Abstract
Background
Given the limited availability of healthcare resources and the recent introduction of new anti-osteoporosis drugs, the interest in the cost effectiveness of drugs in postmenopausal osteoporosis remains and even increases.
Objective
This study aims to identify all recent economic evaluations on drugs for postmenopausal osteoporosis, to critically appraise the reporting quality, and to summarize the results.
Methods
A literature search using Medline, the National Health Service Economic Evaluation database and the Cost-Effectiveness Analysis Registry was undertaken to identify original articles published between January 1, 2008 and December 31, 2013. Studies that assessed cost effectiveness of drugs in postmenopausal osteoporosis were included. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement was used to assess the quality of reporting of these articles.
Results
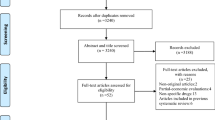
Of 1,794 articles identified, 39 studies fulfilled the inclusion criteria. They were conducted in 14 different countries and nine active interventions were assessed. When compared with no treatment, active osteoporotic drugs were generally cost effective in postmenopausal women aged over 60–65 years with low bone mass, especially those with prior vertebral fractures. Key drivers of cost effectiveness included individual fracture risk, medication adherence, selected comparators and country-specific analyses. Quality of reporting varied between studies with an average score of 17.9 out of 24 (range 7–21.5).
Conclusion
This review found a substantial number of published cost-effectiveness analyses of drugs in osteoporosis in the last 6 years. Results and critical appraisal of these articles can help decision makers when prioritizing health interventions and can inform the development of future economic evaluations.


Similar content being viewed by others
References
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1–2):136. doi:10.1007/s11657-013-0136-1.
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi:10.1359/jbmr.061113.
Hiligsmann M, Kanis JA, Compston J, Cooper C, Flamion B, Bergmann P, et al. Health technology assessment in osteoporosis. Calcif Tissue Int. 2013;93(1):1–14. doi:10.1007/s00223-013-9724-8.
Fleurence RL, Iglesias CP, Johnson JM. The cost effectiveness of bisphosphonates for the prevention and treatment of osteoporosis: a structured review of the literature. Pharmacoeconomics. 2007;25(11):913–33.
Zethraeus N, Borgstrom F, Strom O, Kanis JA, Jonsson B. Cost-effectiveness of the treatment and prevention of osteoporosis—a review of the literature and a reference model. Osteoporos Int. 2007;18(1):9–23. doi:10.1007/s00198-006-0257-0.
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi:10.1007/s00198-012-2074-y.
Body JJ, Bergmann P, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, et al. Evidence-based guidelines for the pharmacological treatment of postmenopausal osteoporosis: a consensus document by the Belgian Bone Club. Osteoporos Int. 2010;21(10):1657–80. doi:10.1007/s00198-010-1223-4.
Hiligsmann M, Boonen A, Dirksen CD, Ben Sedrine W, Reginster JY. Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporotic women. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):19–28. doi:10.1586/erp.12.76.
Hiligsmann M, Vanoverberghe M, Neuprez A, Bruyere O, Reginster JY. Cost-effectiveness of strontium ranelate for the prevention and treatment of osteoporosis. Expert Rev Pharmacoecon Outcomes Res. 2010;10(4):359–66. doi:10.1586/erp.10.53.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. doi:10.1016/j.jval.2013.02.010.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Pharmacoeconomics. 2013;31(5):361–7. doi:10.1007/s40273-013-0032-y.
Si L, Winzenberg TM, Palmer AJ. A systematic review of models used in cost-effectiveness analyses of preventing osteoporotic fractures. Osteoporos Int. 2014;25(1):51–60. doi:10.1007/s00198-013-2551-y.
Akehurst R, Brereton N, Ariely R, Lusa T, Groot M, Foss P, et al. The cost effectiveness of zoledronic acid 5 mg for the management of postmenopausal osteoporosis in women with prior fractures: evidence from Finland, Norway and the Netherlands. J Med Econ. 2011;14(1):53–64. doi:10.3111/13696998.2010.545563.
Borgstrom F, Strom O, Kleman M, McCloskey E, Johansson H, Oden A, et al. Cost-effectiveness of bazedoxifene incorporating the FRAX(R) algorithm in a European perspective. Osteoporos Int. 2011;22(3):955–65. doi:10.1007/s00198-010-1291-5.
Jansen JP, Gaugris S, Bergman G, Sen SS. Cost-effectiveness of a fixed dose combination of alendronate and cholecalciferol in the treatment and prevention of osteoporosis in the United Kingdom and The Netherlands. Curr Med Res Opin. 2008;24(3):671–84. doi:10.1185/030079908x260998.
Kim K, Svedbom A, Luo X, Sutradhar S, Kanis JA. Comparative cost-effectiveness of bazedoxifene and raloxifene in the treatment of postmenopausal osteoporosis in Europe, using the FRAX algorithm. Osteoporos Int. 2014;25(1):325–37. doi:10.1007/s00198-013-2521-4.
Lekander I, Borgstrom F, Strom O, Zethraeus N, Kanis JA. Cost effectiveness of hormone therapy in women at high risks of fracture in Sweden, the US and the UK—results based on the Women’s Health Initiative randomised controlled trial. Bone. 2008;42(2):294–306. doi:10.1016/j.bone.2007.09.059.
Fardellone P, Cortet B, Legrand E, Bresse X, Bisot-Locard S, Vigneron AM, et al. Cost-effectiveness model of using zoledronic acid once a year versus current treatment strategies in postmenopausal osteoporosis. Joint Bone Spine. 2010;77(1):53–7. doi:10.1016/j.jbspin.2009.04.009.
Hiligsmann M, Ethgen O, Bruyere O, Richy F, Gathon HJ, Reginster JY. Development and validation of a Markov microsimulation model for the economic evaluation of treatments in osteoporosis. Value Health. 2009;12(5):687–96. doi:10.1111/j.1524-4733.2008.00497.x.
Berto P, Maggi S, Noale M, Lopatriello S. Risedronate versus alendronate in older patients with osteoporosis at high risk of fracture: an Italian cost-effectiveness analysis. Aging Clin Exp Res. 2010;22(2):179–88. doi:10.3275/6816.
Darba J, Perez-Alvarez N, Kaskens L, Holgado-Perez S, Racketa J, Rejas J. Cost-effectiveness of bazedoxifene versus raloxifene in the treatment of postmenopausal women in Spain. Clinicoecon Outcomes Res. 2013;5:327–36. doi:10.2147/CEOR.S42755.
Ding H, Koinuma N, Stevenson M, Ito M, Monma Y. The cost-effectiveness of risedronate treatment in Japanese women with osteoporosis. J Bone Miner Metab. 2008;26(1):34–41. doi:10.1007/s00774-007-0794-4.
Thompson M, Pasquale M, Grima D, Moehrke W, Kruse HP. The impact of fewer hip fractures with risedronate versus alendronate in the first year of treatment: modeled German cost-effectiveness analysis. Value Health. 2010;13(1):46–54. doi:10.1111/j.1524-4733.2009.00666.x.
Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605–15.
Alzahouri K, Bahrami S, Durand-Zaleski I, Guillemin F, Roux C. Cost-effectiveness of osteoporosis treatments in postmenopausal women using FRAX thresholds for decision. Joint Bone Spine. 2013;80(1):64–9. doi:10.1016/j.jbspin.2012.01.001.
Kanis JA, Adams J, Borgstrom F, Cooper C, Jonsson B, Preedy D, et al. The cost-effectiveness of alendronate in the management of osteoporosis. Bone. 2008;42(1):4–15. doi:10.1016/j.bone.2007.10.019.
Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX–assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19(10):1395–408. doi:10.1007/s00198-008-0712-1.
Pham AN, Datta SK, Weber TJ, Walter LC, Colon-Emeric CS. Cost-effectiveness of oral bisphosphonates for osteoporosis at different ages and levels of life expectancy. J Am Geriatr Soc. 2011;59(9):1642–9. doi:10.1111/j.1532-5415.2011.03571.x.
Salpeter SR, Buckley NS, Liu H, Salpeter EE. The cost-effectiveness of hormone therapy in younger and older postmenopausal women. Am J Med. 2009;122(1):42–52.
Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–81.
Borgstrom F, Strom O, Coelho J, Johansson H, Oden A, McCloskey EV, et al. The cost-effectiveness of risedronate in the UK for the management of osteoporosis using the FRAX. Osteoporos Int. 2010;21(3):495–505. doi:10.1007/s00198-009-0989-8.
Chau D, Becker DL, Coombes ME, Ioannidis G, Adachi JD, Goeree R. Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporosis in Canada. J Med Econ. 2012;15(Suppl 1):3–14. doi:10.3111/13696998.2012.737393.
Hiligsmann M, Reginster JY. Potential cost-effectiveness of denosumab for the treatment of postmenopausal osteoporotic women. Bone. 2010;47(1):34–40. doi:10.1016/j.bone.2010.03.009.
Seeman E, Boonen S, Borgstrom F, Vellas B, Aquino JP, Semler J, et al. Five years treatment with strontium ranelate reduces vertebral and nonvertebral fractures and increases the number and quality of remaining life-years in women over 80 years of age. Bone. 2010;46(4):1038–42. doi:10.1016/j.bone.2009.12.006.
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi:10.1007/s00198-007-0543-5.
Borgstrom F, Strom O, Coelho J, Johansson H, Oden A, McCloskey E, et al. The cost-effectiveness of strontium ranelate in the UK for the management of osteoporosis. Osteoporos Int. 2010;21(2):339–49. doi:10.1007/s00198-009-0971-5.
Borgstrom F, Strom O, Marin F, Kutahov A, Ljunggren O. Cost effectiveness of teriparatide and PTH (1-84) in the treatment of postmenopausal osteoporosis. J Med Econ. 2010;13(3):381–92. doi:10.3111/13696998.2010.499072.
Ivergard M, Strom O, Borgstrom F, Burge RT, Tosteson AN, Kanis J. Identifying cost-effective treatment with raloxifene in postmenopausal women using risk algorithms for fractures and invasive breast cancer. Bone. 2010;47(5):966–74. doi:10.1016/j.bone.2010.07.024.
Strom O, Jonsson B, Kanis JA. Intervention thresholds for denosumab in the UK using a FRAX(R)-based cost-effectiveness analysis. Osteoporos Int. 2013;24(4):1491–502. doi:10.1007/s00198-012-2115-6.
Grima DT, Papaioannou A, Thompson MF, Pasquale MK, Adachi JD. Greater first year effectiveness drives favorable cost-effectiveness of brand risedronate versus generic or brand alendronate: modeled Canadian analysis. Osteoporos Int. 2008;19(5):687–97. doi:10.1007/s00198-007-0504-z.
Hiligsmann M, Rabenda V, Gathon HJ, Ethgen O, Reginster JY. Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int. 2010;86(3):202–10.
Hiligsmann M, Reginster JY. Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics. 2011;29(10):895–911. doi:10.2165/11539980-000000000-00000.
Jonsson B, Strom O, Eisman JA, Papaioannou A, Siris ES, Tosteson A, et al. Cost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22(3):967–82. doi:10.1007/s00198-010-1424-x.
Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy. 2013;11(5):485–97. doi:10.1007/s40258-013-0047-8.
Hiligsmann M, Bruyere O, Reginster JY. Cost-effectiveness of strontium ranelate versus risedronate in the treatment of postmenopausal osteoporotic women aged over 75 years. Bone. 2010;46(2):440–6. doi:10.1016/j.bone.2009.08.052.
Lippuner K, Johansson H, Borgstrom F, Kanis JA, Rizzoli R. Cost-effective intervention thresholds against osteoporotic fractures based on FRAX(R) in Switzerland. Osteoporos Int. 2012;23(11):2579–89. doi:10.1007/s00198-011-1869-6.
Strom O, Borgstrom F, Kleman M, McCloskey E, Oden A, Johansson H, et al. FRAX and its applications in health economics–cost-effectiveness and intervention thresholds using bazedoxifene in a Swedish setting as an example. Bone. 2010;47(2):430–7. doi:10.1016/j.bone.2010.05.020.
Wasserfallen JB, Krieg MA, Greiner RA, Lamy O. Cost effectiveness and cost utility of risedronate for osteoporosis treatment and fracture prevention in women: a Swiss perspective. J Med Econ. 2008;11(3):499–523. doi:10.3111/13696990802332770.
Hiligsmann M, Bruyere O, Reginster JY. Cost-utility of long-term strontium ranelate treatment for postmenopausal osteoporotic women. Osteoporos Int. 2010;21(1):157–65. doi:10.1007/s00198-009-0924-z.
Murphy DR, Smolen LJ, Klein TM, Klein RW. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord. 2012;13:213. doi:10.1186/1471-2474-13-213.
Hiligsmann M, Ben Sedrine W, Reginster JY. Cost-effectiveness of bazedoxifene compared with raloxifene in the treatment of postmenopausal osteoporotic women. J Bone Miner Res. 2013;28(4):807–15. doi:10.1002/jbmr.1819.
Hiligsmann M, Boonen A, Rabenda V, Reginster JY. The importance of integrating medication adherence into pharmacoeconomic analyses: the example of osteoporosis. Expert Rev Pharmacoecon Outcomes Res. 2012;12(2):159–66. doi:10.1586/erp.12.8.
Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R. Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int. 2011;22(10):2565–73. doi:10.1007/s00198-011-1668-0.
Rabenda V, Hiligsmann M, Reginster JY. Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother. 2009;10(14):2303–15. doi:10.1517/14656560903140533.
Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E. A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health. 2011;14(4):571–81. doi:10.1016/j.jval.2010.11.010.
Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239–56. doi:10.1007/s00198-012-1964-3.
Zethraeus N, Ben Sedrine W, Caulin F, Corcaud S, Gathon HJ, Haim M, et al. Models for assessing the cost-effectiveness of the treatment and prevention of osteoporosis. Osteoporos Int. 2002;13(11):841–57. doi:10.1007/s001980200117.
Fleurence RL, Iglesias CP, Torgerson DJ. Economic evaluations of interventions for the prevention and treatment of osteoporosis: a structured review of the literature. Osteoporos Int. 2006;17(1):29–40. doi:10.1007/s00198-005-1943-z.
Ades AE. ISPOR states its position on network meta-analysis. Value Health. 2011;14(4):414–6. doi:10.1016/j.jval.2011.05.001.
Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–37. doi:10.1016/j.jval.2011.01.011.
Stevenson MD, Selby PL. Modelling the cost effectiveness of interventions for osteoporosis: issues to consider. Pharmacoeconomics. 2014. doi:10.1007/s40273-014-0156-8.
Jefferson T, Demicheli V, Vale L. Quality of systematic reviews of economic evaluations in health care. Jama. 2002;287(21):2809–12.
Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics. 2006;24(4):355–71.
Bell CM, Urbach DR, Ray JG, Bayoumi A, Rosen AB, Greenberg D, et al. Bias in published cost effectiveness studies: systematic review. Bmj. 2006;332(7543):699–703. doi:10.1136/bmj.38737.607558.80.
Fleurence RL, Spackman DE, Hollenbeak C. Does the funding source influence the results in economic evaluations? A case study in bisphosphonates for the treatment of osteoporosis. Pharmacoeconomics. 2010;28(4):295–306. doi:10.2165/11530530-000000000-00000.
Hiligsmann M, Dellaert BG, Dirksen CD, van der Weijden T, Goemaere S, Reginster JY, et al. Patients’ preferences for osteoporosis drug treatment: a discrete-choice experiment. Arthritis Res Ther. 2014;16(1):R36. doi:10.1186/ar4465.
Lekander I, Borgstrom F, Strom O, Zethraeus N, Kanis JA. Cost-effectiveness of hormone therapy in the United States. J Womens Health (Larchmt). 2009;18(10):1669–77. doi:10.1089/jwh.2008.1246.
Moriwaki K, Komaba H, Noto S, Yanagisawa S, Takiguchi T, Inoue H, et al. Cost-effectiveness of alendronate for the treatment of osteopenic postmenopausal women in Japan. J Bone Miner Res. 2013;28(2):395–403. doi:10.1002/jbmr.1755.
Acknowledgments
No funding has been received for the conduct of this study and/or preparation of this manuscript.
Conflicts of interest
Mickael Hilisgmann has received research grant and/or consulting fees from Amgen, Pfizer, Novartis, Servier and SMB. John Kanis has received consulting fees, advisory board fees, lecture fees, and/or grant support from the majority of companies concerned with skeletal metabolism. Jean-Yves Reginster has received consulting fees, paid advisory boards, lecture fees, and/or grant support from Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed, NPS, Theramex, UCB, Merck Sharp and Dohme, Rottapharm, IBSA, Genevrier, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Novo- Nordisk, and Bristol Myers Squibb. Stuart Silverman has served as an advisor for Amgen, Lilly, Novartis and Pfizer/Wyeth; has served as a consultant for Amgen, Genentech, Lilly, Novartis and Pfizer/Wyeth; and has received research support from Lilly and Pfizer/Wyeth. Annelies Boonen has received educational grants to my department from Merck, Abbot, Amgen and Pfizer as well as speaker honoraria from UCB and Pfizer. Caroline Wyers, Bram Ramaekers, Wafa Ben Sedrine and Silvia Evers have no conflicts of interest relevant to the content of this study.
Authors’ contributions
MH: study rationale and design, literature search, literature selection, quality assessment of studies, interpretation and reflection, writing of the manuscript, guarantor of the study. SE: quality assessment of studies, interpretation and reflection, reviewing of the manuscript. WBS: literature search, literature selection, reviewing of the manuscript. JK: interpretation and reflection, reviewing of the manuscript. BR: quality assessment of studies, reviewing of the manuscript. JYR: interpretation and reflection, reviewing of the manuscript. SS: literature selection, quality assessment of studies, reviewing of the manuscript. CW: literature selection, quality assessment of studies, reviewing of the manuscript. AB: study rationale and design, quality assessment of studies, interpretation and reflection, reviewing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiligsmann, M., Evers, S.M., Ben Sedrine, W. et al. A Systematic Review of Cost-Effectiveness Analyses of Drugs for Postmenopausal Osteoporosis. PharmacoEconomics 33, 205–224 (2015). https://doi.org/10.1007/s40273-014-0231-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0231-1