Abstract
Background
Sacubitril/valsartan (S/V) improves outcomes in patients with heart failure with reduced ejection fraction (HFrEF). Data about the immediate, short-, and intermediate-term hemodynamic effects of S/V are limited.
Methods
In this prospective observational study, 37 outpatients with chronic HFrEF were treated with S/V according to current guideline recommendations. Next to clinical, laboratory and echocardiographic parameters, haemodynamic variables were assessed non-invasively by use of inert gas rebreathing and bioimpedance cardiography at baseline and at 2-week, 3-month and 6-month follow-up. The course of variables throughout the study and the relationship between variables were analysed using fractional polynomials.
Results
S/V treatment resulted in short- and intermediate-term improvements in NYHA functional class (2.3 ± 0.6 at baseline vs. 1.9 ± 0.5 at 6-month follow-up, p = 0.14), 6-min walk test (453 ± 110 vs. 528 ± 98 m, p = 0.02), ejection fraction (31 ± 9 vs. 36 ± 12%, p = 0.13), pulmonary artery pressure (39 ± 10 vs. 31 ± 10 mmHg, p = 0.02), and NT-proBNP values (1702 (782–2897 vs. 1004 (599–1627) ng/L, p = 0.03). In addition, S/V caused immediate decreases in systemic vascular resistance index (SVRI) and systolic blood pressure (SBP), which were associated with a simultaneous drop in stroke volume (SV) and cardiac index (CI). However, while SVRI and SBP remained at low levels during further treatment, SV and CI restored rapidly and increased to slightly higher levels thereafter.
Conclusion
The vasodilative effects of S/V result in immediate reductions in SVRI, SBP, SV and CI. However, S/V induces reverse cardiac remodelling, which is apparent shortly after treatment initiation and leads to improvements of clinical, functional, echocardiographic, laboratory and haemodynamic variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This prospective observational study provides insights into the short- and intermediate-term haemodynamic effects of sacubitril/valsartan (S/V) initiation in ambulatory patients with chronic stable heart failure. |
We found that S/V sacubitril/valsartan mediated vasodilation causes immediate reductions in systemic vascular resistance index, systolic blood pressure, stroke volume and cardiac index. |
S/V induces reverse cardiac remodelling, which is apparent shortly after treatment initiation and leads to improvements in clinical, functional, echocardiographic, laboratory and haemodynamic variables. |
1 Introduction
Since publication of the PARADIGM-HF trial in 2014, sacubitril/valsartan (S/V) has emerged as a new therapeutic strategy for patients with heart failure with reduced ejection fraction (HFrEF) [1]. In PARADIGM-HF, S/V reduced the composite of cardiovascular death or hospitalization for heart failure (HF) by 20% as compared to enalapril in a cohort of 8442 patients with symptomatic HFrEF [1]. A recent meta-analysis of six randomized controlled trials involving 14,959 patients with HFrEF confirmed the benefits of S/V on HF outcomes as compared with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) [2]. In addition, observational studies have reported favourable effects of S/V on HF symptoms, NT-proBNP values and echocardiographic parameters of cardiac function [3,4,5,6].
S/V is a molecule that combines the moieties of valsartan (an ARB) and sacubitril (a neprilysin inhibitor) in a single substance. Neprilysin is a neutral endopeptidase that degrades some vasoactive peptides, including natriuretic peptides, bradykinin and adrenomedullin. In HF, the natriuretic peptide system is activated, leading to vasodilation, natriuresis and a reduction in aldosterone release and sympathetic tone. Inhibition of neprilysin by LBQ657, the active metabolite of sacubitril, increases the levels of these peptides and thus leads to a prolonged duration of their favourable effects. Because neprilysin also breaks down angiotensin II, inhibiting neprilysin will result in an unfavourable accumulation of angiotensin II. For this reason, sacubitril is combined with valsartan, which blocks the angiotensin II type-1 receptor, inhibiting angiotensin II and the release of aldosterone. Thus, S/V improves the imbalance between the renin-angiotensin-aldosterone and natriuretic peptide systems [7].
While the mechanism of action of S/V is well characterized, its immediate effects on cardiac function, haemodynamics and biomarkers of HF as well as their potential correlations with each other require further investigation. In particular, data on the haemodynamic effects of S/V are scarce, since the gold standard methods for measuring haemodynamic variables, thermodilution and the direct Fick’s method, require invasive access. Inert gas rebreathing and bioimpedance cardiography have emerged as new approaches that allow non-invasive measurement of a wide range of haemodynamic variables including the cardiac index. Studies have shown high precision and acceptable agreement between haemodynamic variables measured by inert gas rebreathing or bioimpediance cardiography with those obtained by thermodilution [8, 9].
In the present research project, we explored the immediate, short-term and intermediate-term effects of S/V initiation on cardiac function, non-invasive measures of haemodynamics and biomarkers and their correlations with each other in outpatients with chronic HFrEF.
2 Methods
2.1 Study Design and Patient Selection
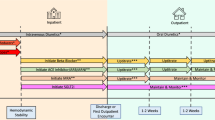
This was a monocentric, prospective, open-label, single-arm, observational study on therapy initiation with S/V within the limits of currently established, officially consented indication criteria. Patients attending the HF outpatients’ clinic of a tertiary University Hospital in Germany for evaluation of HF were offered inclusion in the present study if they fulfilled the following criteria: (a) age ≥ 18 years, (b) diagnosis of HFrEF, (c) indication for HF treatment with S/V according to guidelines and medical standards, (d) stable medication within at least 4 weeks prior to inclusion, (e) stable body weight (± 2.0 kg) within at least 4 weeks prior to inclusion, (e) physically and mentally capable of participating in the study, and (f) written informed consent for participation in the local HF register.
The diagnosis of HF was established according to published guidelines on the basis of typical symptoms and signs resulting from an objective abnormality of cardiac structure or function on echocardiography, cardiac magnetic resonance imaging, or left heart catheterisation [10]. All patients had a left ventricular ejection fraction (LVEF) < 45%. The decision to initiate S/V therapy was at the discretion of the referring physician in the HF outpatients’ clinic. Following guideline recommendations and drug approval, patients were deemed eligible for S/V therapy if they reported HF symptoms despite individually optimized treatment with an ACEI, beta-blocker and mineralocorticoid receptor antagonist [10]. All patients included in the present study have consented to participation in the local HF register. The HF register is an on-going, prospective longitudinal observational study. Since 1996, all patients attending the HF outpatients’ clinic for the evaluation of HF have been asked to provide written informed consent for their data to be recorded and used for research purposes. Less than 1% of patients refused to participate. The proceedings of the register conform with the guidelines of the Declaration of Helsinki and were approved by the local Ethics Committee, including its sub-studies on new diagnostic tools, biomarker sampling and share of scientific information.
Exclusion criteria were: (a) legal incapacity or imprisonment, (b) pregnancy, (c) an estimated glomerular filtration rate (eGFR) < 15 mL/min/m2, (d) severe hepatic failure, biliary cirrhosis or cholestasis (classified as Child-Pugh C), (e) known intolerance of S/V or other components of the drug, (f) history of angioedema, (g) concurrent treatment with aliskiren in patients with diabetes mellitus or impaired renal function (eGFR < 60 mL/min/1.73 m2), (h) serum potassium level > 5.4 mmol/L, and (i) systolic blood pressure (SBP) < 100 mmHg.
2.2 Study Procedures
At baseline (T0), patients were asked for written informed consent. Next to patient history and physical examination, evaluations included 6-min walk-test, electrocardiogram (ECG), echocardiography, blood chemistry, and non-invasive assessment of haemodynamic variables by use of inert gas rebreathing and bioimpedance cardiography.
Echocardiography was performed by an experienced cardiologist in our HF outpatients’ clinic. Echocardiographic parameters included LVEF, left ventricular internal end-diastolic diameter (LVEDD), left ventricular internal end-systolic diameter (LVESD), estimated systolic pulmonary artery pressure (sPAP), inspiratory collapse of the inferior vena cava (yes vs. no), mitral annular plane systolic excursion (MAPSE), and parameters of diastolic left ventricular function (E/E′ and E/A).
Haemodynamic measurements were performed after 10 min of rest using Innocor®, Innovision, Denmark for inert gas rebreathing and CardioScreen 2000, Medis GmbH, Germany for bioimpedance cardiography. Rebreathings were performed in a closed system, which consisted of a three-way respiratory valve connecting a facemask, an anti-static rubber bag, and an infrared photo-acoustic gas analyser. Patients rebreathed a gas mixture of nitrous oxide (N2O) (0.5%) and sulphur hexafluoride (0.1%) in oxygen, diluted with atmospheric air, from an anaesthesia bag of size 3–6 L, depending on the patient’s sex, height and age. The rebreathing manoeuvre was started after a normal expiration at a breathing rate of 20/min. A constant ventilation rate was ensured by having the patient breathe in synchrony with a graphical tachometer on the computer screen, and a constant ventilation volume was ensured by requesting the patient to empty the rebreathing bag completely with each breath. Rebreathing was typically performed over 5–8 breaths, of which the last 2–3 breaths were used for the calculation of pulmonary blood flow and cardiac index. Between the measurements an interval of 5 min was strictly adhered to in order to guarantee the complete elimination of N2O. The details of cardiac index calculations using Innocor® inert gas rebreathing have been described previously [11]. Bioimpedance cardiography was performed by placing four pairs of standard electrocardiographic electrodes on both sides of the neck and both sides of the inferior aspect of the thorax at the level of the xiphoid process with an inter-electrode gap of 5 cm. Verification of the correct signal quality was accomplished by visualization of the ECG, the impedance waveform and its first derivative. Detailed descriptions of bioimpedance cardiography parameters and their calculations have been published elsewhere [12].
S/V was initiated with a dose of 24 mg/26 mg bid or 49 mg/51 mg bid according to the discretion of the referring physician. Patients with previous ACEI therapy were instructed to await a 36-h wash-out period before starting S/V treatment.
Evaluations were repeated at subsequent study visits after 2-week, 3-month, and 6-month follow-up.
2.3 Statistics
All tests were two-tailed, and p < 0.05 was regarded as statistically significant. Variables are presented as mean ± standard deviation, median (interquartile range) or number (%) as appropriate. To compare frequencies, a Chi-squared test or Fisher’s exact test were performed as appropriate. To test the significant differences between two groups, the paired Student’s t test was used for normally distributed variables. For variables not following a normal Gaussian distribution, the Mann–Whitney U test was applied. Repeated-measures ANOVA and the Kruskal–Wallis test were used to compare continuous data between more than two groups.
The distribution of variables measured at each study visit is shown using violin plots. The course of variables throughout the study and the relationship between different variables were analysed using fractional polynomials. Fractional polynomials are a flexible approach to modelling nonlinear and asymmetric relationships since they allow the data to determine the best fitting functional form [13].
3 Results
3.1 Patient Characteristics and Follow-Up
We enrolled a total of 37 patients who met the inclusion criteria outlined above. Mean age at baseline was 61 ± 13 years and 29 patients (78%) were male. Eighteen patients (49%) had HF of ischaemic origin, and the median NT-proBNP concentration was 1702 (782–2897) pg/mL. All but two patients were in NYHA (New York Heart Association) functional class II or III, and functional capacity as measured by the 6-min walk-test distance was mildly reduced (453 ± 110 m). At baseline, all patients were treated with a beta-blocker and ACEI/ARB, and all but one received a mineralocorticoid receptor antagonist. In addition, the majority of patients (70%) were treated with a loop diuretic with a median dose of 40 (40–75) mg furosemide per day. In 32 patients (86%), S/V treatment was initiated with 24/26 mg twice daily (bid), whereas five patients (14%) started treatment with 49/51 mg bid. The complete baseline characteristics of included patients are shown in Table 1.
Subsequent study visits were performed after a median of 14 (14–21) days (T1), 106 (95–126) days (T2), and 196 (183–210) days (T3), respectively. The number of patients with available data from follow-up visits T1, T2 and T3 were 32, 22 and 17, respectively.
3.2 Heart Failure (HF) Symptoms and Functional Capacity
During the study, seven patients (22%) experienced an improvement of at least one NYHA class. The distribution of NYHA classes at each study visit is shown in Fig. 1. There was a statistically non-significant decrease in mean NYHA functional class from 2.3 ± 0.6 at baseline to 1.9 ± 0.5 at 6-month follow-up (p = 0.14 for T0 vs. T3, Fig. 2). In addition, functional capacity as measured by 6-min walk-test distance improved from 453 ± 110 m at baseline to 528 ± 98 m at 6-month follow-up (p = 0.02 for T0 vs. T3, Online Supplementary Material (OSM) eFig. 1). Benefits were apparent early after start of S/V treatment and continued to increase during the study. Clinical HF burden was more severe in patients receiving low-dose S/V treatment (OSM eFig. 2 and OSM eFig. 3). At 6-month follow-up, there was a trend to a decrease in loop diuretic dose from 40 (40–75)/55 ± 39 to 40 (20–40)/42 ± 22 mg furosemide dose equivalent (p = 0.07). Detailed results are presented in OSM eTable 1.
3.3 Echocardiography
During the study, we observed a trend to an improvement in LVEF (31 ± 9% at T0 vs. 36 ± 12% at T3, p = 0.13) and a significant reduction in sPAP (39 ± 10 mmHg at T0 vs. 31±10 mmHg at T3, p = 0.02, OSM eFigs. 4–7), with benefits being visible after 3 months of treatment. The relationship between S/V dosing and LVEF or sPAP, respectively, was non-linear (OSM eFigs. 8 and 9). The proportion of patients with an inspiratory collapse of the vena cava inferior increased from 86% at baseline to 100% at T3 (p = 0.13). However, other echocardiographic parameters including left-ventricular end-diastolic and end-systolic diameters did not change over the study (OSM eTable 2).
3.4 Blood Chemistry
While the majority of laboratory variables were stable over time, we observed a 42% decrease in NT-proBNP values after 14 days of S/V treatment. NT-proBNP values remained at lower levels thereafter. The distribution of NT-proBNP values at each study visit is shown in OSM eFig. 10, while Fig. 3 shows the course of NT-proBNP values throughout the study. As depicted in OSM eFig. 11, the decrease in NT-proBNP concentrations was greater in patients with high-dose S/V therapy. A decrease in NT-proBNP was associated with an almost linear increase in LVEF (OSM eFig. 12). Detailed results of blood chemistry analyses are presented in OSM eTable 3.
3.5 Haemodynamic Variables
Haemodynamic measurements obtained by inert gas rebreathing or bioimpedance cardiography at each study visit are presented in OSM eTable 4 and OSM eTable 5, respectively. S/V initiation caused a prompt and sustained decrease in systemic vascular resistance index (SVRI, Fig. 4), and an immediate drop in cardiac index and stroke volume (Fig. 5, OSM eFig. 13). However, cardiac index and stroke volume recovered rapidly and increased to slightly higher levels thereafter. High-dose S/V therapy was associated with a higher cardiac index and a higher stroke volume (OSM eFig. 14 and eFig. 15). In addition, a high cardiac index was associated with low NT-proBNP values and a high LVEF (Fig. 6, OSM eFig. 16). NT-proBNP reductions following S/V initiation were greater in patients with a low cardiac index (OSM eFig. 17).
3.6 Safety and Tolerability
During the study period, one patient (3%) died. S/V treatment was discontinued in four patients (11%) because of hyperkalaemia (n = 1) or rash (n = 3). A total of 16 patients (43%) withdrew consent for study participation, predominantly because of the COVID-19 pandemic. Median S/V dose at 6-month follow-up was 200 (200–400) mg, which corresponds to a median dose equivalent of 50 (50–100)%. A total of nine patients (24%) achieved the S/V target dose of 97/103 mg bid.
After initiation of S/V therapy, there was a significant decrease in SBP from 117 ± 16 mmHg to 111 ± 12 mmHg at 2-week follow-up (p = 0.02). SBP remained at low levels thereafter (Fig. 7). The relationship between SBP and SVRI was U-shaped (OSM eFig. 18). There were two cases of severe arterial hypotension (SBP < 90 mmHg), one in a patient taking 49/51 mg S/V bid and one in a patient treated with 24/26 mg bid. As further blood pressure measurements yielded SBP values > 90 mmHg, treatment was continued in both cases. Mean serum potassium levels were 4.5 ± 0.3 mmol/L at baseline and 4.4 ± 0.3 mmol/L at 6-month follow-up (p = 0.44). Renal function remained stable throughout the study (OSM eTable 3).
4 Discussion
The present study explored the immediate, short- and intermediate-term effects of S/V initiation on HF symptoms, cardiac function, biomarkers and haemodynamics in 37 outpatients with chronic HFrEF. We observed an early and lasting improvement in both HF symptoms and functional capacity after initiation of S/V. Our results are supported by a large number of observational studies in patients with HFrEF [3, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. In a recent review on real-world data of S/V therapy, 22 out of 25 studies reported improvements in NYHA functional class, although ten of these studies did not specify the level of statistical significance [3]. In addition, we noticed a 42% decrease in median NT-proBNP concentrations after 2 weeks of treatment. This reduction was greater than the 28% reduction reported in PARADIGM-HF after 8–10 weeks of treatment [29]. The PARALLEL-HF trial, a randomised, double-blind comparison of S/V with enalapril in Japanese patients with HFrEF, reported a 21%, 23% and 30% decrease in NT-proBNP after 2 weeks, 4 weeks and 6 months of treatment with S/V, respectively [30]. In real-world studies, the percentage decrease in NT-proBNP levels varied considerably from 4 to 70% across 20 studies, and a statistically significant decrease (p < 0.05) was observed in nine studies [3, 15, 19, 21, 23, 25, 28, 31,32,33]. In the present study, we observed an almost linear inverse relationship between changes in NT-proBNP and LVEF. This finding is supported by several mostly small observational studies [5, 34,35,36,37,38,39,40,41]. In PROVE-HF, a multicentre observational prospective study of 794 patients with HFrEF initiated on S/V, there was a 44% reduction in NT-proBNP values and a 9.4% increase in LVEF at 12-month follow-up [5]. Similar to our study, most of the NT-proBNP reduction occurred by the first follow-up visit 2 weeks after initiation. The study found a weak yet significant correlation between reductions in NT-proBNP levels and improvements in markers of cardiac volume and function at 12 months.
Contrary to prior reports [6, 34,35,36,37,38, 41], we did not observe a significant improvement in echocardiographic LV systolic or diastolic diameters or diastolic function during S/V treatment. However, reductions in previous studies were numerically small and it is possible that the sample size in the present study was too small to detect slight differences in LV diameters or measures of diastolic function. Notably, LV diameters remained stable despite a reduction in loop diuretic dose during the study. In addition, we observed a significant reduction of sPAP after start of S/V therapy. A similar finding was reported from other observational studies [6, 35, 37, 39, 40].
In the present study, non-invasive measurements of haemodynamics revealed that the decrease in SBP following S/V initiation was associated with a simultaneous decrease in cardiac index, stroke volume and SVRI. However, stroke volume and cardiac index recovered rapidly after start of treatment and increased to slightly higher levels thereafter, whereas SVRI and SBP remained at low levels. We can only speculate about the mechanisms behind this striking finding. We hypothesise that immediate changes in haemodynamic variables and SBP may be caused by direct vasodilatory effects of S/V, whereas intermediate increases in stroke volume and cardiac index may result from reverse cardiac remodelling induced by S/V. To the best of our knowledge, the present study is the first to explore the short-term and intermediate-term haemodynamic effects of S/V in outpatients with chronic HFrEF. A study of 25 patients with acutely decompensated HF reported stable invasive measurements of cardiac index immediately after start of S/V therapy [42]. However, study patients were critically ill, requiring intravenous vasoactive medication, and haemodynamic measurements were only available in the first days after start of treatment. Thus, results may not be transferable to patients with stable chronic HFrEF. A recent observational study of 60 outpatients with HFrEF reported a significant increase in cardiac index as determined by echocardiography after up to 2 years of S/V treatment [40]. However, as the first follow-up assessment of cardiac index was performed 6 months after therapy initiation, the study does not provide any data on short-term haemodynamic effects of S/V. It is here that our study expands current knowledge.
In the present study, we observed that benefits of S/V tended to be higher in patients with high-dose S/V treatment. Similarly, a dose-dependent effect on change in LVEF was noted in two observational studies, with higher S/V doses leading to more reverse remodelling [34, 43]. In PARADIGM-HF, S/V dose reduction was associated with a higher risk of major cardiovascular events [44]. However, the magnitude of benefit for patients on lower doses of S/V relative to those on lower doses of enalapril was similar to that of patients who remained on target doses of both drugs. Thus, although patients who do not tolerate a high S/V dose may be at higher cardiovascular risk, patients may still benefit from S/V therapy.
In our study, the median S/V dose at 6-month follow-up was considerably lower than that administered in PARADIGM-HF. Because a run-in period was designed in PARADIGM-HF, patients who did not tolerate target doses of HF drugs were excluded from the trial, and study patients would directly be assigned to target doses after randomisation. In contrast, in real-world practice, S/V is usually initiated at a low dose with subsequent up-titration to prevent adverse events. However, real-world studies show unanimously that most patients do not achieve the S/V target dose [3, 4, 45]. In clinical practice, the main adverse event and deterrent to up-titration is symptomatic hypotension, followed by rising serum potassium levels and worsening renal function [3, 46,47,48]. Hypotension was also one of the most frequent causes for dose reduction in PARADIGM-HF participants [44]. The TITRATION trial compared the tolerability and safety of S/V up-titration over 3 and 6 weeks in 498 patients with HFrEF [49]. In TITRATION, 76% of patients achieved and maintained the S/V target dose over 12 weeks irrespective of the titration regimen as compared to only 24% in the present study. While most baseline characteristics of patients included in TITRATION were similar to those in our cohort, mean baseline SBP in TITRATION was significantly higher than in our study (131 ± 16 vs. 117 ± 16 mmHg). Therefore, patients may have better tolerated the SBP-lowering effect of S/V, resulting in more successful up-titration.
Despite the significant decrease in SBP, S/V treatment was well tolerated, with four patients (11%) terminating therapy due to side effects. Treatment discontinuation was less frequent than in the PARADIGM-HF and TITRATION trials, where discontinuation rates were 18.8% and 13.9%, respectively [1, 49]. In real-world studies, S/V discontinuation rates varied largely from 2 to 35.7% [3,4,5]. It is likely that physicians in our HF outpatients’ clinic follow a conservative dose-escalation strategy that focuses on treatment tolerability instead of dose escalation.
5 Limitations
Our study has several limitations. First, the number of included patients is rather small, which may impede statistical analyses. Second, patient selection may be biased since we included patients from a sole university hospital outpatients’ clinic. However, baseline characteristics of study participants resemble those of patients enrolled in PARADIGM-HF, suggesting that study patients may be representative of ambulatory HFrEF cohorts. Third, the explanatory power of our study is limited by a high drop-out rate. Because of the COVID-19 pandemic, almost half of the study patients (n = 16, 43%) withdrew their consent for further study participation. Although our HF outpatients’ clinic has offered routine care to all HF patients since the start of the COVID-19 pandemic, the fear of infection made a significant number of HF patients cancel their regular check-ups. Similarly, a US nationwide representative survey reported that an estimated 40.9% of US adults have avoided medical care during the pandemic because of concerns about COVID-19 [50]. Fourth, our study lacks a control group. However, as HF treatment with S/V is recommended by international guidelines, a placebo-controlled trial may be considered unethical. Lastly, we cannot comment on the accuracy of bioimpedance cardiography and inert gas rebreathing as we did not perform invasive verification of haemodynamic measurements. However, both methods have previously shown acceptable agreement with haemodynamic values obtained by thermodilution [8, 9, 51] and high precision [9, 11, 51, 52] – a prerequisite for the interpretation of serial measurements of haemodynamic variables.
6 Conclusion
In outpatients with chronic HFrEF, initiation of S/V causes an immediate decrease in SBP, SVRI, stroke volume and cardiac index. While SBP and SVRI remain at low levels during further treatment, stroke volume and cardiac index recover rapidly and increase to slightly higher levels thereafter. S/V initiation results in early and lasting improvements in HF symptoms, functional capacity, echocardiographic parameters of cardiac function and natriuretic peptides.
References
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Zhang H, Huang T, Shen W, Xu X, Yang P, Zhu D, et al. Efficacy and safety of sacubitril–valsartan in heart failure: a meta-analysis of randomized controlled trials. ESC Heart Fail. 2020;7:3841–50.
Proudfoot C, Studer R, Rajput T, Jindal R, Agrawal R, Corda S, et al. Real-world effectiveness and safety of sacubitril/valsartan in heart failure: a systematic review. Int J Cardiol. 2021;15(331):164–71.
Giovinazzo S, Carmisciano L, Toma M, Benenati S, Tomasoni D, Sormani MP, et al. Sacubitril/valsartan in real-life European patients with heart failure and reduced ejection fraction: a systematic review and meta-analysis. ESC Heart Fail. 2021;8(5):3547–56.
Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril–svalsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322(11):1085–95.
Abumayyaleh M, Demmer J, Krack C, Pilsinger C, El-Battrawy I, Behnes M, et al. Hemodynamic effects of sacubitril/valsartan in patients with reduced left ventricular ejection fraction over 24 months: a retrospective study. Am J Cardiovasc Drugs. 2022;22(5):535–44.
Abumayyaleh M, El-Battrawy I, Behnes M, Borggrefe M, Akin I. Current evidence of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Future Cardiol. 2020;16(4):227–36.
Sobanski P, Sinkiewicz W, Kubica J, Blazejewski J, Bujak R. The reliability of noninvasive cardiac output measurement using the inert gas rebreathing method in patients with advanced heart failure. Cardiol J. 2008;15(1):63–70.
Koobi T, Kaukinen S, Ahola T, Turjanmaa VM. Non-invasive measurement of cardiac output: whole-body impedance cardiography in simultaneous comparison with thermodilution and direct oxygen Fick methods. Intensive Care Med. 1997;23(11):1132–7.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Saur J, Fluechter S, Trinkmann F, Papavassiliu T, Schoenberg S, Weissmann J, et al. Noninvasive determination of cardiac output by the inert-gas-rebreathing method—comparison with cardiovascular magnetic resonance imaging. Cardiology. 2009;114(4):247–54.
Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congest Heart Failure. 2003;9(5):241–50.
Wong ES, Wang BC, Garrison LP, Alfonso-Cristancho R, Flum DR, Arterburn DE, et al. Examining the BMI-mortality relationship using fractional polynomials. BMC Med Res Methodol. 2011;28(11):175.
Lau CW, Martens P, Lambeets S, Dupont M, Mullens W. Effects of sacubitril/valsartan on functional status and exercise capacity in real-world patients. Acta Cardiol. 2019;74(5):405–12.
Moliner-Abos C, Rivas-Lasarte M, Pamies Besora J, Fluvia-Brugues P, Sole-Gonzalez E, Mirabet S, et al. Sacubitril/valsartan in real-life practice: experience in patients with advanced heart failure and systematic review. Cardiovasc Drugs Ther. 2019;33(3):307–14.
Ekici B, Yaman M, Kucuk M, Dereli S, Yenercag M, Yigit Z, et al. Angiotensin receptor neprilysin inhibitor for patients with heart failure and reduced ejection fraction: real-world experience from Turkey (ARNi-TR). Turk Kardiyol Dern Ars. 2021;49(5):357–67.
Cosentino ER, Degli Esposti D, Miceli R, Bentivenga C, Landolfo M, Fg Cicero A, et al. Sacubitril/valsartan improves both functional and echocardiographic parameters in patients with chronic heart failure with reduced ejection fraction. Curr Med Res Opin. 2019;35(sup1):9–12.
Pugliese NR, Fabiani I, Zywicki V, Mazzola M, D’Agostino A, Galeotti GG, et al. Effects of sacubitril/valsartan on B-type natriuretic peptide circulating levels and loop diuretic dose in a case series of stabilized heart failure patients with left ventricular ejection fraction </=35. Curr Med Res Opin. 2019;35(sup3):13–8.
Morillas-Climent H, Seller-Moya J, Vicedo-Lopez A, Galcera-Jornet E, Alania-Torres E, Rodriguez-Pichardo Y, et al. Evolution of functional class, biochemical and echocardiographic parameters and clinical outcomes after sacubitril/valsartan initiation in daily practice. J Comp Eff Res. 2019;8(9):685–97.
Vicent L, Esteban-Fernandez A, Gomez-Bueno M, De-Juan J, Diez-Villanueva P, Iniesta AM, et al. Sacubitril/valsartan in daily clinical practice: data from a prospective registry. J Cardiovasc Pharmacol. 2019;73(2):118–24.
de Diego C, Gonzalez-Torres L, Nunez JM, Centurion Inda R, Martin-Langerwerf DA, Sangio AD, et al. Effects of angiotensin–neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm. 2018;15(3):395–402.
Gonzalez-Torres L, De Diego C, Centurion R, et al. Angiotensin-neprilysin inhibition further reverses cardiac remodeling as compared to angiotensin inhibition in reduced heart failure patients. Clin Cardiol J. 2018;2(1):6–9.
Goh V, Koh N, Teoh C, Hon J, Teo L, Lim C, et al. ARNI use leads to improved NYHA class and NT-proBNP levels in a Singaporean cohort. Eur J Heart Fail. 2018;20:81.
Meloche C, Pandrangi P, Parker J, Maley M, Twydell B, Mueller S, et al. The use of sacubitril/valsartan in an advanced heart failure clinic is associated with decreased heart failure hospitalizations, improved left ventricular ejection fraction and functional class. J Card Fail. 2019;25(8, Supplement):S59.
Agostinho JPR, Rodrigues T, Santos R, Cunha N, Rigueira J, Nunes-Ferreira A. Prescription of sacubitril/valsartan in a real-world population. Eur J Heart Fail. 2019;21:228–9.
Martens P, Beliën H, Dupont M, Mullens W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail. 2018;5(3):275–83.
Canu A, Maurin V, Dos Santos P, Picard F. Results of a single center experience on 200 consecutive patients treated with Entresto (sacubitril/valsartan). Eur J Heart Fail. 2017;19:413–4.
Koh NN, Goh V, Teoh C, Teo L, Lim C, Sim D. Tolerability and short-term outcomes of angiotensin receptor-neprilysin inhibitor (ARNI) use in a Singaporean cohort. Eur J Heart Fail. 2017;19:232.
Myhre PL, Vaduganathan M, Claggett B, Packer M, Desai AS, Rouleau JL, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019;73(11):1264–72.
Tsutsui H, Momomura SI, Saito Y, Ito H, Yamamoto K, Sakata Y, et al. Efficacy and safety of sacubitril/valsartan in Japanese patients with chronic heart failure and reduced ejection fraction—results from the PARALLEL-HF study. Circ J. 2021;85(5):584–94.
Pharithi RB, Ferre-Vallverdu M, Maisel AS, O’Connell E, Walshe M, Sweeney C, et al. Sacubitril–valsartan in a routine community population: attention to volume status critical to achieving target dose. ESC Heart Fail. 2020;7(1):159–67.
Galvan Ruiz M, Sanmamed Giron M, Singh M, Groba Marco M, Garcia Salvador J, Caballero Dorta E. Use of sacubitril–valsartan in elderly patients. Subanalysis of the SAVE-RLife study. Eur J Heart Fail. 2019;21:238.
Wachter R, Viriato D, Klebs S, Grunow SS, Schindler M, Engelhard J, et al. Early insights into the characteristics and evolution of clinical parameters in a cohort of patients prescribed sacubitril/valsartan in Germany. Postgrad Med. 2018;130(3):308–16.
Almufleh A, Marbach J, Chih S, Stadnick E, Davies R, Liu P, et al. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis. 2017;7(6):108–13.
Bayard G, Da Costa A, Pierrard R, Romeyer-Bouchard C, Guichard JB, Isaaz K. Impact of sacubitril/valsartan on echo parameters in heart failure patients with reduced ejection fraction a prospective evaluation. Int J Cardiol Heart Vasc. 2019;25: 100418.
Castrichini M, Manca P, Nuzzi V, Barbati G, De Luca A, Korcova R, et al. Sacubitril/valsartan induces global cardiac reverse remodeling in long-lasting heart failure with reduced ejection fraction: standard and advanced echocardiographic evidences. J Clin Med. 2020;9(4):1–13.
Landolfo M, Piani F, Esposti DD, Cosentino E, Bacchelli S, Dormi A, et al. Effects of sacubitril valsartan on clinical and echocardiographic parameters of outpatients with heart failure and reduced ejection fraction. Int J Cardiol Heart Vasc. 2020;31: 100656.
Gu W, Xu C, Li Z, Li ZZ. Echocardiographic changes in elderly patients with heart failure with reduced ejection fraction after sacubitril–valsartan treatment. Cardiovasc Diagn Ther. 2021;11(5):1093–100.
Polito MV, Silverio A, Rispoli A, Vitulano G, Auria F, De Angelis E, et al. Clinical and echocardiographic benefit of sacubitril/valsartan in a real-world population with HF with reduced ejection fraction. Sci Rep. 2020;10(1):6665.
Armentaro G, D’Arrigo G, Magurno M, Toscani AF, Condoleo V, Miceli S, et al. Impact of sacubitril/valsartan on clinical and echocardiographic parameters in heart failure patients with reduced ejection fraction: data from a real life 2-year follow-up study. Front Pharmacol. 2021;12: 733475.
Liu LW, Wu PC, Chiu MY, Tu PF, Fang CC. Sacubitril/valsartan improves left ventricular ejection fraction and reverses cardiac remodeling in taiwanese patients with heart failure and reduced ejection fraction. Acta Cardiol Sin. 2020;36(2):125–32.
Martyn T, Faulkenberg KD, Albert CL, Il’giovine ZJ, Randhawa VK, Donnellan E, et al. Acute hemodynamic effects of sacubitril-valsartan in heart failure patients receiving intravenous vasodilator and inotropic therapy. J Card Fail. 2021;27(3):368–72.
Martens P, Belien H, Dupont M, Vandervoort P, Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther. 2018;36(4): e12435.
Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18(10):1228–34.
Wachter R, Fonseca AF, Balas B, Kap E, Engelhard J, Schlienger R, et al. Real-world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail. 2019;21(5):588–97.
Nordberg Backelin C, Fu M, Ljungman C. Early experience of sacubitril–valsartan in heart failure with reduced ejection fraction in real-world clinical setting. ESC Heart Fail. 2020;7(3):1049–55.
Dashwood A, Vale C, Laher S, Chui F, Rheault H, Gan J, et al. Impact of patient and model of care factors on titration and tolerability of sacubitril/valsartan: an early Australian real-world experience. Heart Lung Circ. 2020;29(11):1688–95.
Chang HY, Feng AN, Fong MC, Hsueh CW, Lai WT, Huang KC, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74(4):372–80.
Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016;18(9):1193–202.
Czeisler MÉMK, Clarke KE, et al. Delay or avoidance of medical care because of COVID-19—related concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1250–7.
Agostoni P, Cattadori G, Apostolo A, Contini M, Palermo P, Marenzi G, et al. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: a new tool for heart failure evaluation. J Am Coll Cardiol. 2005;46(9):1779–81.
Trinkmann F, Berger M, Hoffmann U, Borggrefe M, Kaden JJ, Saur J. A comparative evaluation of electrical velocimetry and inert gas rebreathing for the non-invasive assessment of cardiac output. Clin Res Cardiol. 2011;100(10):935–43.
Acknowledgements
We thank Maike Hornig for her assistance with data collection.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Novartis Pharma GmbH, Nürnberg, Germany. The sponsor was involved in the design of the study and approved the submission of the present article for publication. Data collection, analysis and interpretation as well as writing of the report were solely performed by the authors of the present article.
Competing interests
Hanna Fröhlich, Norbert Frey, Bent Estler, Mirjam Mäck, Philipp Schlegel, Jan Beckendorf, Lutz Frankenstein and Tobias Täger declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
Authors contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HF, LF and TT. The first draft of the manuscript was written by HF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Heidelberg.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Data availability statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fröhlich, H., Frey, N., Estler, B. et al. Haemodynamic Effects of Sacubitril/Valsartan Initiation in Outpatients with Chronic Heart Failure. Am J Cardiovasc Drugs 22, 695–704 (2022). https://doi.org/10.1007/s40256-022-00549-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00549-2