Abstract
Summary
A novel cost-effectiveness model framework was developed to incorporate the elevated fracture risk associated with a recent fracture and to allow sequential osteoporosis therapies to be evaluated. Treating patients with severe osteoporosis after a recent fracture with a bone-forming agent followed by antiresorptive therapy can be cost-effective compared with antiresorptive therapy alone. Incorporating these novel technical attributes in economic evaluations can support appropriate policy and reimbursement decision-making.
Purpose
To develop a cost-effectiveness model accommodating increased fracture risk after a recent fracture and treatment sequencing.
Methods
A micro-simulation cost-utility model was developed to accommodate both treatment sequencing and increased risk with recent fracture. The risk of fracture was estimated and simulated using the FRAX® algorithms combined with Swedish registry data on imminent fracture relative risk. In the base-case cost-effectiveness analysis, a sequential treatment starting with a bone-forming agent for 12 months followed by an antiresorptive agent for 48 months initiated immediately after a major osteoporotic fracture (MOF) in a 70-year-old woman with a T-score of 2.5 or less was compared to an antiresorptive treatment alone for 60 months. The model was populated with data relevant for a UK population reflecting a personal social service perspective.
Results
The cost per additional quality-adjusted life year (QALY) gained in the base-case setting was estimated at £34,584. Sensitivity analyses revealed the sequential treatment to be cost-saving compared with administering a bone-forming treatment alone. Without simulating an elevated fracture risk immediately after a recent fracture, the cost per QALY changed from £34,584 to £62,184.
Conclusion
Incorporating imminent fracture risk in economic evaluations has a significant impact on the cost-effectiveness when evaluating fracture prevention treatments in patients with osteoporosis who sustained a recent fracture. Bone-forming treatment followed by antiresorptive therapy can be cost-effective compared to antiresorptive therapy alone depending on treatment acquisition costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis results in approximately 9 million fractures annually worldwide [1]. The total monetary burden of osteoporosis in 27 EU countries, including both fracture-associated costs and pharmacological interventions, was estimated at €37 billion in 2010 [2]. In addition, osteoporotic fractures account for around 2 million disability-adjusted life years lost annually in Europe [1].
A previous fracture is a major risk factor for future fractures [3,4,5,6]. The risk of suffering a subsequent fracture following a first fragility fracture changes over time and is highest within the first 1 to 2 years following the initial fragility fracture [3,4,5,6]. During this period, 12–34% of women experience a subsequent fracture, with vertebral fractures particularly increasing the re-fracture risk [3, 5]. This temporal elevated fracture risk that is associated with recency of a fracture is termed “imminent risk” [7].
The majority of available osteoporosis therapies decrease bone resorption (antiresorptive agents) [8]. However, an increase in bone mass can primarily be achieved with a few treatments called bone-forming agents [9]. These bone-forming treatments have the potential to reduce the risk of fracture faster and to a higher degree than antiresorptive agents [10,11,12,13,14].
The International Osteoporosis Foundation (IOF) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) recently recommended that increased fracture risk should be differentiated into “high risk” and “very high risk” [7]. High and very high risk is categorised by fracture risk and the thresholds depend on age. The rationale for the more refined characterisation of risk was to help direct appropriate bone-forming interventions to those designated at very high risk. Bone-forming treatments include teriparatide, romosozumab and, in some countries, abaloparatide. Intervention thresholds for the very high-risk group, i.e. at what 10-year major osteoporotic fracture (clinical vertebral, forearm, hip or humerus fracture, MOF) probability should a bone-forming treatment be initiated, were determined from a clinical perspective. However, it is also important to ensure that initiating treatment at the intervention thresholds can be considered cost-effective. To be able to assess the cost-effectiveness of bone-forming agents at the intervention threshold, it is necessary to have a model that can accommodate the specific characteristics of bone-forming treatments and any additional risk associated with a recent fracture. Bone-forming agents, such as romosozumab, are seen as appropriately administrated in sequence with another osteoporosis drug. For example, 1 year with a bone-forming agent followed by a switch to an antiresorptive treatment from the second year onwards to maintain the improvement in bone mineral density (BMD) [11, 15].
The objective of this paper is to present a novel cost-effectiveness model framework that incorporates both the risk associated with a recent fracture and treatment sequencing. This paper describes the structure of the economic model. Furthermore, it reports results on the potential impact on the cost-effectiveness of the example where a bone-forming agent followed by an antiresorptive agent is compared against an antiresorptive agent alone.
Methods and materials
Model structure and simulation technique
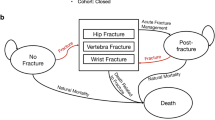
The health states in the model and the possible transitions between these states are shown in Fig. 1. All patients begin in the “at risk” health state, where the simulated patient is a 70-year-old woman with a T-score of − 2.5 and a recent MOF. At the end of each cycle, a patient has a probability of incurring a fracture (any), remaining in the same health state without a new fracture, or dying. If a patient dies, she moves to the “death” state. The model is run using a micro-simulation technique in which patients are simulated individually in the model. This technique is chosen since changes in fracture risk, mortality and patient’s disease progression related to (re-)occurrence of fractures are highly individualised and depends on time and historical fracture events and therefore need to be tracked individually during the course of the simulation to allow for an accurate depiction of individual fracture risk, multiple fractures and treatment patterns (e.g. sequencing and treatment persistence).
Markov micro-simulation model structure. Footnotes: All patients begin in the “at risk of fracture” state, and, at the end of each cycle, a patient has a probability of incurring a fracture (any), remaining in a health state without a new fracture, or dying. “Death” is an absorbing state from any of the other states (“at risk of fracture”, “vertebral fracture”, “hip fracture” and “non-hip, non-vertebral fracture”). If a patient dies, she moves to the “death” state and remains there for the rest of the simulation
Modelling fracture risk
The risk of fracture for a specific target patient population in the model depends on three elements:
-
I.
The risk for an individual in the general population of incurring a fracture;
-
II.
The increased fracture risk associated with osteoporosis (the relative risk) compared to the general population and
-
III.
A risk reduction, if any, attributed to treatment.
General population fracture risk
The general population risk of fractures required for the model is differentiated by age, sex and fracture type and is derived from published sources. Fracture incidence used in this analysis reflected a UK population and is described in Supplementary Table 1.
Increased risk of fracture for target patient population
The increased fracture risk for the target patient population is estimated using the FRAX® algorithm [16]. In addition, with lacking UK data, algorithms derived from a Swedish retrospective real-world study were used to estimate imminent fracture risk [17].
FRAX®
FRAX® is a fracture risk assessment tool that estimates a patient’s fracture risk and can be used to inform intervention decisions for patients at increased risk of fracture [16, 18]. Its use is currently recommended in more than 80 osteoporosis treatment guidelines worldwide [19]. The FRAX® algorithms estimate the 10-year probability of hip and MOF. The fracture risk is based on a number of clinical risk factors: age, gender, BMD, prior fractures, parental hip fracture history, body mass index, ethnicity, smoking, alcohol use, glucocorticoid use, rheumatoid arthritis and secondary osteoporosis [16, 18]. In addition to the 10-year probabilities, FRAX® can also produce the relative risk (RR) of hip fracture, MOF as well as RR of pre-fracture mortality compared to gender- and age-matched controls. The RRs derived can, thus, be used to adjust the population fracture risk for any combination of the clinical risk factors (CRFs) included in FRAX® in the model. The implementation of FRAX® in health economic modelling is described in more detail in Ström et al. [20]. Also, the National Institute for Health and Care Excellence (NICE) has used FRAX® for fracture risk estimation in their recent health technology assessments (HTAs) of osteoporosis treatments [21].
However, a novel FRAX®-related model feature is a functionality that allows for updating the FRAX® relative fracture risk and mortality at pre-specified intervals during the simulation (every year, every 5th or 10th year) related to increasing age and decreasing BMD. In previous cost-effectiveness models, the RR calculated at baseline has been kept constant during the whole simulation [20, 22, 23]. Keeping the RR of fracture constant over time overestimates the fracture risk because with increasing age (all else equal) the RR of fracture decreases. Thus, with this modification, the fracture risk trajectory over the lifetime for a patient is more realistically simulated.
Other CRFs are kept constant in the model due to a lack of data indicating that the prevalence of those factors changes with age.
Imminent fracture risk
While it is well established that a fragility fracture increases the risk of a subsequent fracture over a patient’s lifetime, recent studies have shown that the increase in RR is not constant over time and varies by age and number of fractures [5, 17]. A limitation of the FRAX® algorithm is that it does not capture this time-dependent elevated fracture risk after a recent fracture.
In the model, the relative imminent fracture risk is updated each time a fracture is experienced. Fracture risk at any time point during the model simulation is estimated as a function of the general population risk, the RR estimated by FRAX® for a given patient profile excluding the prior fracture CRF and the maximum of the time-dependent RR of an imminent fracture and the RR of fracture as estimated by FRAX® including the prior fracture CRF.
Data on the imminent fracture risk for the model was derived from a Swedish real-world data study [17]. In this study, women with fractures were matched to controls without a prior fracture, based on gender and birth year. Survival regression analysis was used to estimate the incidence functions of hip, vertebral and MOF (after 1st, 2nd and 3rd fracture), with the first subsequent incident fragility fracture as the failure event. Relative risks for each time period (0–6, 7–12, 13–18, 19–24, 25–36, 37–48, 49–60 months), age group (50–64, 65–75, 75+) and fracture site (any, hip and vertebral) were estimated by interacting exposure status and age with a time period. Separate survival regressions for different types of recent fractures of recent fracture (hip, vertebral and any) were estimated.
Figure 2 provides an example of how the fracture risk trajectory is estimated at different time points in a patient without a fracture at baseline.
-
T0: At this point, the patient has no fracture history. The simulated fracture risk corresponds to the normal population’s risk adjusted for the patient profile’s CRFs according to FRAX®.
-
T1: The patient suffers her first fracture. The simulated risk corresponds to the normal population’s risk adjusted for the patient profile’s CRFs according to FRAX®, and the maximum of time-dependent recent (first) fracture RR and the RR of having fracture history according to FRAX®.
-
T2: The patient suffers her second fracture. The simulated risk corresponds to the normal populations’ risk adjusted for the patient profile’s CRFs according to FRAX® and the maximum of time-dependent recent (second) fracture RR and the RR of having fracture history according to FRAX®.
Modelling the intervention
In its simplest form, a relative fracture risk reduction due to treatment is applied to the target patient population fracture risk during the treatment period. This period of risk reduction is usually followed by a period where the treatment effect is declining (the residual effect after treatment discontinuation). Treatment persistence is important to consider in cost-effectiveness models in osteoporosis [24, 25]. These features (residual effect and treatment persistence) have been included in published osteoporosis cost-effectiveness models and are included in this economic model as well. In addition, a few novel features (i.e. treatment sequencing and imminent risk) are incorporated into this novel economic model, which were required to more appropriately reflect characteristics of osteoporosis disease and to capture the impact of bone-forming agents.
Treatment sequencing
The model accommodates functionality to specify the treatment sequence and the timing of how or when the patient switches treatment. The implemented switch “triggers” are either a fracture (any fracture site) or a specific time point (months since treatment start). Only patients who are persistent with the first treatment will be switched to the next treatment in the sequence. This assumption is made due to the difficulty to distinguish the reason for non-persistence in available data. After a treatment switch, patients have the probability of non-persistence corresponding to the time since the start of the entire treatment regimen. No additional treatments were initiated when a fracture occurred.
Patient population
The model allows for a simulation of patient profiles based on the FRAX® CRFs in combination with the recency of prior fracture, as described above. A recent fracture may be narrowed down to a specific fracture site, including hip, vertebral or MOF. Overall, 13 risk factors need to be defined to run the model simulation; the number of potential patient profiles is almost inexhaustive. However, when performing cost-effectiveness analyses of osteoporosis treatments, it is more relevant to assess broader patient groups that are in line with treatment guidelines or the indication of the drugs. For example, 70-year-old women with a recent vertebral fracture, a T-score of − 2.5 and all other CRFs set at the average for this patient group.
To assess the cost-effectiveness for such broader patient groups, the model runs simulations on a larger set of patient profiles that on aggregate are representative of the target patient population. Such data sets of representative patient profiles for a specific target patient population were drawn from a prevalence and correlation matrix of CRFs from the FRAX® cohort. The default number of patient profiles drawn from the FRAX® matrix was after calibration set to 8000, which was deemed sufficiently large to represent the most possible combinations of risk factors. The distribution of risk factors is described in Supplementary Table 2.
Time horizon and cycle length
Osteoporosis is a chronic disease with long-term consequences after a fracture, so a lifetime time horizon was deemed appropriate. All patients are individually followed through the model from the patient’s age at treatment initiation to their time of death or age of 100 years, whichever comes first.
In most cost-effectiveness (CE) models of osteoporosis treatments, the cycle length has been 6 months or 1 year [22, 26]. For fast-acting bone-forming agents which are mainly intended to be given for shorter periods (i.e. 12–24 months), a 1-year model cycle would be too long as it would only allow for one transition during a 12-month treatment course and miss potentially meaningful achievements in the first 6 months of treatment in which patients are at high risk of subsequent fracture following a recent fracture [5]. In the economic model, the cycle length is flexible and may be changed at a specific time point during the simulation. As a default, a 6-month cycle length during the entirety of the time horizon was used as it was deemed sufficiently short to capture an imminent increase in fracture risk and any meaningful short-term treatment effect.
Data inputs
The model was populated with economic and epidemiological data relevant to a female UK population.
The age- and gender-specific all-cause mortality rates for the general population in the UK were based on the years 2012–2014 [27]. The model calculates the absolute risk of death by applying the normal UK population mortality to the excess mortality 1 year and subsequent years, respectively, after hip, vertebral and other fractures, down adjusted for comorbidities [28, 29]. Increased mortality after a fracture was assumed to persist for 8 years [22].
Available data indicates that bone-forming agents have a better persistence profile compared with antiresorptives [30, 31]. Approximately 50% of patients discontinue treatment with alendronate, administered orally daily or weekly, after 1 year [30, 32]. Persistence with a bone-forming agent was assumed to be 80% during the first year of treatment. Following bone-forming treatment, patients switching to antiresorptive were assumed to have a persistence (percentage of patients on treatment) corresponding to the persistence of patients on alendronate after 1 year of treatment [33].
The impact on the quality of life during the first and subsequent years after hip, vertebral and NHNV fractures were based on EuroQol-5 dimension (EQ-5D) data from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) [34]. This data source was chosen because ICUROS is to date the largest prospective study collecting quality of life data designed to be appropriate for health economic analysis (Supplementary Table 3 and Supplementary Table 4).
Costs of hip, vertebral and NHNV fractures were taken from a study of postmenopausal women in the UK [35, 36] (Supplementary Table 5). The cost of NHNV fractures was calculated by weighting the cost of wrist fractures and the cost of other NHNV fractures based on the population incidence of these fractures [37]. Hip and vertebral fractures are assumed to incur costs in subsequent years (£115 and £357, respectively) [21].
Because no specific treatment strategy was evaluated, an annual treatment acquisition cost of £5000 was assumed for the bone-forming agent and £20 for the antiresorptive agent, in line with treatment acquisition costs of existing reimbursed antiresorptive treatments. In sensitivity analyses, the annual price of the bone-forming agent was varied to £3000 and £7000. In addition, a physician visit every second year and a dual-energy X-ray absorptiometry (DXA) scan every fourth year were assumed and included in the treatment-related costs.
Costs and effects were discounted within the analysis at 3.5% per annum in accordance with NICE guidelines [38]. All costs are presented in 2019 prices.
Model outputs
The primary outcome in the economic model is the cost-effectiveness of a defined treatment versus an alternative treatment strategy reported both as the cost per quality-adjusted life year gained and cost per life year gained. Other outcomes include estimates of life years (LYs), number of fractures avoided and number needed to treat (NNT) to avoid one hip or vertebral fracture.
Analyses
The main purpose of the analysis was to show the potential impact of the novel features of the model framework and not conduct a cost-effectiveness analysis for a specific treatment strategy. Therefore, the intervention treatment strategy was a sequential treatment starting with a bone-forming agent for 12 months initiated immediately after a MOF followed by an antiresorptive agent for 48 months. As a starting point to evaluate the impact of the novel model features on cost-effectiveness results, the intervention treatment strategy was compared to an antiresorptive treatment for 60 months (“base case”).
To explore the impact of treatment sequencing on cost-effectiveness results, the intervention treatment strategy was compared with the two following alternatives: non-sequential bone-forming treatment for 18 and 24 months. To explore the impact of imminent fracture risk on cost-effectiveness results, the intervention treatment strategy was compared with the three following alternatives: sequential treatment as the main strategy but treatment initiated 6, 12 and 24 months after the fracture. As an additional scenario, the impact of deactivating the imminent fracture risk algorithm, thus neglecting the demonstrated imminent fracture risk after a recent fracture, was explored.
The analyses were run based on a set of common assumptions. The patient population tested was assumed to be women starting treatment at an age of 70 years. Compared with no treatment, bone-forming treatment was assumed to reduce the relative risk of fractures by 50% for hip fractures, 60% for vertebral fractures and 40% for other fractures. This treatment efficacy (relative risk reduction from bone-forming treatment) was assumed to be maintained during the entirety of the treatment duration (12 months + 48 months). The relative risk reduction for antiresorptive treatment in isolation was assumed to be 30%, 50% and 20% for hip, vertebral and other fractures, respectively. The relative risk reductions were chosen to be similar to a recent network meta-analysis of bone-forming and antiresorptive treatments for osteoporosis [39]. After treatment discontinuation, the treatment effect was assumed to linearly decline over a period equal to time on treatment (“offset time”). For the treatment sequence (bone-forming agent to antiresorptive), the offset time for both drugs was modelled jointly, referring to the discontinuation of the sequence.
Additional sensitivity analyses were run to explore the impact of imminent risk and treatment sequencing based on the new model features: recent fracture was assumed to be hip or vertebral fracture alone and the starting age of treatment varied between 60 and 80 years. The results from the sensitivity analyses are presented comparing the bone-forming agent for 12 months followed by an antiresorptive agent for 48 months to antiresorptive treatment for 60 months.
Results
Base case
Base case results for the treatment sequence of 12-month bone-forming treatment followed by 48 months of antiresorptive treatment compared with 60-months antiresorptive treatment are presented in Table 1. The bone-forming agent treatment sequence was associated with higher treatment costs and lower fracture-related costs. The total incremental cost was £2978, with an increase in QALYs of 0.086 yielding an incremental cost-effectiveness ratio (ICER), expressing the additional cost per additional QALY gained, of £34,584.
Impact of treatment sequencing
Modelling the intervention treatment strategy as sequence (12-month bone-forming followed by 48-month antiresorptive) had a significant impact on the cost-effectiveness results when compared to a non-sequential bone-forming agent for 18 and 24 months, respectively (Table 2). Compared with bone-forming agent only for 18 months, sequential treatment was associated with incremental QALYs of 0.05 at a lower cost. The comparison with 24-months bone-forming agent showed lower incremental QALYs (0.031) compared with 18-months bone-forming, but to a lower incremental cost.
Impact of imminent fracture risk
Initiation of treatment immediately after fracture, when the fracture risk is highest, was associated with more QALYs and lower costs compared with initiating treatment 6, 12 or 24 months after the initial fracture (Table 2). Deactivating the imminent fracture risk algorithm, i.e. assuming that fracture risk corresponds to any historical fracture and is non-time dependent, was associated with lower incremental QALYs and higher incremental costs compared with the base-case scenario.
Sensitivity analyses
Lower age at treatment initiation was associated with worse cost-effectiveness compared with the base case age of 70 years, in a population with T-score − 2.5 or less and MOF (Fig. 3). Higher age was however associated with better cost-effectiveness. Decreasing the price of the bone-forming agent led to an improved cost-effectiveness compared with the base case, due to lower total cost but did not change the QALYs gained (Table 3). A higher price of bone-forming treatment consequently led to a decreased cost-effectiveness.
Simulating the patients to only initiate treatment after a recent hip fracture was associated with decreased cost-effectiveness as opposed to the base case where patients were simulated to initiate treatment after incurring any MOF.
Discussion
The overall objective of this study was to present a cost-effectiveness model framework that incorporates both recency of fracture and treatment sequencing. These two novel components have so far not been captured in existing osteoporosis modelling approaches. These features have been shown to be important to consider in osteoporosis management and are therefore expected to enable to more accurately capture the progression of osteoporosis patients in any economic evaluation. This paper assesses the impact on economic evaluations when evolving cost-effectiveness modelling in osteoporosis to incorporate these novel features with the example of estimating the cost-effectiveness of bone-forming agents against antiresorptive treatments. Bone-forming agents may be of particular benefit for patients with a recent fracture, where rapid BMD improvement is needed to interrupt a potential fracture cascade, and where sequential antiresorptive treatments can maintain the improved BMD over time. This important clinical evolution was demonstrated in this research to also impact the economic value assessment of osteoporosis treatments as the incremental cost-effectiveness ratio almost doubled (from £34,584 to £62,184) when the novel imminent fracture risk was deactivated. Additionally, under the assumption that the relative risk reduction of the bone-forming agent can be maintained during the sequential treatment with an antiresorptive, the cost-effectiveness results show that a treatment sequence is the dominating strategy compared with a bone-forming agent as a standalone treatment. The results further highlight the importance of starting treatment as early as possible after a fracture occurs. Immediate intervention compared to delayed treatment start was cost-saving in all explored scenarios.
The objective of this study was to present a model framework for estimating the cost-effectiveness of bone-forming agents and not to determine the cost-effectiveness of a specific treatment. In order to evaluate a specific treatment or treatment sequence, the economic model would need to be modified to accommodate the specific characteristics of the intervention and its comparators (i.e. drug price and relative efficacy) in more detail. However, the results presented in this study provides a good indication that treatment with a bone-forming agent followed by an antiresorptive compared to antiresorptive only in patients at imminent risk of fracture could be considered cost-effective despite the substantial price difference. In a recent publication by Kanis et al. [7], the potential added value of a treatment sequence with a bone-forming agent followed by an antiresorptive was calculated as fractures saved. Over a 10-year time frame they estimated that the number of saved fractures increased from 5.7 at a starting age of 50 years to 126.6 at 90 years of age.
There are a considerable number of publications that have estimated the cost-effectiveness of various interventions for treatment and prevention of osteoporotic fractures [22, 40, 41]. Only a few publications have estimated the cost-effectiveness of treatments in a sequence [42, 43]. In Mori et al., the cost per QALY gained of sequential teriparatide/alendronate compared with alendronate alone in osteoporotic women with prior vertebral fracture was greater than $280,000 in a US setting [43]. Le et al. estimated the cost per QALY gained of sequential abaloparatide/alendronate compared with placebo/alendronate in osteoporotic women with a prior vertebral fracture to $188,891 in the USA [42]. Neither of these studies are directly comparable to this study as the study design differs in several aspects (e.g. patient groups, time horizon and comparators) and perhaps, most importantly, they do not consider the imminent risk of fracture. The modelling approach of this research is to the best of knowledge the first study presenting a cost-effectiveness model for osteoporosis treatments that include imminent fracture risk and allows for treatment sequencing at the same time.
Other cost-effectiveness studies on bone-forming agents are available [23, 41, 44] but it is not meaningful to compare these with the results in this study because they use different comparators, are based on other countries and do not consider imminent fracture risk or treatment sequencing.
The algorithms for time-dependent fracture risk in patients with recent fracture (i.e. imminent fracture risk) were derived from a Swedish retrospective real-world data study [3, 17]. Preferably, as much data as possible should be country specific in cost-effectiveness analyses. However, sufficiently detailed and comprehensive data required to estimate imminent fracture risk functions are scarce in most countries. Sweden is one of few countries that can provide population-based patient-level data of such granularity that is required to calculate algorithms for imminent fracture risk appropriate for economic modelling. When using the Swedish data on imminent fracture risk in other countries, this relies on the assumption that the relative risk of recent fracture versus no recent fracture is similar between countries. The validity of this assumption is supported by other studies [5]. Recently, granular data have become available from Iceland which have been used to provide adjustments to conventional estimates of fracture probability using FRAX [7]. In addition to the recency of fracture, probability adjustment was age dependent, decreasing with age in both men and women. Probability ratios also varied according to the site of sentinel fracture with higher ratios for hip and vertebral fracture than for humerus or forearm fracture. These observations may permit refinements in modelling with significant implications for cost-effectiveness.
The imminent fracture risk data from Söreskog et al. incorporated in the model were adjusted for a range of observable confounders (such as prior drug use impacting fracture risk, secondary osteoporosis and comorbidities) [17]. However, not all risk factors that are included in FRAX® were available in the study, such as BMD T-score. Therefore, the risk contribution of a recent fracture, when added on top of the risk estimated by FRAX®, may have been overestimated and should be considered a limitation of the cost-effectiveness model.
Mortality was estimated as a function of normal population mortality and excess mortality one and subsequent years after the fracture, respectively. Since excess mortality was estimated as the cumulative number of deaths during the first year after fracture, mortality risk immediately after fracture may have been underestimated.
The model has the capability of running probabilistic sensitivity analysis (PSA) as well. However, results were not presented because PSA is a standard model functionality and not a novel feature and the deterministic sensitivity analyses provided in this study serve the purpose of this research in a sufficient manner.
In this study, the cost-effectiveness was estimated for a bone-forming agent that might correspond to some overall perception of the characteristics of this class of compounds. However, in future reimbursement applications, the model should be tailored to assess the cost-effectiveness of a specific bone-forming drug in its intended indications in relevant countries. In addition, another future use of the model would be to calculate cost-effectiveness intervention thresholds (i.e. 10-year MOF probabilities at which the bone-forming agent becomes cost-effective) to support the clinical intervention thresholds as recently suggested for very high fracture risk patients by IOF and ESCEO [7].
Conclusion
Incorporating imminent fracture risk in osteoporosis cost-effectiveness modelling has a significant impact on the cost-effectiveness when evaluating fracture prevention treatments in a patient with a recent fracture. Bone-forming agents in a sequence with antiresorptive treatment can be cost-effective compared with antiresorptive therapy despite the difference in treatment acquisition costs.
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Banefelt J, Åkesson KE, Spångéus A, Ljunggren O, Karlsson L, Ström O, Ortsäter G, Libanati C, Toth E (2019) Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int 30(3):601–609
Bliuc D, Nguyen ND, Nguyen TV, Eisman JA, Center JR (2013) Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res 28(11):2317–2324
Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA (2017) Imminent risk of fracture after fracture. Osteoporos Int 28(3):775–780
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68(1):99–102
Kanis JA, Harvey NC, McCloskey E, Bruyere O, Veronese N, Lorentzon M, Cooper C, Rizzoli R, Adib G, Al-Daghri N, Campusano C, Chandran M, Dawson-Hughes B, Javaid K, Jiwa F, Johansson H, Lee JK, Liu E, Messina D, Mkinsi O, Pinto D, Prieto-Alhambra D, Saag K, Xia W, Zakraoui L, Reginster J (2020) Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int 31(1):1–12
Sozen T, Ozisik L, Basaran NC (2017) An overview and management of osteoporosis. Eur J Rheumatol 4(1):46–56
Reginster JY, Neuprez A, Beaudart C, Buckinx F, Slomian J, Disteche S, Bruyere O (2014) Bone forming agents for the management of osteoporosis. Panminerva Med 56(2):97–114
Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A, Lespessailles E, Minisola S, Body JJ, Geusens P, Moricke R, Lopez-Romero P (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391(10117):230–240
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377(15):1417–1427
Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, Almasri J, Farah W, Sarigianni M, Muthusamy K, Al Nofal A, Haydour Q, Wang Z, Murad MH (2019) Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab 104(5):1623–1630
Lou S, Lv H, Yin P, Li Z, Tang P, Wang Y (2019) Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 30(1):59–70
Diez-Perez A, Marin F, Eriksen EF, Kendler DL, Krege JH, Delgado-Rodriguez M (2019) Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone 120:1–8
Bone HG, Cosman F, Miller PD, Williams GC, Hattersley G, Hu M-y, Fitzpatrick LA, Mitlak B, Papapoulos S, Rizzoli R (2018) ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. The Journal of Clinical Endocrinology & Metabolism 103(8):2949–2957
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19(4):385–397
Soreskog E, Strom O, Spangeus A, Akesson KE, Borgstrom F, Banefelt J, Toth E, Libanati C, Charokopou M (2020) Risk of major osteoporotic fracture after first, second and third fracture in Swedish women aged 50 years and older. Bone 134:115286
Kanis JA (2008) Assessment of osteoporosis at the primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK
Kanis JA, Harvey NC, Johansson H, Liu E, Vandenput L, Lorentzon M, Leslie WD, McCloskey EV (2020) A decade of FRAX: how has it changed the management of osteoporosis? Aging Clin Exp Res 32(2):187–196
Strom O, Borgstrom F, Kleman M, McCloskey E, Oden A, Johansson H, Kanis JA (2010) FRAX and its applications in health economics--cost-effectiveness and intervention thresholds using bazedoxifene in a Swedish setting as an example. Bone 47(2):430–437
Davis S (2015) Bisphosphonates for preventing osteoporotic fragility fractures (including a partial update of NICE technology appraisal guidance 160 and 161). Assessment report. Table 32., National Institute for Health and Care Excellence (NICE), Editor
Jonsson B, Strom O, Eisman JA, Papaioannou A, Siris ES, Tosteson A, Kanis JA (2011) Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 22(3):967–982
Borgstrom F, Strom O, Marin F, Kutahov A, Ljunggren O (2010) Cost effectiveness of teriparatide and PTH(1-84) in the treatment of postmenopausal osteoporosis. J Med Econ 13(3):381–392
Strom O, Borgstrom F, Kanis JA, Jonsson B (2009) Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int 20(1):23–34
Hiligsmann M, Reginster JY, Tosteson ANA, Bukata SV, Saag KG, Gold DT, Halbout P, Jiwa F, Lewiecki EM, Pinto D, Adachi JD, Al-Daghri N, Bruyere O, Chandran M, Cooper C, Harvey NC, Einhorn TA, Kanis JA, Kendler DL, Messina OD, Rizzoli R, Si L, Silverman S (2019) Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos Int 30(1):45–57
Borgstrom F, Johnell O, Jonsson B, Zethraeus N, Sen SS (2004) Cost effectiveness of alendronate for the treatment of male osteoporosis in Sweden. Bone 34(6):1064–1071
Office for National Statistics National Life Tables, Great Britain. 2012-2014. Central rate of mortality, females
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32(5):468–473
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15(2):108–112
Landfeldt E, Strom O, Robbins S, Borgstrom F (2012) Adherence to treatment of primary osteoporosis and its association to fractures--the Swedish Adherence Register Analysis (SARA). Osteoporos Int 23(2):433–443
Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R (2017) Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 12(1):22
Jonsson E, Eriksson D, Åkesson K, Ljunggren Ö, Salomonsson S, Borgström F, Ström O (2015) Swedish osteoporosis care. Arch Osteoporos 10(1):24
Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, Jick S (2012) Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause 19(1):33–40
Svedbom A, Borgstom F, Hernlund E, Strom O, Alekna V, Bianchi ML, Clark P, Curiel MD, Dimai HP, Jurisson M, Kallikorm R, Lember M, Lesnyak O, McCloskey E, Sanders KM, Silverman S, Solodovnikov A, Tamulaitiene M, Thomas T, Toroptsova N, Uuskula A, Tosteson ANA, Jonsson B, Kanis JA (2018) Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures-results from the ICUROS. Osteoporos Int 29(3):557–566
Gutierrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, Shannon P, Gitlin M, Robbins S (2012) Clinical burden and incremental cost of fractures in postmenopausal women in the United Kingdom. Bone 51(3):324–331
Gutierrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, Shannon P, Robbins S, Gitlin M (2011) Study of the incremental cost and clinical burden of hip fractures in postmenopausal women in the United Kingdom. J Med Econ 14(1):99–107
Barrett JA, Baron JA, Beach ML (2003) Mortality and pulmonary embolism after fracture in the elderly. Osteoporos Int 14(11):889–894
National Institute for Health and Care Excellence (NICE) (2013), Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 24 Oct 2019
Simpson EL, Martyn-St James M, Hamilton J, Wong R, Gittoes N, Selby P, Davis S (2020) Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: a systematic review and network meta-analysis. Bone 130:115081
Borgstrom F, Jonsson B, Strom O, Kanis JA (2006) An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting: based on the results of the SOTI and TROPOS trials. Osteoporos Int 17(12):1781–1793
Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY (2020) Cost-effectiveness of sequential treatment with abaloparatide followed by alendronate vs. alendronate monotherapy in women at increased risk of fracture: a US payer perspective. Semin Arthritis Rheum 50:394–400
Le QA, Hay JW, Becker R, Wang Y (2019) Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann Pharmacother 53(2):134–143
Mori T, Crandall CJ, Ganz DA (2019) Cost-effectiveness of sequential teriparatide/alendronate versus alendronate-alone strategies in high-risk osteoporotic women in the US: analyzing the impact of generic/biosimilar teriparatide. JBMR Plus 3(11):e10233
Murphy DR, Smolen LJ, Klein TM, Klein RW (2012) The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord 13:213
Availability of data and material
Not applicable.
Funding
This study was funded by UCB Pharma and Amgen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.A.K. reports grants from Amgen, Eli Lilly and Radius Health and consulting fees from Theramex. J.A.K. is the architect of FRAX® but has no financial interest. E.S., I.L., F.B. and O.S. are employees of Quantify Research which was contracted and paid by UCB, a pharmaceutical company marketing products for osteoporosis, to conduct the study. The authors did not receive direct payment as a result of this work outside of their normal salary payments. D.W., C.S. and M.C. are employees of UCB Pharma. B.S. is an employee of Amgen (Europe).
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 25.7 kb).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Söreskog, E., Borgström, F., Lindberg, I. et al. A novel economic framework to assess the cost-effectiveness of bone-forming agents in the prevention of fractures in patients with osteoporosis. Osteoporos Int 32, 1301–1311 (2021). https://doi.org/10.1007/s00198-020-05765-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05765-7