Abstract
Purpose of Review
Fluid retention or congestion is a major cause of symptoms, poor quality of life, and adverse outcome in patients with heart failure (HF). Despite advances in disease-modifying therapy, the mainstay of treatment for congestion—loop diuretics—has remained largely unchanged for 50 years. In these two articles (part I: loop diuretics and part II: combination therapy), we will review the history of diuretic treatment and the current trial evidence for different diuretic strategies and explore potential future directions of research.
Recent Findings
We will assess recent trials including DOSE, TRANSFORM, ADVOR, CLOROTIC, OSPREY-AHF, and PUSH-AHF amongst others, and assess how these may influence current practice and future research.
Summary
There are few data on which to base diuretic therapy in clinical practice. The most robust evidence is for high dose loop diuretic treatment over low-dose treatment for patients admitted to hospital with HF, yet this is not reflected in guidelines. There is an urgent need for more and better research on different diuretic strategies in patients with HF.
Similar content being viewed by others
Introduction
Fluid retention leading to peripheral and pulmonary congestion is the hallmark of symptomatic heart failure (HF) and is associated with poor quality of life and adverse outcome regardless of left ventricular ejection fraction (LVEF) [1]. Most patients admitted to hospital with HF have severe venous congestion (anasarca); the treatment for which is diuretics [2,3,4].
The majority of out-patients with chronic HF (and all patients admitted to hospital with HF) are treated with diuretics [5], but there is little consensus on the specific goals of diuretic therapy [6, 7]. The European Society of Cardiology HF guidelines recommend that “persistent congestion” is excluded before discharge in patients admitted to hospital with HF [6, 8]. However, many patients are discharged with residual congestion[9], including in some clinical trials [10•], and signs of congestion on ultrasound are common in the absence of signs on examination [11, 12].
But what is the point of achieving decongestion? The National Institute of Health and Care Excellence (NICE) guidelines state that diuretics should be used to improve symptoms [13], whereas the American Heart Association (AHA) guidelines recommend diuretics to improve symptoms and prognosis [14]. Aside from one meta-analysis of small placebo-controlled trials before the modern era of neuro-hormonal inhibition in HF [15], there are scant data to support either position.
There has only been a handful of modern-day trials of diuretics and diuretic strategies. Few have been powered to detect differences in outcome, and none has shown convincing symptomatic benefit. The “standard care” arm in each of the studies has been highly variable, often using low doses of intravenous (IV) furosemide. Perhaps as a result, the gold standard of treatment for fluid retention in HF (loop diuretic)—the dose of which often determined by physician preference—has remained unchanged for the last 60 years [16]. Here, we review the evidence to date for current practice of treatment with loop diuretics, and, in a separate article, examine future trends and possibilities.
A Short History of Diuretic Treatment
The first detailed account of diuretic therapy for patients with HF was in 1785 [17]. William Withering reported improvements in breathing and peripheral oedema in patients with ‘dropsy’ (anasarca) who were treated with the leaves of Digitalis purpurea. Withering noted increased urine output as a side effect of treatment rather than a mechanism by which the patients were improving [6•]. Approximately 20 years later, the diuretic effects of mercury salts when given to patients with syphilis were described [18].
Digoxin and mercurial diuretics remained the only effective treatments for HF [19], until the discovery of acetazolamide [20], spironolactone [21], and thiazide diuretics in the 1950s [22]. All three were less toxic and better tolerated than mercury salts which quickly fell out of fashion [23, 24]. A decade later, 4-chloro-N-(2-furyl-methyl)-5-sulphmoyl anthranillic acid, or furosemide, was discovered, marking the last major advance in diuretic therapy for patients with HF [16].
Early trials showed that oral or IV administration of furosemide (and other loop diuretics) in patients with HF caused diuresis and was associated with improved symptoms compared to placebo [25,26,27,28]. Loop diuretics led to a larger and quicker diuresis than either acetazolamide, or spironolactone alone, and had an additive diuretic effect when given in combination with either agent (Table 1) [16, 29].
Compared to thiazide diuretics, loop diuretics tended to induce a greater diuresis and greater symptomatic improvement, with a lower risk of hypotension, hypokalaemia, or hyponatraemia [30,31,32,33,34,35]. Amiloride and triamterene inhibit sodium–potassium co-transporters in the distal convoluted tubule, which increases sodium excretion and reduces potassium excretion—‘potassium-sparing’ diuretics [36, 37]. Both were used only in combination with loop or thiazide diuretics to counter the excess potassium loss seen in early studies [38]. They have been superseded by spironolactone which has a similar (mild) potassium-sparing diuretic effect but profound prognostic benefits in patients with HF and a reduced ejection fraction (HeFREF) [39].
Loop diuretics have remained the cornerstone of the treatment of venous congestion in patients with HF since the 1960s. However, there is very little evidence to support their use [15], and even less on which to base recommendations on dosing or administration.
Loop Diuretics
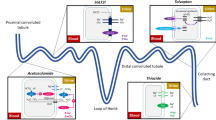
Loop diuretics are organic anions and are highly protein-bound in the serum [16]. They are actively excreted into the urinary space, entering the basolateral membrane of cells in the proximal convoluted tubule (PCT) through organic anion transporters 1 and 2 (OAT1 and OAT2) and are moved into the urinary space via the multidrug resistance-associated protein 4 (MRAP4) (Fig. 1) [40]. They compete with chloride ions to bind to and inhibit the sodium–potassium-chloride co-transporter (NKCC) on the apical membrane of the thick ascending limb of the loop of Henle. The NKCC reabsorbs filtered Na+, K+, and Cl− ions in a 1:1:2 ratio [41]. Inhibition of the NKCC thus increases the urinary concentrations of each electrolyte and reduces the ion concentration in the renal medulla, causing natriuresis and diuresis [42].
Common side effects or complications of loop diuretics include renal dysfunction [43] and electrolyte abnormalities (hyponatraemia, hypokalaemia, hypochloraemia, and metabolic alkalosis) [44,45,46,47], all of which are associated with worse outcomes. In particular, renal dysfunction and hypochloraemia (amongst other mechanisms) may contribute to loop diuretic resistance [48, 49]. Renin release is governed, in part, by urinary sodium and chloride concentrations detected in the PCT by NKCC co-transporters in the macula densa (MD). By inhibiting the NKCC2 in the MD, loop diuretics increase activation of the renin–angiotensin–aldosterone system (RAAS) [50,51,52]. While activation of the RAAS is commonly thought to result from abnormal haemodynamics in patients with HF, plasma concentrations of renin and aldosterone may be normal in patients with symptomatic HF who are not taking diuretics, increasing only after loop diuretics have been started: [53, 54] it is possible that loop diuretics are the primary driver of renin-angiotensin aldosterone system (RAAS) activation in patients with HF.
Which Loop Diuretic to Use?
Furosemide is the most commonly used loop diuretic in both in- and out-patients [55, 56]. However, there are important and, possibly, clinically significant differences in the pharmacokinetics of oral furosemide, bumetanide, and torsemide (Table 2) [29, 57, 58].
There have been a few head-to-head comparisons of loop diuretics in patients with HF. Small, open-label RCTs with short-term follow-up found few differences in symptoms or diuresis between furosemide and bumetanide [59,60,61]. However, others suggested that mortality, hospitalisation, symptoms, and quality of life were better with torsemide compared to furosemide [62,63,64]; a definitive trial followed (Table 3).
The TRANSFORM (Effect of Torsemide vs Furosemide After Discharge on All-Cause Mortality in Patients Hospitalized With Heart Failure) trial randomised 2859 patients to either oral furosemide or torsemide at the point of discharge following admission with HF, the dose of which was determined by the treating clinician [65•]. Most patients had HeFREF (70%) and were taking a loop diuretic prior to admission (66%). The primary endpoint was all-cause mortality, and the secondary endpoints included all-cause mortality or all-cause hospitalisation. Most patients were taking 80 mg of oral furosemide equivalents per day (the mean dose of oral loop diuretic on discharge was 79 mg of furosemide equivalents in both arms).
There was no difference in either the primary or secondary endpoint during a median follow-up of 17 months. Perhaps, the most notable finding of TRANSFORM was the dire prognosis following a hospitalisation with HF: 26% of patients in each arm died after a median follow-up of 17 months, and nearly half of patients had either died or were re-admitted in the first year following discharge.
There was no benefit of one treatment over the other in sub-group analysis. No data was collected on symptoms, diuresis, or congestion at different time points, so we do not know if the prognosis was poor due to inadequate diuresis, or residual congestion at the point of discharge. Regardless, given the high event rate and neutral findings, it is probable that the type of loop diuretic used is not important [66].
What Dose to Use?
Not all patients with HF need diuretic treatment. Optimal medical therapy may negate the need for loop diuretic treatment in some patients with HeFREF. Angiotensin-converting enzyme inhibitors [67], sacubitril valsartan [68], mineralocorticoid receptor antagonists [69], and sodium-glucose co-transporter 2 inhibitors [70], all have either a mild diuretic effect or reduce the need for loop diuretic treatment.
Diuretic withdrawal in patients with no or minimal congestion who are receiving optimal disease-modifying therapy is associated with improvements in renal function and reductions in plasma renin concentration without worsening symptoms or an increased risk of hospitalisation during short-term follow-up [71, 72]. However, diuretic withdrawal will not be suitable for all euvolaemic patients.
One small study of medication withdrawal (including diuretics) in stable out-patients with HF and a reduced ejection fraction (HeFREF) found worsening symptoms and doubling of serum natriuretic peptide concentration after 48 h [73]. In the only randomised controlled trial of diuretic withdrawal in stable out-patients with HeFREF, all of whom had NYHA class I symptoms and were receiving less than 80 mg of furosemide equivalents per day, 1 in 4 needed to restart loop diuretic during 90-day follow-up [74]. For those without congestion, the optimal dose may be that which prevents the recurrence of congestion, which will vary between patients and may change over time.
Dosing of furosemide for patients with congestion, either oral or IV, is contentious. Observational studies have found that higher doses are associated with the worse outcome both in patients hospitalised with HF [75, 76] and out-patients with chronic HF [1, 55, 77]. However, these analyses are confounded by the need to treat more severe disease with higher doses of diuretic.
Some observational data suggest that loop diuretics are associated with improved survival in those with more severe congestion [78, 79] and that increasing the dose of loop diuretic is associated with improved survival, regardless of the dose used, if the severity of congestion also improves [80].
For patients hospitalised with HF who were taking a loop diuretic prior to admission, the ESC and AHA guidelines recommend starting patients on 1–2 × their usual daily dose of oral diuretics in divided doses given IV [8, 14]. The NICE guidelines for acute HF merely recommend IV doses “higher” than that of the oral dose on admission [81].
Most patients who are prescribed a loop diuretic take no more than 80 mg per day of oral furosemide equivalents [82,83,84,85], but up to 50% of patients are not taking a loop diuretic on admission [86, 87]. In these circumstances, guidelines recommend low doses of IV furosemide (20–40 mg per day) [8, 14]. Thus, the starting dose of IV furosemide for patients admitted to the hospital could range anywhere between 20 and 160 mg per day.
The Diuretic Optimisation Strategies Evaluation (DOSE) trial attempted to clarify the optimal diuretic dosing strategy for patients admitted to hospital with HF and the best mode of administration (bolus vs. continuous infusion) [88]. Patients were randomised to either low dose (usual dose of oral loop diuretic) vs. high dose (2.5 × usual dose of oral loop diuretic) and to either continuous infusion or twice daily bolus administration of loop diuretic (Table 4). The co-primary endpoints were the change in symptoms measured on a visual analogue scale of 0 to 100 and the change in serum creatinine from randomisation to 72 h.
The trial was neutral in that neither dosing nor administration strategy was superior in respect to the primary endpoints, but there were a number of interesting, clinically relevant findings (Table 4):
-
1.
Patients in the high-dose arm (median daily dose 258 mg) had greater weight loss, greater net fluid loss, and greater improvement in breathlessness than patients in the low-dose arm (median daily dose 119 mg) after 72 h.
-
2.
Patients in the high-dose arm were more likely to change to oral diuretics and less likely to require intensification of diuretic treatment at 48 h than were patients in the low-dose arm
-
3.
Despite the greater diuresis with high-dose diuretic compared to low dose, the adverse event rate was higher in the low-dose arm.
-
4.
Patients in the bolus dosing arm were approximately twice as likely to require either an increase in loop diuretic dose or the addition of a thiazide diuretic at 48 h as patients in the continuous infusion arm. Despite these differences, there was no difference in weight loss, fluid loss, freedom from congestion, or improvement in symptoms between continuous or bolus dosing.
The results suggest that (1) high-dose treatment (2.5 × the oral dose on admission) induces a greater diuresis than does low-dose treatment, without a greater risk of adverse effects and (2) that continuous infusion may cause a similar diuresis to bolus dosing but without the need for treatment intensification. Due to the short half-life of IV loop diuretics, bolus dosing may allow for a period between doses during which renal sodium resorption may increase (and diuresis decrease) [89]. Other studies have found a greater diuresis and greater improvements in clinical congestion with continuous infusions over bolus dosing [90, 91].
Concerns over side effects or complications of high-dose IV loop diuretic are commonplace but may lead to an over-cautious approach to diuretic treatment. In the CARESS trial of ultrafiltration (UF) versus diuretic therapy for patients admitted to hospital with HF and worsening renal function, the diuretic therapy arm allowed for up to 720 mg of IV furosemide per day plus 10 mg of metolazone per day. The co-primary endpoint was a change in serum creatinine and a change in weight at 96 h. Both groups lost a similar amount of weight, but renal function improved in the diuretic therapy arm, and hyponatraemia or hypokalaemia occurred in only 3% of patients. Although not powered to detect a difference in clinical outcome, there was a trend towards a higher rate of all-cause hospitalisation or death in the UF arm [92].
Surprisingly, the DOSE trial has had little influence on guidelines or clinical trial protocols which continue to allow bolus administration of low doses (doses equal to the oral dose taken on admission) and, in some cases, discourage the use of continuous infusions [93].
Perhaps, the most useful lessons of the DOSE and TRANSFORM studies are that it is not the type of loop diuretic you use that matters, but what you do with it (in terms of dosing and administration strategy) that counts.
Strategies of Loop Diuretic Dosing and Administration
Some have suggested that patients respond to loop diuretics in an ‘all-or-none’ fashion, with conditions such as chronic kidney disease causing some patients to have a higher threshold to induce diuresis than others [94]. While all trial data to date demonstrate this is as a gross over-simplification, the data indicate that response to treatment is subject to a law of diminishing returns: doses above a certain threshold do not induce a much greater diuresis, and loop diuretic efficacy (volume of urine produced per 40 mg of furosemide) [95] falls with increasing doses (Fig. 2) [96]. Identifying patients unlikely to respond to diuretic therapy early during treatment may allow early titration of treatment in some and avoid the use of excessively high doses in others.
Loop diuretic efficacy in recent diuretic trials. Daily furosemide equivalents reported in text in DOSE, ADVOR, and TACTICS trials and estimated from reported cumulative dose in CLOROTIC, EMPA-RESPONSE-AHF, EMPAG-HF, PUSH-AHF, and OSPREY. Daily urine output reported in PUSH-AHF, CLOROTIC, and EMPA-RESPONSE-AHF trials; estimated from reported cumulative urine output at 72 h in the DOSE and TACTICS trials and at 96 h in the OSPREY trial; estimated from figures in the ADVOR and EMPAG trials
Natriuresis-Guided Loop Diuretic Treatment
Poor ‘natriuretic response’ (low urine sodium concentration after administration of IV loop diuretic) is associated with a greater risk of worsening renal function, inadequate treatment of congestion, loop diuretic resistance, and poor prognosis in patients treated with IV loop diuretic [97,98,99]. Natriuretic-guided dosing of loop diuretics—increasing dose of loop diuretic to achieve a given urine sodium concentration—has featured in ESC HF recommendations since 2019, but the efficacy of natriuresis-guided diuretic treatment was not assessed in an RCT until 2023 [8].
In the PUSH-AHF trial, 310 patients admitted to the hospital with HF were randomised to either natriuresis-guided therapy or standard care [100•]. In both arms, the initial dose of loop diuretic was based on renal function and prior loop diuretic use and was given as a bolus. Patients randomised to the natriuresis-guided arm had urine sodium concentration tested at 2, 6, 12, 18, and 24 h. The bolus dose was doubled (with a maximum dose of 5 mg bumetanide (200 mg furosemide)) at any time point if there was poor natriuretic response (defined as urinary sodium concentration < 70 mmol/L). The primary endpoint was total natriuresis after 24 h of treatment (Table 4).
Unsurprisingly, titrating loop diuretic dose based on urine sodium concentration was associated with greater natriuresis compared to standard therapy. Urine output at 24 and 48 h was also greater in the natriuresis-guided arm. This, again, was unsurprising. Eighty-five percent of patients in the natriuresis-guided arm had their diuretic dose doubled during the first 24 h, and, despite similar median initial dose of a loop diuretic (160 mg furosemide equivalents in both arms), patients in the natriuresis-guided arm received almost twice as much diuretic during admission as those in the standard care arm (26 mg of bumetanide over 7-day hospitalisation period, ~ 4 mg per day = 160 mg furosemide equivalents vs. 15 mg bumetanide over 7-day hospitalisation, and ~ 2 mg per day = 80 mg furosemide per day).
Natriuresis-guided treatment was stopped after 24 h, and, perhaps as a result, the differences in natriuresis and urine output were lost after 72 h of treatment. There was no difference in the adverse event rate, length of hospitalisation, re-admission with HF, or mortality between the two groups. Although hypotension is a concern when giving bolus doses of up to 200 mg IV furosemide, these data were not reported. The non-randomised ENACT-HF trial reported similar results which have been presented but not published [101].
The practicalities of repeated testing of urine sodium concentration in a busy ward environment aside, the PUSH-AHF and ENACT-HF trials demonstrate (as the DOSE trial did a decade earlier) that the larger the dose of loop diuretic you give, the greater the diuresis you induce (Fig. 3). Only a minority of patients had an adequate natriuretic response to the initial dose of diuretic, even though approximately half were not taking loop diuretic prior to admission. These trials, and natriuretic-guided diuretic treatment as a strategy, do not advance our understanding of how to use diuretics. It is unlikely that natriuresis-guided treatment will become the standard care for most HF specialists in busy healthcare systems.
Seventy-two-hour urine output in diuretic trials. Daily furosemide equivalents reported in text in the DOSE, ADVOR, and TACTICS trials and estimated from reported cumulative dose in the CLOROTIC, EMPA-RESPONSE-AHF, EMPAG-HF, PUSH-AHF, and OSPREY trials. Seventy-two-hour urine output reported in the DOSE and TACTICS trials; estimated from urine output reported at 24 h in the PUSH-AHF, CLOROTIC, and EMPA-RESPONSE-AHF trials, at 48 h in the ADVOR trial, and at 96 h in the OSPREY trial; estimated from figures in the EMPAG trial
Out-patient Parenteral Loop Diuretic Treatment
Administering IV loop diuretic to ambulatory out-patients is a common practice in the USA, and there is an increasing use of so-called ‘furosemide lounges’ (whereby patients attend an ambulatory unit, are cannulated, given an IV bolus of loop diuretic, and return home) in Europe. Avoiding hospitalisation has obvious benefits for the patient and healthcare systems, but supporting data are almost non-existent. Some observational reports suggest that either IV or subcutaneous (SC) furosemide given either at home or in a furosemide lounge, can cause weight loss, improve symptoms and signs of congestion, and reduce admission to hospital in most, but not all patients [102,103,104,105].
There has been one small RCT of treatment in a furosemide lounge versus conventional in-patient care (N = 24) in patients deemed to require at least 2 days of IV loop diuretic therapy. After 60 days, patients treated in the furosemide lounge accrued numerically more days alive and out-of-hospital compared to those treated as an in-patient (47 vs. 59; P = 0.13). However, more patients treated in the furosemide lounge were readmitted after 60 days (6 vs. 2; P = 0.31) [106]. No firm conclusions can be drawn from the small sample size, and a larger trial is planned [107].
SC furosemide infusions are commonly used in patients with advanced HF and palliative care needs for whom hospitalisation is unsuitable or unwanted [108]. Data from small randomised studies suggest that subcutaneous furosemide may have a similar diuretic effect to IV furosemide [109,110,111]: larger studies are planned (EudraCT 2020–004833-19) [112].
However, caution is required when interpreting trials of out-patient strategies. Days alive out-of-hospital at a given point in time may be an appealing primary endpoint [102, 112], but it is problematic. Randomising patients to out-patient parenteral treatment immediately shortens the length of the index hospitalisation. The median length of stay in a hospital in the UK is 8 days [4], and patients randomised to out-patient treatment will get a head start. If patients require longer treatment in the out-patient setting than they do as an in-patient due to insufficient diuresis, the days spent out-of-hospital will be greater, but disability due to symptoms, and the inconvenience of increased urine output, health care visits, and treatment equipment will be prolonged. Another problem is that any potential harm of early discharge may take longer than 30 days to manifest; days alive and out-of-hospital may be similar or greater in the early discharge arm at more distant time points.
Treatment in the out-patient setting is only safe if patients are able to cope with the increased burdens of transport, increased urine output at home, and the equipment for IV or SC furosemide infusions. Patients who are deemed unable to cope are likely to be excluded from the trials. The vast majority of patients admitted to hospital with HF are aged over 75, with moderate to severe peripheral oedema, NYHA class III or IV symptoms, and multiple co-morbidities [4]. It is unclear what proportion of patients admitted to hospital with HF would be suitable for parenteral treatment in the out-patient setting. Eligibility may depend far more on social circumstances than on patient or disease characteristics.
Finally, there are very few data comparing parenteral diuretic therapy to increased oral therapy. In the only head-to-head RCT in patients with HF (N = 10), there was a little difference in the urine output 8 h after treatment between those receiving 80 mg furosemide subcutaneously or orally (1550 mL (range 1353 to 1866 ml) vs. 1833 ml (range 1623 to 2726 ml), respectively, P not reported) [110]. Whether increasing oral diuretic is as effective as parenteral treatment given at home is unknown but may be preferable from a health economic and patient convenience point-of-view.
Summary and Conclusion
In 1964, the English physician Wilfred Stokes who used up to 300 mg of furosemide per day wrote that ‘Dosage [of furosemide] is largely arbitrary, governed by limits of known safety, experience, and recommendation.’ [16] Over half a century later, very little has changed.
The results of the DOSE trial are largely ignored by clinical guidelines but demonstrate that high-dose loop diuretic given via a continuous infusion induces a greater diuresis without the need for treatment intensification than does low-dose treatment. Natriuretic-guided dosing of diuretic in PUSH-AHF also found that most patients fail to respond to a low-dose diuretic (even if loop diuretic naïve) and that giving higher doses induces a greater diuresis without an increase in adverse events.
Hospitalisation with severe fluid retention is a common cause of morbidity in patients with HF and for some, high-dose loop diuretic treatment alone may be sufficient. However, hospitalisation often lasts many days, at great cost to the patient and healthcare system. Early discharge and use of parenteral treatment at home or in a furosemide lounge may shorten hospital stay but may only be suitable for a minority of patients. Data from randomised trials on the safety and efficacy of the furosemide lounge is non-existent. An alternative approach may be to speed up in-patient diuresis using combination therapy—loop diuretic plus an adjunctive diuretic treatment—which we will consider in part 2 of this review.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Pellicori P, Cleland JG, Zhang J, Kallvikbacka-Bennett A, Urbinati A, Shah P, Kazmi S, Clark AL. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther. 2016;30(6):599–609. https://doi.org/10.1007/s10557-016-6697-7.
Shoaib A, Mamas MA, Ahmad QS, et al. Characteristics and outcome of acute heart failure patients according to the severity of peripheral oedema. Int J Cardiol. 2019;285:40–6. https://doi.org/10.1016/j.ijcard.2019.03.020.
Shoaib A, Waleed M, Khan S, et al. Breathlessness at rest is not the dominant presentation of patients admitted with heart failure. Eur J Heart Fail. 2014;16(12):1283–91. https://doi.org/10.1002/ejhf.153.
National Institute for Cardiovascular Outcomes Research. National Heart Failure Audit. 2022 summary report. Available from: NHFA-DOC-2022-FINAL.pdf (nicor.org.uk) [Accessed 22/09/2023].
Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(12):1574–85. https://doi.org/10.1002/ejhf.813.
• Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137–55. https://doi.org/10.1002/ejhf.1369. A useful summary of agreed “best practice” with diuretic treatment from the ESC
Butler J, Gheorghiade M, Metra M. Moving away from symptoms-based heart failure treatment: misperceptions and real risks for patients with heart failure. Eur J Heart Fail. 2016;18(4):350–2. https://doi.org/10.1002/ejhf.507.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Skibelund AK, ESC Scientific Document Group. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;25:ehad195. https://doi.org/10.1093/eurheartj/ehad195.
Rubio-Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Givertz MM, Bloomfield DM, Dittrich H, Damman K, Pérez-Calvo JI, Voors AA. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. 2018;1(258):185–91. https://doi.org/10.1016/j.ijcard.2018.01.067.
• Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, Tartaglia K, Chenot F, Moubayed S, Dierckx R, Blouard P, Troisfontaines P, Derthoo D, Smolders W, Bruckers L, Droogne W, Ter Maaten JM, Damman K, Lassus J, Mebazaa A, Filippatos G, Ruschitzka F, Dupont M, ADVOR Study Group. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387(13):1185–95. https://doi.org/10.1056/NEJMoa2203094. Largest trial of a diuretic agent in patients admitted to hospital with heart failure; best evidence to date that acetazolamide may be a usefull add-on diuretic treatment
• Pellicori P, Shah P, Cuthbert J, et al. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail. 2019;21(7):904–16. https://doi.org/10.1002/ejhf.1383. Observational work that demonstrates many patients that have congestion without signs or symptoms on examination
Cuthbert JJ, Pellicori P, Flockton R, et al. The prevalence and clinical associations of ultrasound measures of congestion in patients at risk of developing heart failure. Eur J Heart Fail. 2021;23(11):1831–40. https://doi.org/10.1002/ejhf.2353.
National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management NICE guideline [NG106]. Available from: Overview | Chronic heart failure in adults: diagnosis and management | Guidance | NICE. [Accessed: 03/10/2023].
Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published correction appears in Circulation. 2022 May 3;145(18):e1033] [published correction appears in Circulation. 2022 Sep 27;146(13):e185] [published correction appears in Circulation. 2023 Apr 4;147(14):e674]. Circulation. 2022;145(18):e895–1032. https://doi.org/10.1161/CIR.0000000000001063.
Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ. Diuretics for heart failure. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003838. https://doi.org/10.1002/14651858.CD003838.pub3. Update in: Cochrane Database Syst Rev. 2016;4:CD003838.
Stokes W. A new effective diuretic - Lasix. Br Med J. 1964;2(5414):910–4.
Withering W. An account of the foxglove and some of its medical uses: with practical remarks on dropsy and other diseases. London: GG J & J Robinson; 1785.
Stringham JS. On the diuretic effects of mercury in a case of syphilis. Med Phys J. 1807;18(104):331–3.
Evans W, Paxon T. A comparison of the mercurial diuretics used in heart failure. Br Heart J. 1941;3(2):112–20.
Friedberg CK, Taymor R, Minor JB, Halpern M. The use of diamox, a carbonic anhydrase inhibitor, as an oral diuretic in patients with congestive heart failure. N Engl J Med. 1953;248(21):883–9. https://doi.org/10.1056/NEJM195305212482102.
Carruthers BM, Ledray RD, Seraglia M, Mcintosh HW, Walsh GC. Effect of an aldosterone antagonist (spironolactone) on patients with severe congestive heart failure. Can Med Assoc J. 1963;89(13):633–41.
Schreiner GE, Bloomer HA. Effect of chlorothiazide on the edema of cirrhosis, nephrosis, congestive heart failure and chronic renal insufficiency. N Engl J Med. 1957;257(21):1016–22.
Ford RV, Rochelle JB 3rd. The differing mechanisms of action of mercurials, carbonic anhydrase inhibitors, and chlorothiazide as diuretic agents. J Lab Clin Med. 1959;53(1):53–63.
Hollander W, Chobanian AV, Wilkins RW. Relationship between diuretic and antihypertensive effects of chlorothiazide and mercurial diuretics. Circulation. 1959;19(6):827–38.
Kourouklis C, Christensen O, Augoustakis D. Bumetanide in congestive heart failure. Curr Med Res Opin. 1976;4(6):422–31. https://doi.org/10.1185/03007997609111998.
Sherman LG, Liang CS, Baumgardner S, Charuzi Y, Chardo F, Kim CS. Piretanide, a potent diuretic with potassium-sparing properties, for the treatment of congestive heart failure. Clin Pharmacol Ther. 1986;40(5):587–94. https://doi.org/10.1038/clpt.1986.228.
Haerer W, Bauer U, Sultan N, Cernoch K, Mouselimis N, Fehske KJ, Hetzel M, Stauch M, Hombach V. Acute and chronic effects of a diuretic monotherapy with piretanide in congestive heart failure–a placebo-controlled trial. Cardiovasc Drugs Ther. 1990;4(2):515–21. https://doi.org/10.1007/BF01857763.
Kleber FX, Thyroff-Friesinger U. Treatment of mild chronic congestive heart failure with ibopamine, hydrochlorothiazide, ibopamine plus hydrochlorothiazide or placebo. A double-blind comparative study. Cardiology. 1990;77(Suppl 5):67–74. https://doi.org/10.1159/000174699.
Stason WB, Cannon PJ, Heinemann HO, Laragh JH. Furosemide. A clinical evaluation of its diuretic action. Circulation. 1966;34(5):910–20.
Crawford RJ, Allman S, Gibson W, Kitchen S, Richards HH. A comparative study of frusemide-amiloride and cyclopenthiazide-potassium chloride in the treatment of congestive cardiac failure in general practice. J Int Med Res. 1988;16(2):143–9. https://doi.org/10.1177/030006058801600209.
Stewart JH, Edwards KD. Clinical comparison of frusemide with bendrofluazide, mersalyl, and ethacrynic acid. Br Med J. 1965;2(5473):1277–81. https://doi.org/10.1136/bmj.2.5473.1277.
Levy B. The efficacy and safety of furosemide and a combination of spironolactone and hydrochlorothiazide in congestive heart failure. J Clin Pharmacol. 1977;17(7):420–30. https://doi.org/10.1002/j.1552-4604.1977.tb04625.x.
Coodley EL, Nandi PS, Chiotellis P. Evaluation of a new diuretic, diapamide, in congestive heart failure. J Clin Pharmacol. 1979;19(2–3):127–36. https://doi.org/10.1002/j.1552-4604.1979.tb02470.x.
Gabriel R, Baylor P. Comparison of the chronic effects of bendrofluazide, bumetanide and frusemide on plasma biochemical variables. Postgrad Med J. 1981;57(664):71–4. https://doi.org/10.1136/pgmj.57.664.71.
Heseltine D, Bramble MG. Loop diuretics cause less postural hypotension than thiazide diuretics in the frail elderly. Curr Med Res Opin. 1988;11(4):232–5. https://doi.org/10.1185/03007998809114241.
Crosley AP Jr, Ronquillo LM, Strickland WH, Alexander F. Triamterene, a new natruretic agent. Preliminary observations in man. Ann Intern Med. 1962;56:241–51. https://doi.org/10.7326/0003-4819-56-2-241.
Baer JE, Jones CB, Spitzer SA, Russo HF. The potassium-sparing and natriuretic activity of N-amidino-3,5-diamino-6-chloropyrazinecarboxamide hydrochloride dihydrate (amiloride hydrochloride). J Pharmacol Exp Ther. 1967;157(2):472–85.
Richardson A, Bayliss J, Scriven AJ, Parameshwar J, Poole-Wilson PA, Sutton GC. Double-blind comparison of captopril alone against frusemide plus amiloride in mild heart failure. Lancet. 1987;2(8561):709–11. https://doi.org/10.1016/s0140-6736(87)91074-9.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341(10):709–17. https://doi.org/10.1056/NEJM199909023411001.
Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol. 2007;18(1):37–45. https://doi.org/10.1681/ASN.2005090966.
Somasekharan S, Tanis J, Forbush B. Loop diuretic and ion-binding residues revealed by scanning mutagenesis of transmembrane helix 3 (TM3) of Na-K-Cl cotransporter (NKCC1). J Biol Chem. 2012;287(21):17308–17.
Ellison DH, Felker GM. Diuretic treatment in heart failure [published correction appears in N Engl J Med. 2018 Feb 1;378(5):492]. N Engl J Med. 2017;377(20):1964–75.
Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13(8):599–608. https://doi.org/10.1016/j.cardfail.2007.04.008.
Verbrugge FH, Steels P, Grieten L, Nijst P, Tang WH, Mullens W. Hyponatremia in acute decompensated heart failure: depletion versus dilution. J Am Coll Cardiol. 2015;65(5):480–92.
Lombardi CM, Carubelli V, Peveri G, et al. Prognostic significance of serum potassium in patients hospitalized for acute heart failure [published online ahead of print, 2022 May 11]. ESC Heart Fail. 2022. https://doi.org/10.1002/ehf2.13925.
Cuthbert JJ, Brown OI, Urbinati A, et al. Hypochloraemia following admission to hospital with heart failure is common and associated with an increased risk of readmission or death: a report from OPERA-HF. Eur Heart J Acute Cardiovasc Care. 2022;11(1):43–52. https://doi.org/10.1093/ehjacc/zuab097.
Cooper LB, Mentz RJ, Gallup D, Lala A, DeVore AD, Vader JM, AbouEzzeddine OF, Bart BA, Anstrom KJ, Hernandez AF, Felker GM. Serum bicarbonate in acute heart failure: relationship to treatment strategies and clinical outcomes. J Card Fail. 2016;22(9):738–42.
Cox ZL, Testani JM. Loop diuretic resistance complicating acute heart failure. Heart Fail Rev. 2020;25(1):133–45. https://doi.org/10.1007/s10741-019-09851-9.
Cuthbert JJ, Bhandari S, Clark AL. Hypochloraemia in patients with heart failure: causes and consequences. Cardiol Ther. 2020;9(2):333–47. https://doi.org/10.1007/s40119-020-00194-3.
Brater DC. Pharmacokinetics of loop diuretics in congestive heart failure. Br Heart J. 1994;72(2 Suppl):S40–3.
Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274(2 Pt 2):R263–79.
Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10(4):676–87. https://doi.org/10.2215/CJN.12391213.
Anand IS, Veall N, Kalra GS, et al. Treatment of heart failure with diuretics: body compartments, renal function and plasma hormones. Eur Heart J. 1989;10(5):445–50. https://doi.org/10.1093/oxfordjournals.eurheartj.a059508.
Bayliss J, Norell M, Canepa-Anson R, Sutton G, Poole-Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J. 1987;57(1):17–22. https://doi.org/10.1136/hrt.57.1.17.
Khan MS, Greene SJ, Hellkamp AS, et al. Diuretic changes, health care resource utilization, and clinical outcomes for heart failure with reduced ejection fraction: from the CHAnge the Management of Patients With Heart Failure Registry. Circ Heart Fail. 2021;14(11):e008351. https://doi.org/10.1161/CIRCHEARTFAILURE.121.008351.
Bikdeli B, Strait KM, Dharmarajan K, et al. Dominance of furosemide for loop diuretic therapy in heart failure: time to revisit the alternatives? J Am Coll Cardiol. 2013;61(14):1549–50.
Henning R, Lundvall O. Evaluation in man of bumetanide, a new diuretic agent. Eur J Clin Pharmacol. 1973;6(4):224–7. https://doi.org/10.1007/BF00644736.
Knauf H, Mutschler E. Clinical pharmacokinetics and pharmacodynamics of torasemide. Clin Pharmacokinet. 1998;34(1):1–24. https://doi.org/10.2165/00003088-199834010-00001.
Ramsay F, Crawford RJ, Allman S, Bailey R, Martin A. An open comparative study of two diuretic combinations, frusemide/amiloride ('Frumil’) and bumetanide/potassium chloride (“Burinex” K), in the treatment of congestive cardiac failure in hospital out-patients. Curr Med Res Opin. 1988;10(10):682–9. https://doi.org/10.1185/03007998809111119.
Sagar S, Sharma BK, Sharma PL, Wahi PL. A comparative randomized double-blind clinical trial of bumetanide and furosemide in congestive cardiac failure and other edema states. Int J Clin Pharmacol Ther Toxicol. 1984;22(9):473–8.
Konecke LL. Clinical trial of bumetanide versus furosemide in patients with congestive heart failure. J Clin Pharmacol. 1981;21(11):688–90. https://doi.org/10.1002/j.1552-4604.1981.tb05684.x.
Müller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV–efficacy and quality of life. Eur J Heart Fail. 2003;5(6):793–801. https://doi.org/10.1016/s1388-9842(03)00150-8.
Cosín J, Díez J, TORIC investigators. Torasemide in chronic heart failure: results of the TORIC study [published correction appears in Eur J Heart Fail 2002 Oct;4(5):667]. Eur J Heart Fail. 2002;4(4):507–13. https://doi.org/10.1016/s1388-9842(02)00122-8.
Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111(7):513–20. https://doi.org/10.1016/s0002-9343(01)00903-2.
• Mentz RJ, Anstrom KJ, Eisenstein EL, et al. Effect of torsemide vs furosemide after discharge on all-cause mortality in patients hospitalized with heart failure: the TRANSFORM-HF randomized clinical trial. JAMA. 2023;329(3):214–23. The largest blinded randomised trial of different forms of loop diuretic
Täger T, Fröhlich H, Grundtvig M, Seiz M, Schellberg D, Goode K, Kazmi S, Hole T, Katus HA, Atar D, Cleland JGF, Agewall S, Clark AL, Frankenstein L. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure - a multicenter propensity score matched analysis. Int J Cardiol. 2019;15(289):83–90. https://doi.org/10.1016/j.ijcard.2019.01.109.
Good JM, Brady AJ, Noormohamed FH, Oakley CM, Cleland JG. Effect of intense angiotensin II suppression on the diuretic response to furosemide during chronic ACE inhibition. Circulation. 1994;90(1):220–4. https://doi.org/10.1161/01.cir.90.1.220.
Vardeny O, Claggett B, Kachadourian J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail. 2019;21(3):337–41. https://doi.org/10.1002/ejhf.1402.
Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol. 1996;78(8):902–907. https://doi.org/10.1016/s0002-9149(96)00465-1.
Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF trial [published correction appears in Circulation. 2020 Nov 3;142(18):e316]. Circulation. 2020;142(18):1713–24. https://doi.org/10.1161/CIRCULATIONAHA.120.048739.
Galve E, Mallol A, Catalan R, et al. Clinical and neurohumoral consequences of diuretic withdrawal in patients with chronic, stabilized heart failure and systolic dysfunction. Eur J Heart Fail. 2005;7(5):892–8. https://doi.org/10.1016/j.ejheart.2004.09.006.
van Kraaij DJ, Jansen RW, Sweep FC, Hoefnagels WH. Neurohormonal effects of furosemide withdrawal in elderly heart failure patients with normal systolic function. Eur J Heart Fail. 2003;5(1):47–53. https://doi.org/10.1016/s1388-9842(02)00205-2.
Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland JGF. The effects of short-term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail. 2017;19(5):643–9. https://doi.org/10.1002/ejhf.748.
Rohde LE, Rover MM, Figueiredo Neto JA, et al. Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double-blind, multicentre, randomized trial. Eur Heart J. 2019;40(44):3605–12.
Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–8.
Hasselblad V, Stough WG, Shah MR, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–106.
Nuzzi V, Cannatà A, Pellicori P, et al. Diuretic dose trajectories in dilated cardiomyopathy: prognostic implications [published correction appears in Clin Res Cardiol. 2022 Dec 6;:]. Clin Res Cardiol. 2023;112(3):419–30. https://doi.org/10.1007/s00392-022-02126-8.
Faselis C, Lam PH, Patel S, et al. Loop diuretic prescription and long-term outcomes in heart failure: association modification by congestion. Am J Med. 2021;134(6):797–804. https://doi.org/10.1016/j.amjmed.2020.11.019.
Faselis C, Arundel C, Patel S, et al. Loop diuretic prescription and 30-day outcomes in older patients with heart failure. J Am Coll Cardiol. 2020;76(6):669–79. https://doi.org/10.1016/j.jacc.2020.06.022.
Simonavičius J, Maeder MT, Eurlings CGMJ, et al. Intensification of pharmacological decongestion but not the actual daily loop diuretic dose predicts worse chronic heart failure outcome: insights from TIME-CHF. Clin Res Cardiol. 2021;110(8):1221–33. https://doi.org/10.1007/s00392-020-01779-7.
National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management. Clinical guideline [CG187]. November 2021. Available from: https://www.nice.org.uk/guidance/cg187 . Accessed 1/7/2022.
DeVore AD, Hasselblad V, Mentz RJ, et al. Loop diuretic dose adjustments after a hospitalization for heart failure: insights from ASCEND-HF. Eur J Heart Fail. 2015;17(3):340–6. https://doi.org/10.1002/ejhf.235.
Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol. 2017;2(9):950–8. https://doi.org/10.1001/jamacardio.2017.2198.
Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. https://doi.org/10.1056/NEJMoa1005419.
Felker GM, Mentz RJ, Cole RT, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69(11):1399–406. https://doi.org/10.1016/j.jacc.2016.09.004.
Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, Piepoli MF, Crespo-Leiro MG, Lainscak M, Ponikowski P, Filippatos G, Ruschitzka F, Seferovic P, Coats AJS, Lund LH, ESC-EORP-HFA Heart Failure Long-Term Registry Investigators. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2019;21(11):1338–52. https://doi.org/10.1002/ejhf.1492.
Greene SJ, Triana TS, Ionescu-Ittu R, et al. In-hospital therapy for heart failure with reduced ejection fraction in the United States. JACC Heart Fail. 2020;8(11):943–53. https://doi.org/10.1016/j.jchf.2020.05.013.
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM, NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805.
Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WH, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail. 2014;7(5):766–72. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001377.
Frea S, Pidello S, Volpe A, Canavosio FG, Galluzzo A, Bovolo V, Camarda A, Golzio PG, D’Ascenzo F, Bergerone S, Rinaldi M, Gaita F. Diuretic treatment in high-risk acute decompensation of advanced chronic heart failure-bolus intermittent vs. continuous infusion of furosemide: a randomized controlled trial. Clin Res Cardiol. 2020;109(4):417–25.
Ng KT, Yap JLL. Continuous infusion vs. intermittent bolus injection of furosemide in acute decompensated heart failure: systematic review and meta-analysis of randomised controlled trials. Anaesthesia. 2018;73(2):238–47.
Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367(24):2296–304. https://doi.org/10.1056/NEJMoa1210357.
Ter Maaten JM, Beldhuis IE, van der Meer P, et al. Natriuresis-guided therapy in acute heart failure: rationale and design of the Pragmatic Urinary Sodium-based treatment algoritHm in Acute Heart Failure (PUSH-AHF) trial. Eur J Heart Fail. 2022;24(2):385–92. https://doi.org/10.1002/ejhf.2385.
Anisman SD, Erickson SB, Morden NE. How to prescribe loop diuretics in oedema. BMJ. 2019;364:l359. https://doi.org/10.1136/bmj.l359. Published 2019 Feb 21.
Verbrugge FH. Editor’s choice-diuretic resistance in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2018;7(4):379–89. https://doi.org/10.1177/2048872618768488.
Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137–55. https://doi.org/10.1002/ejhf.1369.
Khorramshahi Bayat M, Ngo L, Mulligan A, Chan W, McKenzie S, Hay K, Ranasinghe I. The association between urinary sodium concentration (UNa) and outcomes of acute heart failure: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2022;8(7):709–21. https://doi.org/10.1093/ehjqcco/qcac007.
Biegus J, Zymliński R, Sokolski M, Todd J, Cotter G, Metra M, et al. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail. 2019;21:624–33.
Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, et al. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail. 2016;9:e002370.
• ter Maaten JM, Beldhuis IE, van der Meer P, et al. Natriuresis-guided diuretic therapy in acute heart failure: a pragmatic randomized trial [published online ahead of print, 2023 Aug 28]. Nat Med. 2023. https://doi.org/10.1038/s41591-023-02532-z. Randomised trial of natriuresis-guided loop diuretic treatment
Dauw J, Lelonek M, Zegri-Reiriz I, et al. Rationale and design of the efficacy of a standardized diuretic protocol in acute heart failure study. ESC Heart Fail. 2021;8(6):4685–92.
Ioannou A, Browne T, Jordan S, Metaxa S, Mandal AKJ, Missouris CG. Diuretic lounge and the impact on hospital admissions for treatment of decompensated heart failure. QJM. 2020;113(9):651–6. https://doi.org/10.1093/qjmed/hcaa114.
Payne D. Intravenous diuretic administration in the home environment. Br J Community Nurs. 2021;26(12):599–603. https://doi.org/10.12968/bjcn.2021.26.12.599.
Ryder M, Murphy NF, McCaffrey D, O’Loughlin C, Ledwidge M, McDonald K. Outpatient intravenous diuretic therapy; potential for marked reduction in hospitalisations for acute decompensated heart failure. Eur J Heart Fail. 2008;10(3):267–72. https://doi.org/10.1016/j.ejheart.2008.01.003.
Austin J, Hockey D, Williams WR, Hutchison S. Assessing parenteral diuretic treatment of decompensated heart failure in the community. Br J Community Nurs. 2013;18(11):528–34. https://doi.org/10.12968/bjcn.2013.18.11.528.
Wong KYK, Hughes DA, Debski M, et al. Effectiveness of out-patient based acute heart failure care: a pilot randomised controlled trial. Acta Cardiol. 2023;78(7):828–37. https://doi.org/10.1080/00015385.2023.2197834.
Wong K, Latt NKZ, Debski M, et al. Safety and effectiveness of outpatient based acute heart failure care: a randomised controlled feasibility trial. Heart. 2020;106(suppl 2):A74–5. https://doi.org/10.1136/heartjnl-2020-BCS.96.
Birch F, Boam E, Parsons S, Ghosh J, Johnson MJ. Subcutaneous furosemide in advanced heart failure: service improvement project. BMJ Support Palliat Care. 2023;13(1):112–6. https://doi.org/10.1136/bmjspcare-2020-002803.
Gilotra NA, Princewill O, Marino B, et al. Efficacy of Intravenous furosemide versus a novel, pH-neutral furosemide formulation administered subcutaneously in outpatients with worsening heart failure. JACC Heart Fail. 2018;6(1):65–70. https://doi.org/10.1016/j.jchf.2017.10.001.
Sica DA, Muntendam P, Myers RL, et al. Subcutaneous furosemide in heart failure: pharmacokinetic characteristics of a newly buffered solution. JACC Basic Transl Sci. 2018;3(1):25–34. https://doi.org/10.1016/j.jacbts.2017.10.001. Published 2018 Feb 7.
Osmanska J, Brooksbank K, Docherty KF, et al. A novel, small-volume subcutaneous furosemide formulation delivered by an abdominal patch infusor device in patients with heart failure: results of two phase I studies [published online ahead of print, 2023 Oct 6]. Eur Heart J Cardiovasc Pharmacother. 2023;pvad073. https://doi.org/10.1093/ehjcvp/pvad073.
National Health Service. Health Regulation Authority. Planning and improving research. SUBCUT-HF II. 2022. Available from: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/subcut-hf-ii/ . Accessed 22/09/2023.
Author information
Authors and Affiliations
Contributions
JJC and ALC wrote the manuscript and prepared the tables and figures.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cuthbert, J.J., Clark, A.L. Diuretic Treatment in Patients with Heart Failure: Current Evidence and Future Directions – Part I: Loop Diuretics. Curr Heart Fail Rep 21, 101–114 (2024). https://doi.org/10.1007/s11897-024-00643-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-024-00643-3