical practice and recommendations of the International Society for Bone Densitometry [21], the US Caucasian female BMD reference database was applied to derive T-scores in men [22]. The increased risk of fractures due to a previous fracture was based on a recent large database of Swedish women that reported the risk of a subsequent fracture according to the time since the fractures and the number of fractures [14]. As men have a greater subsequent fracture risk after fractures than women [23], an adjustment was made according to the study of Center et al. [23].
Mortality rates per age for US men (in 2019) were derived from the US National Vital Statistics System. Mortality rates after hip and vertebral fractures were incorporated in the model, consistent with prior economic studies [6, 24]. In line with the study of Tran et al. [25], no mortality excess was incorporated after NHNV fractures. Comorbidities may also contribute to excess mortality; therefore, only 25% of the fracture excess death was considered to be attributable to a fracture event and therefore included in the model, in line with clinical studies [26, 27] and recommendations of the ESCEO-IOF guideline [8].
2.4 Fracture Costs
All healthcare costs were expressed in US$2021 and adjusted by the US consumer price index for medical care if appropriate. Yearly incremental costs of hip, vertebral, and NHNV fractures were extracted from the recent US study of Tran et al. estimating Medicare and commercial costs of fracture patients compared to controls in a sample of 91,925 women aged 50–64 years and 134,265 women aged ≥ 65 years from 2008 to 2017 [15]. Components of costs included inpatient admissions, emergency room visits, outpatient services and visits (such as outpatient office visits, skilled nursing facility services, and other outpatient services), and outpatient pharmacy claims. These fracture costs were adjusted by a proportion factor of 1.11 derived from Williams et al. [28] to consider higher fracture costs in men than women, and higher fracture costs were used for subsequent fractures (compared with the initial fracture, + 107% and + 68% for commercial and Medicare patients, respectively) [29].
In contrast with most previous economic evaluations that estimated the long-term costs of hip fracture based on admission rates to nursing homes following the fracture, this study used yearly incremental costs of hip, vertebral, and NHNV during a 5-year post-fracture follow-up period, according to the same study of Tran et al. [15]. As hip fractures are associated with long-term admission to nursing home and costs [30], the incremental cost of hip fractures in year 5 from Tran et al. was maintained for a lifetime. No cost after the 5-year period was conservatively assumed for vertebral and NHNV fractures (except in a sensitivity analysis). In the case of multiple fractures, only one (the highest) fracture cost was considered.
2.5 Utilities
Utilities data from the report of nationally representative values for the noninstitutionalized US adult population for seven health-related quality-of-life scores (data from 2006 using EQ-5D) [31] were adjusted by the study of Gold et al. [32] (which revealed that patients with a previous fracture have a 13% lower utility than the general population) to derive baseline men utilities according to age. The International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) [33] evaluating the quality of life of 3021 patients (86% of women) across the world with fractures using the EQ-5D questionnaire was used to derive the consequences of hip and vertebral fracture on baseline utilities. As ICUROS only included hip, vertebral, and wrist fractures, the study of Kanis et al. [34] was used for the impact of NHNV on utility. In the absence of ICUROS men-specific utility data, combined data for men and women were used, in line with a recent study suggesting that men and women had a similar quality of life 1 year following fragility fracture [35].
2.6 Treatments
Sequential ABL/ALN was compared to sequential unbranded TPTD/ALN, generic ALN monotherapy, and no treatment. As most US patients with a hip fracture do not start an anti-osteoporosis medication in the year following the fracture [36], no treatment is a relevant comparator treatment. Based on recommendations from the 2016 clinical practice guidelines for osteoporosis from the American Association of Clinical Endocrinologists and American College of Endocrinology [37], patients received 18 months of ABL or TPTD followed by ALN for up to 5 years.
Because of similar BMD gains in men and women [3, 38] and the lack of antifracture efficacy data for ABL in men (as with other anabolic agents), the efficacy on fracture risk was extrapolated from clinical studies in women with postmenopausal osteoporosis (PMO), as done in most previous cost-effectiveness analyses in men with osteoporosis [7]. Similar treatment effects as in previous studies in women with PMO were used [4, 6].
The effects of ABL on the fracture risk reduction was taken from the 43-month ACTIVE/ACTIVExtend intention-to-treat population and were maintained during ALN intake. Abaloparatide was shown to decrease the risk of vertebral fractures by 84% and the risk of NHNV fractures by 58%, assuming a fracture risk reduction for major osteoporotic fractures [39]. As the ACTIVE/ACTIVExtend trials were not statistically designed to reveal a significant risk reduction for hip fracture, the effects of ABL on nonvertebral fractures were conservatively used as a surrogate for hip fracture as recommended by the ESCEO-IOF guideline [8]. Therefore, we assumed that ABL reduced the risk of hip fracture by 37% [39]. Upon initiation of treatment with ALN, it was assumed that the fracture risk would decrease at a similar proportion to estimates from the National Institute for Health and Care Excellence appraisal (TA464) [40]. Alendronate therefore further reduced the risks of hip, vertebral, and NHNV fractures by 33%, 55%, and 19%, respectively.
In line with the ACTIVExtend study suggesting that a bone-forming treatment (i.e., ABL) maintains its effects on fracture risk reduction after switching to ALN, treatment effects of TPTD from the ACTIVE trial was used for the sequence TPTD/ALN. It was further assumed that the effects of ABL or TPTD conservatively linearly decrease in the year following ALN discontinuation, while ALN efficacy declined to zero for a similar period to the treatment period in line with previous economic and clinical studies [41].
The wholesale acquisition cost price from the Online Red Book [42] was used for drug prices, being currently the most commonly used measure of drug costs in US-based cost-effectiveness analyses [43]. Annualized yearly cost of ABL, unbranded TPTD, and generic ALN were thus US$24,600, US$32,340, and US$182, respectively. Total costs of the drugs were adjusted by the number of drugs taken during the ACTIVE trial (estimated to be 81.5% for ABL and 86.7% for TPTD) to allow for the fact that not all drugs were received by patients. Monitoring costs included one physician visit of (US$118) at 6-month intervals during treatment and one BMD measurement at a cost of US$47.5 every 2 years in line with Medicare insurance reimbursement.
Adverse events with medications were included in the analysis. For ABL and TPTD, the effects on hypercalcemia were included. Incidences of 0.37%, 3.41%, and 6.37% were used for no treatment, ABL, and TPTD in line with the ACTIVE trial, respectively. The cost of hypercalcemia in the USA was derived from Liu et al. [44] (US$130 in 2003). Gastrointestinal adverse effects with ALN were also included, using a similar methodology to the National Institute for Health and Care Excellence in the UK [40, 45], suggesting that patients taking ALN used 0.041 extra general practitioner consultations in the first 6 months of treatment, followed by 0.021 general practitioner consultations during each 6-month interval of treatment, as well as a proton-pump inhibitor for each consultation.
Treatment effects and treatment costs were reduced by medication adherence based on the method developed by Liu et al. [44] (see Sect. 1 of the ESM), in line with economic evaluations of ABL in women with PMO [4,5,6]. Persistence levels from the study of Cheng et al. [46] were selected as this study assessed persistence to anabolic (i.e., TPTD) and oral bisphosphonates (including ALN) in the same population comprising 10,863 US women (with a mean age of 66 years) newly initiating medications in 2012. The persistence level to TPTD at 12 months (that was also used for ABL in the model) was estimated at 59.1%, similarly to the findings of a systematic review of studies assessing persistence to TPTD [47] evaluating the median persistence to TPTD at 1 year at 52%. In the model, persistence data at 12 months were used for the full 18-month treatment period of TPTD/ABL to counterbalance the somewhat higher and lower persistence at 6 and 18 months, respectively [47]. Persistence to ALN from Cheng et al. was estimated at 12 months at 35.1%, similarly to another US study from Singer et al. [48] in the USA (39%). In contrast with previous economic evaluations in women with PMO [4,5,6] that assumed a similar persistence rate for the maximum duration of treatment (5 years), a lower persistence to ALN (17.5%) was assumed from year 3 onwards in line with the review of Fatoye et al. [49] and the US study of Singer et al. [48].
2.7 Model Validation
The model was based on a previously published and extensively validated model [12]. The validation of the model was performed on face validity, internal validity, and external validity. For face validity, we consulted with clinical experts in the field of osteoporosis that reviewed and approved all data and model assumptions. For internal validity, various tests on model parameters and modeling assumptions were performed and compared with expected directions. For external validity, we compared the predicted lifetime risk of fractures and expected life expectancy with those reported in epidemiological studies.
2.8 Analyses and Sensitivity Analyses
For each analysis and alternative treatment, based on 1,000,000 first-order simulations, healthcare costs, fracture events, and QALYs were estimated. Incremental cost-effectiveness ratios (ICERs), defined as the difference between healthcare costs divided by the difference in QALYs, were estimated for all interventions compared with the next non-dominated intervention. An intervention is dominated if it provides less QALYs for more costs than another intervention. The Institute for Clinical and Economic Review suggests that interventions with ICERs below US$100,000 per QALY gained are considered a high care value, as well as interventions with ICERs between US$100,000 and US$150,000 that offer considerable other benefits [50].
Multiple analyses were conducted to characterize parameter uncertainty including various ages (from 50 to 90 years). Several one-way sensitivity analyses were directed on model parameters and structure varying fracture incidence (± 25%), fracture costs (± 25%), fracture effects on utilities (± 25%), discount rates (0%, 5%), fracture excess mortality (assuming no excess mortality), shorter time horizon (10 years), and population (assuming men with only T-score ≤−2.5 and no previous fracture). Other sensitivity analyses were conducted assuming that long-term hip fracture costs are based on admission rates to nursing homes that were estimated at 15% in US men aged ≥ 65 years and 12.6% in US men aged < 65 years [51, 52], and if incremental costs of vertebral and NHNV fractures in year 5 were maintained for a lifetime as done for hip fractures. Sensitivity analyses were also conducted on ABL drug price (± 20% and 50%), on hip fracture risk reduction for ABL and TPTD assuming a treatment effect on major osteoporotic fractures and using efficacy data from a recent network meta-analysis [53]. Subsequently, two sensitivity analyses were conducted on the effects of ABL and TPTD after discontinuation assuming (1) a linear decrease up to 3 years following discontinuation and (2) a maintenance of the effects 2 years following discontinuation and a linear decline in the next following 3 years. Finally, complete medication adherence and no adverse events were also assessed.
Probabilistic sensitivity analyses were conducted following guidelines by varying most parameters according to distributions, [54]. Table 4 of the ESM provides the list of distributions. Two hundred second-order simulations of 50,000 patients per alternative treatment were conducted for the probabilistic sensitivity analyses. Cost-effectiveness acceptability curves were estimated to indicate the probability of each alternative treatment being cost effective according to the decision maker’s willingness to pay per QALY gained.
3 Results
3.1 Model Validation
The model performed well during validation, producing fracture incidence and mortality rates that were similar to the data. Expected life expectancies were also very similar to empirical data. The lifetime risks of hip and clinical vertebral fracture were estimated at 10.2% and 8.2% for men aged 50 years, respectively. Furthermore, tests on model parameters and modeling assumptions were coherent with expected results.
3.2 Base-Case Analysis
Lifetime healthcare costs, fractures, QALYs, and the ICER (US$2021 per QALY gained) are presented in Table 2 for US men aged 70 years with a recent fracture and a BMD T-score ≤−2.5. Sequential ABL/ALN therapy was associated with more QALYs for lower costs compared with a similar sequence starting with unbranded TPTD, being therefore dominant compared to TPTD/ALN. The ICERs compared to ALN monotherapy were estimated at US$66,467.
The ICERs of sequential ABL/ALN compared to ABL/ALN for other ages are presented in Table 3 and fell below the US cost-effectiveness threshold of US$150,000 per QALY gained in men aged ≥ 50 years. The ICERs generally decreased with increasing age, except at the age of 90 years. In all age scenarios, unbranded TPTD/ALN and no treatment were dominated (less QALY, more costs) by ABL/ALN and ALN monotherapy, respectively. Table 1 of the ESM provides the lifetime healthcare costs, fractures, QALYs, and ICERs of ABL/ALN compared to each treatment for each age scenario.
3.3 One-Way Sensitivity Analysis
Sequential ABL/ALN was dominant (more QALYs for less costs) compared to sequential unbranded TPTD/ALN (see Table 4 of the ESM) in all sensitivity analyses, except when assuming a 50% higher drug cost of ABL. In that simulation, ABL/ALN led to more costs and QALYs, resulting in an ICER of US$175,441 per QALY gained. No treatment was dominated (less QALYs, more costs) compared to ALN monotherapy in all sensitivity analyses.
The ICER of ABL/ALN compared to ALN monotherapy was most sensitive to ABL drug price, fracture incidence, and fracture costs (Fig. 2a, b). The ICERs of sequential ABL/ALN were below the cost-effectiveness threshold of US$150,000 per QALY gained in most sensitivity analyses, except in men with a BMD T-score ≤−2.5 and no prior fracture where the ICER compared to ALN monotherapy increased to US$189,492. Assuming complete medication adherence led to a higher cost per QALY gained (US$115,391 vs US$66,467).
One-way sensitivity analyses on a model data and structure and b treatment characteristics on the cost per quality-adjusted life-year (QALY) gained of abaloparatide/alendronate (ABL/ALN) compared to ALN monotherapy in men with a recent fracture and a bone mineral density T-score ≤−2.5 aged 70 years. TPTD teriparatide
3.4 Probabilistic Sensitivity Analyses
The cost-effectiveness acceptability curves (reported in Fig. 3 for men aged 70 years and in Fig. 1 of the ESM for the ages of 50, 60, 80, and 90 years) suggests that ABL/ALN was the most cost-effective intervention from a decision maker’s willingness to pay US$75,000 per QALY gained in men aged ≥ 60 years, and from a willingness to pay US$150,000 in men aged 50 years. Figure 4 reports the cost-effectiveness acceptability curves of ABL/ALN compared to ALN monotherapy, ABL/ALN was cost effective at a threshold of US$150,000 per QALY gained in 51%, 89%, 88%, 90%, and 88% of the simulations of men in their 50s, 60s, 70s, 80s, and 90s, respectively. Figure 2 of the ESM reports the cost effectiveness of ABLN/ALN compared to other individual treatments.
4 Discussion
Sequential therapy with ABL followed by ALN was shown to be cost effective for US men at high (and imminent) risk of fracture. Sequential ABL/ALN leads to improved QALYs for less total healthcare costs compared with sequential unbranded TPTD/ALN. Noting the wholesale acquisition cost for US unbranded TPTD for 2022 is approximately 35% lower than branded TPTD, cost savings from ABL/ALN are thus even more favorable compared to branded TPTD/ALN. Furthermore, sequential ABL/ALN was cost effective compared with ALN monotherapy (threshold of US$150,000 per QALY) in US men aged ≥ 50 years at high risk of fractures. The probabilistic sensitivity analyses revealed that there is uncertainty in the cost effectiveness of ABL/ALN compared to ALN monotherapy in men aged 50 years with probabilities to be cost effective near to 50% for both treatments, while the probabilities of ABL/ALN to be cost effective exceed 85% in men aged ≥ 60 years. It is further interesting to observe that, even though no treatment is dominated (less QALY, more costs) in all analyses, only a minority of patients with recent fracture receive an anti-osteoporosis medication in the year following the fracture [36]. ALN monotherapy remains the most prescribed first-line treatment for osteoporosis because of its low cost. However, improved QALYs in a cost-effective approach could be obtained with sequential treatment with an anabolic agent such as ABL first followed by ALN, particularly in men at high risk of fractures.
To our knowledge, this study is the first cost-effectiveness study of ABL in men with osteoporosis as well as the first economic study of sequential treatment in this population. There is increasing evidence supporting the health value of sequential therapy with anabolic therapy (such as ABL) first followed by an antiresorptive agent (ALN) [55, 56], as well as on the cost effectiveness of this strategy compared to antiresorptive monotherapy in women with PMO [4, 6, 56, 57]. The current study therefore extends the economic value of this strategy to men at high risk of fractures.
Although direct comparison between this study and previous studies in women with PMO should be made with caution, as several model parameters and assumptions may differ (e.g., various populations, differences in fracture and incidence data, and evolution of drug prices), our results are in line with these previous studies. Two studies [4, 5] reported that sequential ABL/ALN was dominant compared with sequential TPTD/ALN in women at high risk of fractures. Another study [6] demonstrated that sequential ABL/ALN therapy was cost effective (at a threshold of US$150,000 per QALY) compared to ALN monotherapy for women with a BMD T-score ≤−3.5 and aged ≥ 60 years under full adherence, and in women aged ≥ 50 years under real-world adherence. Interestingly, from studies in both women and men, the cost effectiveness of sequential ABL/ALN compared to ALN monotherapy improved when incorporating real-world adherence, resulting from better adherence to anabolic treatments compared with ALN [46]. Medication adherence has been shown to be an important driver of the cost effectiveness between osteoporosis medications [58].
A sensitivity analysis confirmed the importance of baseline fracture risk on the cost effectiveness of sequential treatment. In men with no previous fracture and a BMD T-score ≤−2.5, sequential ABL/ALN was not cost effective compared to ALN monotherapy, suggesting that a high risk of fractures is needed for sequential treatment to be cost effective. This is consistent with a recent study highlighting that the cost effectiveness of sequential treatment in women is driven by the treatment acquisition costs and is limited to those at very high fracture risk [56]. Other sensitivity analyses showed that the results were robust when varying diverse model parameters and were also sensitive to drug cost, time horizon, and effects on hip fractures.
There are potential limitations of this study. For example, the ACTIVE and ACTIVExtend trials were not statistically designed to reveal a significant risk reduction for hip fracture [4, 6]. Therefore, we conservatively used the risk reduction of nonvertebral fracture (37%) as a surrogate for hip fracture, in line with the ESCEO-IOF guideline. Assuming the risk reduction of major osteoporotic fracture (58%) improved the cost effectiveness of ABL/ALN as shown in a sensitivity analysis. Although ACTIVExtend suggests the maintenance of the effects of ABL after transitioning to ALN for a period of 2 years, further investigation would be of interest to assess this extrapolation to a 5-year ALN period. In addition, more data are needed on real-world adherence to ABL and on the effect of adherence on ABL fracture efficacy. Observational studies could also provide relevant information on the real-world effectiveness of ABL. Recently, Cosman et al. [59] confirmed the real-world effectiveness of ABL versus TPTD on the nonvertebral fracture incidence using data from the US administrative claims database study. Another study [60] reported that most women were satisfied with ABL and found it convenient/easy to prepare and store. Further studies assessing preference and satisfaction to ABL in men with osteoporosis, as well as data on real-world effectiveness in this population would be of interest.
Another potential limitation is that data for fracture risk reduction in men for osteoporosis medications are not available. However, for any drug approved for the treatment of osteoporosis that has shown a reduced fracture incidence in women with PMO, regulators require a bridging study with a placebo comparator for approval in men [61]. This approach is also accepted by payers, and most previous economic evaluations in men with osteoporosis have therefore assumed similar treatment efficacy between men and women [7]. Furthermore, awareness of osteoporosis in men and its consequences is improving, albeit fracture data for men remain limited [62]. Certain relevant detailed data were available only for women (e.g., fracture costs, relative risk of subsequent fracture risk after a recent fracture) and adjustments were done to consider differences between men and women. Future epidemiological and economic studies on the burden and consequences of fractures should systematically include both women and men, and report data separately. Another potential limitation is that assumptions on adherence used in this study were derived from a study in women, while a systematic review reported that worse adherence to oral bisphosphonates was associated with men [63]. Adherence to ALN was conservatively assumed to be similar in patients with and without previous use of anabolic treatment, while one may expect that previous use of anabolic agents may potentially influence adherence to an antiresorptive agent. In the future, as real-world persistence data with sequential ABL/ALN become available, an update of the cost-effectiveness analysis may be of interest. We did not model the cost effectiveness in men by the specific fracture location. It has been shown that some fracture sites such as the hip or vertebrae are associated with a greater relative risk for subsequent fractures [23], leading to improved cost effectiveness of sequential ABL/ALN in men with previous hip or vertebral fractures. However, a study strength was the incorporation of the time-dependent fracture risk [64] and fracture costs in patients with recent fracture. To our knowledge, time-dependent fracture costs have not been used in previous economic models in osteoporosis. In previous models, the long-term costs of hip fractures were based on institutionalization rates, which may modestly contribute to an overestimation of the cost effectiveness of medications. The recent availability of detailed data regarding the time-dependent fracture risks and costs makes it possible to develop better microsimulation models incorporating the time since fractures. Finally, our study used fracture incidence data from white US men. Because of differences in fracture risk according to ethnicity [65], our findings could not be generalizable to the whole US male population, and to non-white US men.
5 Conclusions
This study suggests that sequential ABL/ALN leads to more QALYs for lower costs than to unbranded TPTD/ALN in US men aged ≥ 50 years at high fracture risk and may also be cost effective compared to generic ALN monotherapy in men aged ≥ 50 years when assuming real-world adherence, especially in those aged ≥ 60 years.
References
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75.
Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16(1):82.
Czerwinski E, Cardona J, Plebanski R, Recknor C, Vokes T, Saag KG, et al. The efficacy and safety of abaloparatide-SC in men with osteoporosis: a randomized clinical trial. J Bone Miner Res. 2022;37(12):2435–44.
Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum. 2019;49(2):184–96.
Le QA, Hay JW, Becker R, Wang Y. Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann Pharmacother. 2019;53(2):134–43.
Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide followed by alendronate vs. alendronate monotherapy in women at increased risk of fracture: a US payer perspective. Semin Arthritis Rheum. 2020;50(3):394–400.
Li N, Beaudart C, Cauley JA, Ing SW, Lane NE, Reginster JY, et al. Cost effectiveness analyses of interventions for osteoporosis in men: a systematic literature review. Pharmacoeconomics. 2023. https://doi.org/10.1007/s40273-022-01239-2.
Hiligsmann M, Reginster JY, Tosteson ANA, Bukata SV, Saag KG, Gold DT, et al. Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos Int. 2019;30(1):45–57.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Pharmacoeconomics. 2022;40(6):601–9.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Academy of Managed Care Pharmacy. AMCP format for formulary submissions: guidance on submission of pre-approval and post-approval clinical and economic information and evidence, Version 4.1. 2020. https://www.amcp.org/Resource-Center/format-formulary-submissions/AMCP-Format-for-Formulary-Submissions-4.1. Accessed 14 Apr 2023.
Hiligsmann M, Ethgen O, Bruyere O, Richy F, Gathon HJ, Reginster JY. Development and validation of a Markov microsimulation model for the economic evaluation of treatments in osteoporosis. Value Health. 2009;12(5):687–96.
Hiligsmann M, Reginster JY, Silverman S. The value of a patient-level modeling approach and need for better reporting in economic evaluations of osteoporosis. J Manag Care Spec Pharm. 2020;26(3):334–5.
Soreskog E, Strom O, Spangeus A, Akesson KE, Borgstrom F, Banefelt J, et al. Risk of major osteoporotic fracture after first, second and third fracture in Swedish women aged 50 years and older. Bone. 2020;134: 115286.
Tran O, Silverman S, Xu X, Bonafede M, Fox K, McDermott M, et al. Long-term direct and indirect economic burden associated with osteoporotic fracture in US postmenopausal women. Osteoporos Int. 2021;32(6):1195–205.
Ettinger B, Black DM, Dawson-Hughes B, Pressman AR, Melton LJ 3rd. Updated fracture incidence rates for the US version of FRAX. Osteoporos Int. 2010;21(1):25–33.
Luo C, Qin SX, Wang QY, Li YF, Qu XL, Yue C, et al. Cost-effectiveness analysis of five drugs for treating postmenopausal women in the United States with osteoporosis and a very high fracture risk. J Endocrinol Investig. 2023;46(2):367–79.
Nayak S, Greenspan SL. Cost-effectiveness of 3 versus 6 years of zoledronic acid treatment before bisphosphonate holiday for women with osteoporosis. Osteoporos Int. 2022;33(1):229–38.
Lewiecki EM, Chastek B, Sundquist K, Williams SA, Weiss RJ, Wang Y, et al. Osteoporotic fracture trends in a population of US managed care enrollees from 2007 to 2017. Osteoporos Int. 2020;31(7):1299–304.
Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone. 2000;27(5):585–90.
Watts NB, Leslie WD, Foldes AJ, Miller PD. International Society for Clinical Densitometry Position Development Conference: task force on normative databases. J Clin Densitom. 2013;16(4):472–81.
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89.
Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297(4):387–94.
Hiligsmann M, Reginster JY. Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics. 2011;29(10):895–911.
Tran T, Bliuc D, van Geel T, Adachi JD, Berger C, van den Bergh J, et al. Population-wide impact of non-hip non-vertebral fractures on mortality. J Bone Miner Res. 2017;32(9):1802–10.
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int. 2004;15(2):108–12.
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003;32(5):468–73.
Williams SA, Chastek B, Sundquist K, Barrera-Sierra S, Leader D Jr, Weiss RJ, et al. Economic burden of osteoporotic fractures in US managed care enrollees. Am J Manag Care. 2020;26(5):e142–9.
Weaver J, Sajjan S, Lewiecki EM, Harris ST, Marvos P. Prevalence and cost of subsequent fractures among US patients with an incident fracture. J Manag Care Spec Pharm. 2017;23(4):461–71.
Talevski J, Sanders KM, Lal A, Watts JJ, Beauchamp A, Duque G, et al. A micro-costing analysis of post-fracture care pathways: results from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS). Osteoporos Int. 2022;33(9):1895–907.
Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Mak. 2006;26(4):391–400.
Gold T, Williams SA, Weiss RJ, Wang Y, Watkins C, Carroll J, et al. Impact of fractures on quality of life in patients with osteoporosis: a US cross-sectional survey. J Drug Assess. 2019;8(1):175–83.
Svedbom A, Borgstom F, Hernlund E, Strom O, Alekna V, Bianchi ML, et al. Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures: results from the ICUROS. Osteoporos Int. 2018;29(3):557–66.
Kanis JA, Johansson H, Oden A, Harvey NC, Gudnason V, Sanders KM, et al. Characteristics of recurrent fractures. Osteoporos Int. 2018;29(8):1747–57.
Talevski J, Sanders KM, Watts JJ, Nicholson GC, Seeman E, Iuliano S, et al. Sex differences in recovery of quality of life 12 months post-fracture in community-dwelling older adults: analyses of the Australian arm of the International Costs and Utilities Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos Int. 2022;33(1):67–75.
Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–37.
Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis: 2016: executive summary. Endocr Pract. 2016;22(9):1111–8.
Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722–33.
Bone HG, Cosman F, Miller PD, Williams GC, Hattersley G, Hu MY, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(8):2949–57.
NICE. Bisphosphonates for treating osteoporosis. Technology appraisal guidance [TA464]. Last update: 8 July 2019. https://www.nice.org.uk/guidance/ta464. Accessed 14 Apr 2023.
Hiligsmann M, Evers SM, Ben Sedrine W, Kanis JA, Ramaekers B, Reginster JY, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205–24.
IBM. IBM Micromedex Red Book. https://www.ibm.com/products/micromedex-red-book. Accessed 1 May 2022.
Levy J, Rosenberg M, Vanness D. A Transparent and consistent approach to assess US outpatient drug costs for use in cost-effectiveness analyses. Value Health. 2018;21(6):677–84.
Liu H, Michaud K, Nayak S, Karpf DB, Owens DK, Garber AM. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med. 2006;166(11):1209–17.
Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy. 2013;11(5):485–97.
Cheng LI, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, et al. Persistence and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm. 2015;21(9):824–33.
Koller G, Goetz V, Vandermeer B, Homik J, McAlister FA, Kendler D, et al. Persistence and adherence to parenteral osteoporosis therapies: a systematic review. Osteoporos Int. 2020;31(11):2093–102.
Singer AJ, Liu J, Yan H, Stad RK, Gandra SR, Yehoshua A. Treatment patterns and long-term persistence with osteoporosis therapies in women with Medicare fee-for-service (FFS) coverage. Osteoporos Int. 2021;32(12):2473–84.
Fatoye F, Smith P, Gebrye T, Yeowell G. Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open. 2019;9(4):e027049.
Dubois R. Cost-effectiveness thresholds in the USA: are they coming? Are they already here? J Comp Eff Res. 2016;5(1):9–11.
Leibson CL, Tosteson ANA, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50(10):1644–50.
Tajeu GS, Delzell E, Smith W, Arora T, Curtis JR, Saag KG, et al. Death, debility, and destitution following hip fracture. J Gerontol A Biol Sci Med Sci. 2014;69(3):346–53.
Reginster J, Bianic F, Campbell R, Martin M, Williams SA, Fitzpatrick LA. Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int. 2019;30(7):1465–73.
Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32(2):198–202.
Curtis EM, Reginster JY, Al-Daghri N, Biver E, Brandi ML, Cavalier E, et al. Management of patients at very high risk of osteoporotic fractures through sequential treatments. Aging Clin Exp Res. 2022;34(4):695–714.
Yu G, Tong S, Liu J, Wan Y, Wan M, Li S, et al. A systematic review of cost-effectiveness analyses of sequential treatment for osteoporosis. Osteoporos Int. 2022;34:641–58. https://doi.org/10.1007/s00198-022-06626-1.
Hiligsmann M, Boonen A, Rabenda V, Reginster JY. The importance of integrating medication adherence into pharmacoeconomic analyses: the example of osteoporosis. Expert Rev Pharm Out. 2012;12(2):159–66.
Cosman F, Cooper C, Wang Y, Mitlak B, Varughese S, Williams SA. Comparative effectiveness and cardiovascular safety of abaloparatide and teriparatide in postmenopausal women new to anabolic therapy: a US administrative claims database study. Osteoporos Int. 2022;33(8):1703–14.
Gold DT, Weiss R, Beckett T, Deal C, Epstein RS, James AL, et al. Abaloparatide real-world patient experience study. JBMR Plus. 2021;5(3):e10457.
Kaufman JM, Reginster JY, Boonen S, Brandi ML, Cooper C, Dere W, et al. Treatment of osteoporosis in men. Bone. 2013;53(1):134–44.
Kaufman JM. Management of osteoporosis in older men. Aging Clin Exp Res. 2021;33(6):1439–52.
Yeam CT, Chia S, Tan HCC, Kwan YH, Fong W, Seng JJB. A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos Int. 2018;29(12):2623–37.
Soreskog E, Borgstrom F, Lindberg I, Strom O, Willems D, Libanati C, et al. A novel economic framework to assess the cost-effectiveness of bone-forming agents in the prevention of fractures in patients with osteoporosis. Osteoporos Int. 2021;32(7):1301–11.
Wright NC, Saag KG, Curtis JR, Smith WK, Kilgore ML, Morrisey MA, et al. Recent trends in hip fracture rates by race/ethnicity among older US adults. J Bone Miner Res. 2012;27(11):2325–32.
Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–90.
Acknowledgements
The authors are grateful to the Chair for Biomarkers of Chronic Disease, King Saud University, Riyadh, KSA for its support, and to Interface Science et Recherche ASBL for medical writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study is sponsored by Radius Health, Inc. The sponsor was involved in the design of the study, review of the protocol, and review and author approval of the manuscript. The sponsor had no role in the final selection of data and assumptions, execution and analysis of the model, and the interpretation of the results.
Conflict of interest
Mickaël Hiligsmann has received research grants through his institution from Amgen, Radius Health, and ViiV, consulting fees from UCB and lecture fees from Mylan Pharmaceuticals; Stuart S. Silverman has received grants from Amgen and Radius Health and consulting fees from Amgen and Radius Health. Andrea J. Singer has received research grants paid to her institution from Radius Health and UCB, consulting fees from Agnovos, Amgen, Radius Health, and UCB, and speaking fees from Amgen and Radius Health. Jake Mathew, Yamei Wang, Leny Pearman, and John Caminis are employees and shareholders in Radius Health. Jean-Yves Reginster has received consulting fees or paid advisory boards from IBSA-Genevrier, Mylan, Radius Health, Pierre Fabre, and Teva; lecture fees when speaking at the invitation of a sponsor: IBSA-Genevrier, Mylan, CNIEL, Dairy Research Council, Teva; and grant support from industry (all through institution) from IBSA-Genevrier, Mylan, CNIEL, and Radius Health.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data utilized in this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Authors’ contributions
Concept and design: MH and JYR; protocol review and approval: all authors; acquisition of data: MH and JYR; model analyses: MH and JYR; interpretation of data: MH, SS, AS, JYR; critical revision of the paper for important intellectual content: all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hiligsmann, M., Silverman, S.S., Singer, A.J. et al. Cost-Effectiveness of Sequential Abaloparatide/Alendronate in Men at High Risk of Fractures in the United States. PharmacoEconomics 41, 819–830 (2023). https://doi.org/10.1007/s40273-023-01270-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01270-x