Abstract
The aetiology of heart failure with preserved ejection fraction (HFpEF) is heterogenous and overlaps with that of several comorbidities like atrial fibrillation, diabetes mellitus, chronic kidney disease, valvular heart disease, iron deficiency, or sarcopenia. The diagnosis of HFpEF involves evaluating cardiac dysfunction through imaging techniques and assessing increased left ventricular filling pressure, which can be measured directly or estimated through various proxies including natriuretic peptides. To better narrow down the differential diagnosis of HFpEF, European and American heart failure guidelines advocate the use of different algorithms including comorbidities that require diagnosis and rigorous treatment during the evaluation process. Therapeutic recommendations differ between guidelines. Whilst sodium glucose transporter 2 inhibitors have a solid evidence base, the recommendations differ with regard to the use of inhibitors of the renin–angiotensin–aldosterone axis. Unless indicated for specific comorbidities, the use of beta-blockers should be discouraged in HFpEF. The aim of this article is to provide an overview of the current state of the art in HFpEF diagnosis, clinical evaluation, and treatment.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) has been called a clinical syndrome, not a disease. The word syndrome stems from the Greek συνδρομή (syndromḗ, going together), meaning the joint occurrence of different clinical signs and symptoms. Over the last decades, clinicians have endeavoured to disentangle the HF syndrome yielding the differentiation of HF with reduced (HFrEF), HF with mildly reduced (HFmrEF), and HF with preserved ejection fraction (HFpEF). Even though clinical signs and symptoms overlap grossly between these HF types, the latter type, HFpEF, continues to raise pertinent questions with regard to diagnosis, clinical assessment, and treatment.
It has taken decades to agree on the term HFpEF in international guidelines. In 2008, the European Society of Cardiology (ESC) finally chose to use this term instead of “diastolic HF”, whose use has largely been abandoned over the following years. HFpEF is different from the prototypical form HFrEF for several reasons. It has been suggested that in HFpEF the problem starts in the periphery with hypertension, the metabolic syndrome or the like that spread to the heart, whereas in HFrEF the problem starts in the heart and spreads to the periphery [1]. However, though attractive, this concept has not been proven so far. Women have an overall higher likelihood to develop HFpEF than men (in HFrEF the situation is vice versa), and sex disparities exist (Table 1). From a pathophysiological standpoint, HFpEF develops when the left ventricle (LV) is unable to accept an adequate volume of blood during diastole, at normal diastolic pressures, and at volumes sufficient to maintain an appropriate stroke volume [2]. These abnormalities are caused by a decrease in ventricular relaxation and/or an increase in ventricular stiffness. Till today, there are no established animal models that accurately recapitulate the ventricular complexities leading to HFpEF, yielding difficulties in the development of adequate therapies [3].
The aim of this article is to provide clinicians with practical guidance for the diagnosis, clinical evaluation, and treatment of patients with HFpEF, defined as symptomatic HF with a left ventricular ejection fraction (LVEF) ≥ 50%.
Diagnosis of HFpEF
Clinical diagnosis
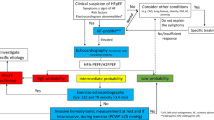
The clinical diagnosis of HFpEF remains challenging due to its heterogeneous aetiology (see “Evaluating the presence of systemic disease”) that recently sparked the idea of different HFpEF phenotypes that may require different treatment strategies with much focus on patients’ comorbidities (Fig. 1) [4]. The introduction of two different diagnostic tools has greatly enhanced our ability to diagnose HFpEF in recent years. The first, the HFA-PEFF algorithm offers a structured approach to the diagnosis of HFpEF by assessing predisposing risk factors, clinical signs and symptoms, objective evidence of cardiac dysfunction, and functional and structural abnormalities [5]. Each criterion is assigned a score, reflecting the probability of HFpEF and its impact on prognosis [6]. A second, recently proposed diagnostic tool is the H2FPEF score [7]. Both algorithms are summarized in Fig. 2. Finally, a diagnostic algorithm has recently been proposed for patients with HFpEF to identify comorbidities involved in the development of dyspnoea and/or other symptoms suggestive of HFpEF as shown in Fig. 3 [8].

Modified from Anker et al. [4] COPD, chronic obstructive pulmonary disease; MR, mitral regurgitation; TR, tricuspid regurgitation
HFpEF phenotypes with upper and lower boundary of the estimated prevalence values.

Modified from Verwerft et al. [8] CAD, coronary artery disease; CMR, cardiac magnetic resonance imaging; CPET, cardiopulmonary exercise test; CT, computed tomography; ECG, electrocardiogram; PVI, pulmonary vein isolation
Components of assessing the contributors to dyspnoea in patients with confirmed diagnosis of HFpEF according to the HFA-PEFF or the H2FPEF score. Cut-off values were ≥ 5 and ≥ 6, respectively.
Factors enhancing the pre-test probability for the diagnosis of HFpEF encompass female sex, advanced age, hypertension, obesity, chronic kidney disease (CKD), diabetes mellitus (DM), and atrial fibrillation (AF). These factors indicate the development of diastolic dysfunction and LV hypertrophy [9]. When patients with typical symptoms such as exertional dyspnoea, fatigue, and reduced exercise tolerance present alongside these risk indicators, HFpEF should be suspected. Clinical signs and symptoms of HFpEF resemble those of other forms of HF, including peripheral oedema, elevated jugular venous pressure, and pulmonary congestion on auscultation or chest radiography [10]. Whilst these findings are not specific to HFpEF, they provide supportive evidence for the diagnosis. Furthermore, orthopnoea and paroxysmal nocturnal dyspnoea are commonly reported symptoms in patients with HFpEF, often resulting from elevated LV filling pressures during recumbency [7].
Objective evidence of cardiac dysfunction is required to confirm the diagnosis of HFpEF. Elevated levels of natriuretic peptides, such as B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP), are suggestive of HFpEF, particularly in patients with predisposing risk factors and clinical signs of HF [11]. However, it is essential to consider other causes of elevated natriuretic peptides, such as acute coronary syndromes, valvular heart disease, AF, and CKD (see “Chronic kidney disease and hyperkalaemia”). Echocardiography remains the cornerstone of functional and structural assessment in patients with suspected HFpEF [12]: LVEF is typically preserved (≥ 50%), left atrial volume index (LAVI) is increased, and there is evidence of diastolic dysfunction leading to elevated cardiac filling pressures [12]. The presence of a hypercontractile phenotype has been suggested to exist in opposition to a normocontractile phenotype, with the former characterized by an LVEF > 60–70% and the latter by an LVEF below this range [13]. Diastolic dysfunction is defined as a shift in the LV end-diastolic pressure–volume (LVEDPVR) relation, and echocardiographic measurements are merely non-invasive approaches. Thus, invasive haemodynamic assessments, such as measuring left ventricular end-diastolic pressure (LVEDP) under exercise or hand-grip testing and right heart catheterization with or without exercise, can offer a more detailed understanding of diastolic function and provide valuable insights into the diagnosis of HFpEF [7]. Moreover, it is important to acknowledge the value of functional testing over plain coronary angiography, because non-obstructive coronary artery disease (CAD) is over-represented in HFpEF, placing more emphasis on coronary microvascular dysfunction [9, 14]. Cut-off values reflecting elevated LV filling pressures are summarized in Table 2, which also shows differences between ESC guidelines for the diagnosis and treatment of HF [10] and the American guidelines issued jointly by the American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) [15]. Whether the one or the other algorithm is superior in clinical practice is still a matter of clinical studies [16, 17].
Exercise testing, including cardiopulmonary exercise testing (CPET), allows for the assessment of functional capacity, ventilatory efficiency, and gas exchange abnormalities during exercise, which may help to identify exercise-induced elevations in pulmonary artery and LV filling pressures, particularly in patients with unexplained dyspnoea [18]. CPET can also be used to differentiate HFpEF from other causes of exercise intolerance, such as pulmonary diseases or deconditioning [19]. Finally, right heart catheterization at exercise, for a meaningful number of patients, allows for the direct measurement of LV filling pressures, pulmonary artery pressures, and cardiac output, providing valuable haemodynamic information to support the diagnosis of HFpEF [20].
Biomarkers
Circulating concentrations of the natriuretic peptides BNP and NT-proBNP are elevated in HFpEF populations, albeit to a lesser extent than in patients with HFrEF [21, 22]. A substantial number of patients with HFpEF present with mildly elevated natriuretic peptide levels located within a diagnostic grey zone and possible differences between men and women (Table 1). Comorbidities have significant influence on natriuretic peptide levels, particularly AF, CKD, and obesity [22]. Whilst AF and CKD are associated with increased natriuretic peptide concentrations, obesity associates with lower levels. In patients with a body mass index (BMI) ≥ 30 kg/m2, it has been suggested to reduce diagnostic natriuretic peptide cut-off levels by 50% [22]. ESC and American HF guidelines and recent consensus documents suggest the use of natriuretic peptides for diagnosing elevated filling pressures in HFpEF in the acute and non-acute settings as shown in Table 2 [10, 15, 22]. In the work-up of patients with suspected acute HF, the same cut-off levels as shown in Table 2 should be used [22]. Elevated BNP and NT-proBNP concentrations are integrated as major or minor criteria in the HFA-PEFF score (Fig. 2) using different cut-off levels for patients in sinus rhythm and AF.5 Of note, elevated natriuretic peptide concentrations have been used as inclusion criteria in the PARAGON, DELIVER, and EMPEROR-Preserved trials (see “SGLT2 inhibitors”) [23,24,25].
The performance of both biomarkers has been questioned in the diagnosis of HFpEF in outpatients with unexplained dyspnoea. Indeed, up to one-third of patients with HFpEF and an elevated pulmonary capillary wedge pressure (PCWP) at rest were found to display a BNP value below the diagnostic threshold [26]. In patients with exercise-induced dyspnoea assessed by CPET, one-third was diagnosed with HFpEF despite circulating NT-proBNP concentrations < 125 ng/l [27]. These patients had higher risk of HF events than patients without HFpEF and with normal NT-proBNP. From a pathophysiological perspective, a small ventricle size in a hypertrophic ventricle, a classical feature of HFpEF, counteracts increased wall stress, which is the main trigger for natriuretic peptide release. The imperfection of natriuretic peptides in the diagnosis of HFpEF is also reflected by not qualifying as a variable for the H2FPEF score [7]. In fact, the HFA-PEFF score may be consistent with a diagnosis of HFpEF, even when natriuretic peptide levels are below the given thresholds (Fig. 2) [5].
Imaging in HFpEF
Among the various imaging techniques, transthoracic echocardiography (TTE) is most valuable in providing information about cardiac structure and function such as LV hypertrophy, LV mass, and diastolic function [5, 10]. Tissue Doppler imaging (TDI) offers insight into diastolic dysfunction by measuring early diastolic relaxation velocity of the mitral annulus (e′). Speckle-tracking echocardiography (STE) enables assessment of myocardial strain and left atrial (LA) function [28]. These measurements aid estimating elevated LV end-diastolic pressure (LVEDP), using parameters such as the E/e′ ratio derived from TDI [28]. Whilst exercise echocardiography can help in certain cases by revealing exercise-induced changes in diastolic function that might not be apparent at rest, its widespread diagnostic use remains controversial with some studies highlighting its limitations in routine use and the limited additional value at increasing the sensitivity for diastolic dysfunction [5, 17].
Cardiac magnetic resonance (CMR) enables high-resolution imaging and detailed tissue characterization [30]. It allows accurate functional assessment, myocardial strain, and fibrosis imaging using techniques such as late gadolinium enhancement (LGE) and T1/T2 mapping. Advanced techniques like 4D flow permit visualization of intracardiac blood flow patterns, whilst parametric mapping methods like native T1 and extracellular volume (ECV) mapping reveal myocardial tissue properties [29, 30]. Tissue characterization by CMR can aid in excluding storage diseases like amyloidosis (see “Evaluating the presence of systemic disease”) [31] and can also help to exclude other potential causes of HF, such as infiltrative cardiomyopathies or myocarditis. Like TTE, CMR imaging is now possible during exercise in order to assess dynamic changes in ventricular function and perfusion during exercise [32]. It can also help in the assessment of elevated LVEDP through the evaluation of LA volumes and function, which are related to diastolic function and filling pressures [33]. Computed tomography (CT) can be useful for assessing cardiovascular (CV) anatomy and detecting coronary artery calcification and potential pulmonary embolism. However, its role in HFpEF is limited due to its low sensitivity for detecting subtle changes in cardiac function [10]. Finally, positron emission tomography (PET) may provide insight into myocardial perfusion, metabolism, and inflammatory burden. It may have a potential role in assessing HFpEF, especially in understanding its pathophysiology, but its clinical use remains limited due to cost and availability [34].
Clinical evaluation and risk assessment
Evaluating the presence of systemic disease
The majority of HFpEF cases are attributable to common CV risk factors and comorbidities like hypertension, DM, and obesity. Still, the possibility of non-CV systemic aetiology should be considered in situations outlined in Table 3, in which secondary HFpEF may be present [5]. Indeed, most systemic diseases have extracardiac symptoms, such as polyneuropathy in ATTR amyloidosis, macroglossia in AL amyloidosis, and stroke-like episodes or seizures in mitochondrial disease. Specific systemic diseases that may be present in HFpEF are summarized in Table 4. Comorbidities and secondary HFpEF as a result of systemic disease should be assessed using a structured approach as outlined in Fig. 3.
Cardiac amyloidosis is among the comparatively frequent systemic diseases in secondary HFpEF. It results from misfolded proteins that accumulate in various organs: light-chain proteins in AL amyloidosis [35] and transthyretin either in wild-type (ATTRwt) or hereditary (ATTRh) amyloidosis. Diagnostic extracardiac “red flags” in ATTR amyloidosis are bilateral carpal tunnel syndrome, ruptured biceps and tendons, lumbar spinal stenosis, and polyneuropathy, whereas macroglossia, nephrotic syndrome, and periorbital haematoma frequently occur with AL amyloidosis [36]. Cardiac involvement diagnosis is based on LV hypertrophy and typical strain patterns on TTE (see “Imaging in HFpEF”). If suspected, immediate blood testing for light-chain abnormalities should be performed, in addition to imaging with CMR or technetium-diphosphonate (DPD) scintigraphy, in cases of ATTR amyloidosis followed by genetic testing. Treatment of ATTR cardiomyopathy with tafamidis reduces disease progression and HF hospitalization in New York Heart Association classes I and II, whereas AL amyloidosis requires haematological treatment [10]. Fabry disease is a rare X-linked lysosomal storage disorder that manifests as hypertrophic cardiomyopathy, cryptogenic stroke, or end-stage renal disease. Extracardiac symptoms include angiokeratoma, vertigo, and gastrointestinal issues. Enzyme replacement therapy has demonstrated benefits. Finally, mitochondrial disorders [37], which are rare genetic diseases affecting organs reliant on mitochondrial oxidative metabolism, can lead to cardiomyopathy and/or conduction defects. The age of disease onset can aid in diagnosis, with many genetic disorders presenting early in life (Fig. 4).

Modified from Kubo et al. [154] ATTRwt, wild-type transthyretin amyloidosis; ATTRh, hereditary transthyretin amyloidosis; HNCM, hypertrophic non-obstructive cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy
Relative prevalence and HFpEF patients’ age at onset caused by systemic disease.
Diabetes mellitus
Patients with DM exhibit a 2–fourfold increase in the risk of developing HF [38,39,40,41], whose aetiology embraces coronary artery disease, arterial hypertension, direct or indirect effects of hyperglycaemia, and obesity-associated factors [42, 43]. Likewise, longer duration of DM, increased BMI, and CKD are associated with HF [44, 45]. A large European registry found that ~ 36% of all outpatients with HF have DM [46], with numbers rising to 50% in decompensated patients [47]. HFrEF and HFpEF are equally common in individuals with DM, but HFpEF is more likely to remain undetected [48,49,50]. In the Atherosclerosis Risk In the Community (ARIC) study, worsening dysglycaemia was associated with increased LV mass, worse diastolic function, and subtle reduction in LV systolic function. For every 1% increase in glycated haemoglobin, LV mass was higher by 3.0 g, E/e′ by 0.5, and global longitudinal strain by 0.3% suggesting dysglycaemia to contribute to subclinical impairment in cardiac structure and function [51]. It is important to acknowledge that the coexistence of DM and HF is associated with increased CV mortality [52, 53]. In patients with acute decompensated HF, the presence of DM is associated with increased intrahospital [47] and 1-year mortality [54] as well as rehospitalization rate [47]. These results were confirmed also in the EMPEROR-Preserved and DELIVER trials [24, 25]. Altogether, individuals with DM should be regularly assessed for the presence of typical HF signs and symptoms. If HF is suspected, additional diagnostic tests are recommended (Table 4). Vice versa, all patients with HF should be screened for the presence of DM.
Atrial fibrillation
According to the Framingham study, 55% of patients with HFrEF have AF during their lifetime; the corresponding number for HFpEF reaches 62%. Of these, 32% have AF before the diagnosis of HFpEF, in 18% the two diagnoses coincide, and 12% receive the diagnosis of AF after the diagnosis of HFpEF [55]. AF is independently associated with an increased risk of HF hospitalization and death in HFpEF and HFrEF, but the association in HFpEF is stronger [56]. The presence of AF is the most relevant indicator that HF symptoms (e.g. dyspnoea) are caused by HFpEF and therefore a prominent component in the H2FPEF algorithm (Fig. 2) [7]. The HFA-PEFF algorithm uses natriuretic peptide testing instead of AF [5]. The response to HF treatment with the SGLT2i empagliflozin [23] and dapagliflozin [57] is similar in patients with and without AF. In patients with clinically stable HF in the CABANA trial that predominantly recruited patients with HFpEF, treatment of AF pulmonary vein isolation (in comparison to drug therapy) was associated with clinically relevant improvements in survival, freedom from AF recurrence, and quality of life [58].
Chronic kidney disease and hyperkalaemia
CKD affects at least 40–50% of all patients with HF with somewhat higher prevalence in HFpEF than in HFrEF [59]. In the DELIVER trial, 49% of patients randomized to dapagliflozin or placebo had an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 [60]. In the EMPEROR-preserved trial, 53.5% of all patients had CKD, defined as eGFR < 60 ml/min/1.73 m2 or urine albumin to creatinine ratio > 300 mg/g [61]. The effect of CKD on mortality appears to be less pronounced in HFpEF than in HFrEF [59]. CKD itself has been associated with a higher incidence of HFpEF, as renal dysfunction can contribute to fluid overload and increased cardiac stress [62]. Hyperkalaemia has been associated with worse prognosis and an increased likelihood of suboptimal medical therapy, particularly with regard to inhibitors of the renin–angiotensin–aldosterone axis such as mineralocorticoid receptor antagonists (MRAs). Other predisposing risk factors of hyperkalaemia include DM, advanced age, CKD, and high baseline potassium values. The potassium binder patiromer has shown efficacy in patients with HF both in lowering potassium levels and in enabling MRA therapy [63], and subanalyses of large trials suggest similar effectiveness for sodium zirconium cyclosilicate [64], but no trial data are available specifically for patients with HFpEF and thus further investigations are needed.
Iron deficiency
The ESC guidelines for the management of HF define iron deficiency (ID) irrespective of the respective type as present when serum ferritin is < 100 ng/ml or when serum ferritin is of 100–299 ng/ml with transferrin saturation (TSAT) < 20%.10 Further, the guidelines recommend that “all patients with HF are periodically screened for anaemia and ID”. Both points are important because treatment studies showing the effectiveness of intravenous iron repletion with ferric carboxymaltose and ferric derisomaltose are available for patients with HFrEF and HFmrEF only. Using these criteria, several studies investigated ID in patients with HFpEF. A large meta-analysis published in 2019 including data of 1424 patients with HFpEF found a prevalence of ID of 59% [65]. Later studies either confirmed these data at a prevalence of 57.5% or found higher values at 75%. Overall, the prevalence of ID appears to be higher in patients with decompensated as compared to compensated HF [66, 67]. Since iron is crucially involved in erythropoiesis and present in all enzymes of the respiratory chain in myocardium and skeletal muscle, there is a high likelihood of ID having an impact on exercise capacity. Study results in patients with HFpEF and ID, however, have been inconsistent: ID was associated with lower peak oxygen consumption in three of four studies, with lower 6-min walking test distance in two of three studies and with lower quality of life in two studies [65]. Barandiarán Aizpurua et al. [68] found a significant association between ID and reduced exercise capacity, but this was lost after multivariable adjustment. Using data from 300 patients with HFpEF, the latter study found that patients with ID have worse prognosis with a higher likelihood of reaching the combined endpoint of all-cause mortality or hospitalization for HF after 4 years of follow-up. The results of the double-blind, randomized FAIR-HFpEF trial are currently awaited [69]. Retrospective analyses suggest functional improvement after application of ferric carboxymaltose [70].
Valvular heart disease
Valvular heart disease is common in HFpEF. Functional tricuspid regurgitation (TR) affects 20–50%, [4, 71, 72] functional mitral regurgitation (MR) up to 20% of all patients with HFpEF [48, 73]. Isolated functional TR refers to atrial origin and is often associated with AF [74]. Patients with severe TR in HF experience symptoms such as dyspnoea and venous congestion [74], and TR has been identified as an independent predictor of adverse outcomes and mortality [75, 76]. Transcatheter tricuspid valve intervention (TTVI) has gained attention recently as a safer alternative to isolated tricuspid valve surgery [10, 77, 78]. Studies have shown the safety and positive impact of TTVI on quality of life, hospitalization, and survival, especially in HFpEF [78,79,80,81]. Ongoing trials aim to validate this new therapy. Similarly, functional MR is associated with increased cardiovascular morbidity and mortality and is often caused by atrial distention [73]. Early studies and registry analyses suggest that transcatheter mitral valve interventions can be safe and provide functional, clinical, and symptomatic benefits, but this needs confirmation through randomized controlled trials [82].
Wasting and frailty
Both cachexia and sarcopenia are prevalent conditions in patients with HF and can increase the risk of developing frailty [83]. Cachexia refers to the involuntary weight loss that occurs in the presence of chronic illnesses like HF, whilst sarcopenia specifically refers to the loss of skeletal muscle mass and impaired skeletal muscle function [84]. These terms, occasionally accompanied by the loss of bone mineral density [85], collectively fall under the category of body wasting. Sarcopenia may, but does not necessarily, precede cachexia [86]. A recent meta-analysis has shown that sarcopenia is more prevalent in patients with HFrEF than HFpEF (28 vs. 18%) and has an overall higher prevalence among patients HF in Asia (35%) than in Europe (31%) or the Americas (25%) [87]. In patients with HFpEF, sarcopenia is an independent predictor of death [88]; however, this association may be less pronounced in HFpEF than in HFrEF [89]. Clinical implications of sarcopenia in HFpEF embrace lower 6-min walking distance, lower peak oxygen consumption on spiroergometry, and lower quality of life [90]. Cytokine expression patterns contributing to sarcopenia development appear to differ between HFrEF and HFpEF [91]. Finally, according to a recent ESC position paper [92], frailty is defined as “a multidimensional dynamic state, independent of age, that makes the individual with HF more vulnerable to the effect of stressors”. It affects up to 45% of all patients with HF with body wasting being one of the chief contributing factors to become frail [10]. Multidisciplinary interventions including exercise training and nutritional support might be beneficial for affected patients [93, 94].
Treatment
The treatment of HFpEF requires a holistic approach that identifies potentially treatable comorbidities as discussed above and in Fig. 3. Apart from pharmacological treatment discussed below and in Table 3, patient counseling encompasses a holistic approach addressing diet, physical activity, and psychological well-being [95, 96]. Dietary recommendations often focus on sodium restriction to manage fluid retention and optimize blood pressure control. Engaging in regular, tailored physical activity is encouraged, emphasizing activities suitable for the individual’s fitness level. A multidisciplinary approach involving psychology is essential, recognizing the emotional impact of HF. Counseling may address stress management, coping strategies, and fostering a positive mindset to enhance overall quality of life.
ACEi, ARBs, and ARNI
Interpretation of the results of trials blocking the renin-angiotensin-system (RAS) differs significantly between ESC and American HF guidelines (Table 3). Whilst in HFmrEF, there is general agreement that RAS blockade is indicated based on subanalyses of large randomized trials, recommendations for RAS blockade in HFpEF are currently provided in the American HF guidelines only [15]. None of the trials in HFpEF with the angiotensin-converting enzyme inhibitor (ACEi) perindopril, or the angiotensin-receptor blockers (ARBs) candesartan and irbesartan, met its primary endpoint [97,98,99]. In case of candesartan, a retrospective analysis of the CHARM program revealed that this ARB reduces outcomes only in HFrEF and HFmrEF, but not HFpEF [100]. Nevertheless, many patients with HFpEF are treated with these drugs for the management of comorbidities such as hypertension and CKD.
In the PARAGON trial in patients with an LVEF > 45%, the angiotensin-receptor neprilysin inhibitor (ARNI) sacubitril/valsartan did not significantly reduce the primary endpoint of CV death and total HF hospitalizations [23]. It is interesting to acknowledge a number of secondary analyses that provided valuable insight into subgroups of patients. One subanalysis of the PARAGON-HF trial suggested that women with HFpEF are more likely to benefit from ARNI treatment than men [101]. In both sexes, there seemed to be effectiveness in HFmrEF, and higher LVEF values were not associated with benefit when compared to valsartan. However, in a pre-specified participant-level pooled analysis of the PARAGLIDE-HF and PARAGON-HF trials in patients with LVEF > 45% and a recent HF decompensation, sacubitril/valsartan reduced the risk of worsening HF and CV death significantly and quite early after randomization [102]. Benefits of sacubitril/valsartan were more pronounced in patients with LVEF ≤ 60% (p for interaction = 0.021). Thus, ARNI treatment may be of value in HFpEF patients with LVEF ≤ 60% and a recent decompensation [103]. Similarly, the STRONG-HF trial demonstrated that after a hospitalization for acute HF rapid uptitration of oral medications including ACEi/ARB/ARNI and close follow-up did reduce mortality and HF rehospitalization as well as quality of life independent of LVEF [104]. These results suggest that patients with HFpEF should be treated with RAS blockade at least after a worsening HF event.
Beta-blockers
Βeta-blockers are widely prescribed in patients with HFpEF, although evidence for beneficial effects in these patients is poor. In a large cohort of 19,083 patients with HFpEF from the Swedish HF registry, 83% were on β-blockers [105]. This may in part be related to comorbidities such as AF, hypertension, or previous myocardial infarction (MI). However, β-blockers are no longer first choice for hypertension, and in this cohort AF and post-MI prevalence was lower than 50% and 30%, respectively. Therefore, many patients may receive β-blockers primarily under the intention to treat HFpEF, driven partially by the pathophysiological concept that LV filling may be improved by longer diastole at lower heart rates. On the contrary, myocardial relaxation is supported by sympathetic tone-mediated cyclic AMP, which is decreased by β-blockers and prolonged diastolic filling results in increased LV end-diastolic volume and wall stress. Moreover, a significant number of patients with HFpEF suffer from chronotropic incompetence, which may worsen further under β-blocker treatment [106].
In the SENIORS trial [107], nebivolol showed a reduction of combined all-cause mortality or CV hospitalization in patients with HFrEF, HFmrEF, and HFpEF with no differences between groups. In registries or secondary analyses from randomized trials, however, results are not consistent. A propensity score–matched cohort study using the Swedish Heart Failure Registry showed a statistically significant, numerically larger survival of 45 compared to 42% after 5 years of β-blocker treatment in HFpEF patients (HR of 0.93, p = 0.04) [105]. However, survival free from HF hospitalization was not different between both groups suggesting that HF hospitalization may at best not improve with β-blockers. This would be consistent with a recent secondary analysis of the TOPCAT trial [108]. Here, β-blocker use was associated with a higher risk of HF hospitalization among patients with HFpEF and a LVEF ≥ 50% (HR 1.74, p < 0.01). Thus, without further randomized data to treat or not to treat HFpEF patients with β-blockers is an individual decision. There is no recommendation to treat HFpEF patients with β-blockers and it seems appropriate to stop β-blocker treatment to check for symptomatic improvement and increased exercise capacity [107].
MRAs
Activation of the mineralocorticoid receptor (MR) in CV cells such as cardiomyocytes, endothelial, and vascular smooth muscle cells as well as myeloid cells is a well-described phenomenon contributing to the pathophysiology of HF independent of LVEF [109, 110]. In addition to the data in HFrEF where MR antagonists (MRA) have a class IA indication according to all HF guidelines, numerous pre-clinical and clinical studies have demonstrated beneficial effects of MRAs in HFpEF and HFmrEF [110]. The class IIB recommendation for use of spironolactone in ESC and American guidelines is based on a post hoc analysis of the TOPCAT trial (spironolactone in HF with LVEF ≥ 45%), which suggested that in a subgroup of patients with LVEF 44–49%, spironolactone reduced the risk of the primary endpoint (CV death, HF hospitalization, or resuscitated sudden death), mostly due to a reduction in CV mortality with spironolactone [111].
In patients with HFpEF, European and American HF guidelines diverge [112]: Whilst the latter assigns a IIB recommendation to spironolactone for selected patients with HFpEF, particularly those at the lower end of the LVEF spectrum, to decrease hospitalizations, such statement has not been included in the 2021 ESC guideline. Support for the recommendation for spironolactone in HFpEF comes from subanalyses of the TOPCAT trial: Particularly in American patients, spironolactone reduced the primary endpoint [113]. Moreover, patients included on the basis of elevated natriuretic peptide levels at baseline as a clear indictor for manifest HF did benefit from the use of spironolactone [114]. To further support the use of spironolactone in HFpEF, it needs to be acknowledged that the drug exerts beneficial effects on several comorbidities such as hypertension, LV hypertrophy, CV fibrosis, fluid retention, right HF, (re)occurrence of AF, and the metabolic syndrome [110]. Three randomized controlled trials with MRAs are currently ongoing in patients with HFpEF and HFmrEF, including SPIRIT-HF (EudraCT 2017–000697-11) and SPIRRIT (NCT02901184) using spironolactone as well as FINEARTS investigating the novel non-steroidal MRA finerenone against placebo.
SGLT2 inhibitors
SGLT2i have emerged as a novel treatment option for patients with HF, irrespective of underlying subtype [24, 25, 115,116,117,118,119,120,121,122]. In patients with HFpEF, both dapagliflozin and empagliflozin have shown beneficial effects on CV mortality and HF hospitalization in EMPEROR-Preserved and DELIVER, [24, 25] yielding a class I A recommendation in the 2023 focused update of the 2021 ESC HF guidelines [123]. Further prognostic benefits on the progression of CKD were shown in the EMPA-KIDNEY and DAPA-CKD trials [124, 125]. Of note, in all studies, the impact on CV hospitalization rates outperformed mortality–lowering benefits of SGLT2i.
SGLT2i act through inhibition of the SGLT2 in the proximal renal tubulus leading to increased glucosuria and diuresis [120, 121, 125], effects described in patients with chronic stable and acute decompensated HF [24, 25, 115,116,117,118,119,120,121, 126,127,128]. SGLT2i do not increase sympathetic or neurohumoral drive in patients with HF but do enhance effects of loop diuretics [121]. A definite cardiac mechanism of action remains elusive; peripheral effects have been linked to increased glucosuria, improved diuretic responsiveness, and renal protection [121, 129, 130]. Indeed, SGLT2i induce glucosuria associated with a caloric deficit of 250–300 kilocalories per day [131]. This process increases cardiac free fatty acid oxidation and circulating ketone body levels including beta-hydroxybutyrate, an alternative energy source that can be utilized by cardiomyocytes [132]. Whilst other hypotheses have been proposed, enhanced ketone body metabolism under SGLT2i may in part explain the beneficial effects of SGLTi in HF through a shift in cardiac energy substrates utilization [131]. Of note, whilst initial evidence from animal studies and patient cohorts supported this hypothesis, a recent randomized trial in subjects with HF showed that 12 weeks of treatment with empagliflozin 10 mg did not affect cardiac energy metabolism assessed by NMR and did not result in changes in circulating metabolites compared to placebo [133]. Thus, the beneficial cardiac effects of SGLT2i might be mediated through secondary effects without metabolic mediators.
Device therapy
Elevated LA pressure during rest or exercise is considered a key pathophysiological feature of HFpEF. Elevated LA pressure results in increased PCWP and pulmonary venous hypertension. It has been shown that elevated PCWP during rest or exercise is inversely related to exercise performance136 and is correlated with mortality [135, 136]. Accordingly, reducing PCWP in HFpEF is a potential therapeutic target that may be achieved by diuretics and vasodilators, alternatively by a shunt from the left to the right atrium (RA). A number of devices and procedures have been developed to create a left to right shunt. The overall efficacy of the most recent REDUCE LAP-HF II Study was neutral [137]. Analysis of treatment on total HF events showed differential effects in pre-specified subgroups and identified pulmonary vascular function during exercise as the primary determinant for beneficial or harmful effects of the shunt. In a subsequent systematic post hoc statistical analysis, patients with normal exercise pulmonary vascular resistance (PVR ≤ 1.74 WU) without a pacemaker derived a significant clinical benefit from the shunt with a 55% reduction in the rate of HF events. The Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score showed a 5.5-point higher increase from baseline in shunt patients, with 40% of shunt patients showing more than a 20-point increase [138]. Accordingly, the currently ongoing confirmatory Responder-HF study is a randomized, multicenter, double-blind, sham-controlled trial to evaluate clinical outcome in patients corresponding to the Responder cohort identified from the post hoc analysis of the REDUCE LAP-HF II study.
Acute heart failure in patients with HFpEF
Acute HF is defined as a rapid or gradual worsening or onset of the HF syndrome urging patients to pursue medical attention [10]. Patients are referred to an emergency medical department or seek outpatient support. The prognosis of acute decompensated HFpEF and HFrEF is comparable [139]. Haemodynamic congestion, as shown with measurements of the diameter of the inferior vena cava and LA area, is comparable in acute decompensated HFpEF and HFrEF patients [140].
The recommended diagnostic work-up for new onset acute HF in HFpEF and HFrEF patients is similar [10] and outlined in Table 5. The basic therapeutic approach to acute HF in patients with HFpEF and HFrEF overlaps grossly and includes the administration of loop diuretics, vasodilators, inotropes, vasopressors, renal replacement therapy, and mechanical support as needed and where appropriate [10]. In the recently published ADVOR trial, acetazolamide was associated with a higher diuretic response and shortened length of hospital stay independent of LVEF [141]. In the ROPA-Dop trial, low-dose dopamine did not improve renal function in patients hospitalized for acute HFpEF, and continuous intravenous loop diuretic treatment was associated with worsening renal function compared to intermittent delivery [142]. Ultrafiltration was not effective in acute HF independent of LVEF. In fact, in those patients with an LVEF > 40%, ultrafiltration resulted in worsened renal function [143]. So far, no specific therapeutic approach has exclusively been established for acute decompensated HFpEF. The rehospitalization rate is higher in patients with previous HFpEF-related hospitalization and comparable to HFrEF patients with no previous HF-related hospitalization [139]. Interestingly, an SGLT2i was recently shown to reduce CV mortality and worsening HF in patients with and without previous HF hospitalization [144]. Beneficial effects of SGLT2i including enhanced diuresis and improved diuretic efficiency in subjects with acute decompensated HF seem to be independent of the underlying form of cardiomyopathy and include patients with and without preserved LVEF [127, 145, 146].
Outlook
It is important to note that classification of HF according to LVEF is purely arbitrary and in its present form not based on physiological or clinical data. Historically, HFrEF was defined as LVEF < 35% that was later changed to < 40% to allow facilitated inclusion into clinical trials. Recently, two studies showed different behaviors of patients with HF symptoms [147, 148]. Patients with an LVEF > 60% showed a left shift of the pressure–volume relationship representing a form of contracture [148]. In contrast, patients with lower LVEF values < 60% shifted to the right with higher volumes at similar filling pressures representing the features of HFrEF.150,151 Therefore, another classification based on these pathophysiological studies has recently been proposed [149].
Given the complex pathophysiology and clinical presentation of HFpEF, the future of diagnosing and treating HFpEF lies in integrated approaches, combining data from multiple clinical and imaging modalities to provide a holistic view of the condition. The incorporation of artificial intelligence (AI) and machine learning in HFpEF imaging has the potential to revolutionize the field. AI-driven algorithms can help in identifying novel imaging biomarkers specific to HFpEF by analyzing large datasets from various imaging modalities [150, 151]. These advanced techniques can facilitate the automatic segmentation and quantification of cardiac structures, reducing the time and effort required for manual image analysis. AI can also be used to enhance the interpretation of echocardiographic and CMR images, leading to better diagnostic accuracy and more comprehensive risk assessment [152, 153]. These approaches might also inform about novel and individualized treatments of this heterogenous syndrome.
Conclusions
The clinical diagnosis of HFpEF is a multifaceted process that relies on the integration of predisposing risk factors, clinical signs and symptoms, biomarkers, imaging findings, and exercise testing including CPET. The diagnosis HFpEF should not be rejected on the basis of normal natriuretic peptides levels in patients with unexplained dyspnoea, because this will lead to missed diagnoses and delayed treatments. The HFA-PEFF algorithm offers a practical and structured approach to aid clinicians in this diagnostic process. Accurate diagnosis is essential to guide management and treatment strategies, ultimately improving patient outcomes. Besides, it remains important to accurately identify and treat comorbidities contributing to the HFpEF syndrome. Advances in modern imaging techniques and the development of future tools hold great promise for improving the diagnosis and management of HFpEF. As our understanding of HFpEF evolves, so too will our ability to harness these cutting-edge technologies to benefit patients and optimize care.
Abbreviations
- ACC:
-
American College of Cardiology
- ACEi:
-
Angiotensin converting enzyme inhibitor
- AF:
-
Atrial fibrillation
- AHA:
-
American Heart Association
- AI:
-
Artificial intelligence
- ARB:
-
Angiotensin-receptor blocker
- ARIC:
-
Atherosclerosis Risk In the Community
- ARNI:
-
Angiotensin-receptor neprilysin inhibitor
- ATTRh:
-
Hereditary transthyretin amyloidosis
- ATTRwt:
-
Wild-type transthyretin amyloidosis
- BMI:
-
Body mass index
- BNP:
-
B-type natriuretic peptide
- CAD:
-
Coronary artery disease
- CKD:
-
Chronic kidney disease
- CMR:
-
Cardiovascular magnetic resonance
- COPD:
-
Chronic obstructive pulmonary disease
- CPET:
-
Cardiopulmonary exercise test
- CT:
-
Computed tomography
- CV:
-
Cardiovascular
- DM:
-
Diabetes mellitus
- DPD:
-
Diphosphonate scintigraphy
- ECV:
-
Extracellular volume
- eGFR:
-
Estimated glomerular filtration rate
- ESC:
-
European Society of Cardiology
- HF:
-
Heart failure
- HFmrEF:
-
Heart failure with mildly reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFSA:
-
Heart Failure Society of America
- HFrEF:
-
Heart failure with reduced ejection fraction
- HNCM:
-
Hypertrophic non-obstructive cardiomyopathy
- HOCM:
-
Hypertrophic obstructive cardiomyopathy
- ID:
-
Iron deficiency
- LA:
-
Left atrial
- LAVI:
-
Left atrial volume index
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle/ventricular
- LVEDP:
-
Left ventricular end-diastolic pressure
- LVEF:
-
Left ventricular ejection fraction
- MR:
-
Mitral regurgitation
- MRA:
-
Mineralocorticoid-receptors antagonist
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- PCWP:
-
Pulmonary capillary wedge pressure
- PET:
-
Positron emission tomography
- PVI:
-
Pulmonary vein isolation
- SGLT2:
-
Sodium glucose transporter 2
- STE:
-
Speckle-tracking echocardiography
- TDI:
-
Tissue Doppler imaging
- TR:
-
Tricuspid regurgitation
- TTE:
-
Transthoracic echocardiography
- TTVI:
-
Transcatheter tricuspid valve intervention
References
Ferrari R, Balla C, Fucili A. (2016) Heart failure: an historical perspective. Eur Heart J Suppl 18(Supplement G). G3–G10
Zile MR, Brutsaert DL (2002) New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 5:1387–1393
Roh J, Houstis N, Rosenzweig A (2017) Why don’t we have proven treatments for HFpEF? Circ Res 120:1243–1245
Anker SD, Usman MS, Anker MS, Butler J, Böhm M, Abraham WT et al (2023) Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association (HFA) and the European Heart Rhythm Association (EHRA) of the ESC, and the European Society of Hypertension (ESH). Eur J Heart Fail 25:936–955
Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E et al (2019) How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 40:3297–3317
Hashemi D, Mende M, Trippel TD, Petutschnigg J, Hasenfuss G, Nolte K et al (2022) Evaluation of the HFA-PEFF Score: results from the prospective DIAST-CHF cohort. ESC Heart Fail 9:4120–4128
Reddy NV, Carter RE, Obokata M, Redfield MM, Borlaug BA (2018) A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138:861–870
Verwerft J, Soens L, Wynants J, Meysman M, Jogani S, Plein D et al (2023) Heart failure with preserved ejection fraction: relevance of a dedicated dyspnoea clinic. Eur Heart J 44:1544–1556
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H et al (2021) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726
Maisel AS, Daniels LB (2012) Breathing not properly 10 years later: what we have learned and what we still need to learn. J Am Coll Cardiol 60:277–282
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314
Rosch S, Kresoja KP, Besler C, Fengler K, Schöber AR, von Roeder M, Lücke C, Gutberlet M, Klingel K, Thiele H, Rommel KP, Lurz P (2022) Characteristics of heart failure with preserved ejection fraction across the range of left ventricular ejection fraction. Circulation 146:506–518
Dryer K, Gajjar M, Narang N, Lee M, Paul J, Shah AP, Nathan S, Butler J, Davidson CJ, Fearon WF, Shah SJ, Blair JEA (2018) Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 314:H1033–H1042
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM et al (2022) AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145:e895–e1032
Reddy YNV, Kaye DM, Handoko ML, van de Bovenkamp AA, Tedford RJ, Keck C et al (2022) Diagnosis of heart failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol 7:891–899
Lanzarone E, Baratto C, Vicenzi M, Villella F, Rota I, Dewachter C et al (2023) Haemodynamic validation of the three-step HFA-PEFF algorithm to diagnose heart failure with preserved ejection fraction. ESC Heart Fail 10:2588–2595
Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R (2017) Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol 70:1618–1636
Guazzi M, Wilhelm M, Halle M, Van Craenenbroeck E, Kemps H, de Boer RA et al (2022) Exercise testing in heart failure with preserved ejection fraction: an appraisal through diagnosis, pathophysiology and therapy - a clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Heart Fail 24:1327–1345
Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM (2010) Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3:588–595
Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P et al (2002) Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol 41:2010–17
Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N et al (2019) Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 21:715–731
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP et al (2019) Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 381:1609–1620
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M et al (2012) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461
Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF et al (2022) Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 387:1089–1098
Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M et al (2012) Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol 110:870–876
Verbrugge FH, Omote K, Reddy YNV, Sorimachi H, Obokata M, Borlaug BA (2022) Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur Heart J 43:1941–1951
Telles F, Marwick TH (2018) Imaging and management of heart failure and preserved ejection fraction. Curr Treat Options Cardiovasc Med 20:90
Quarta G, Gori M, Iorio A, D’Elia E, Moon JC, Iacovoni A et al (2020) Cardiac magnetic resonance in heart failure with preserved ejection fraction: myocyte, interstitium, microvascular, and metabolic abnormalities. Eur J Heart Fail 22:1065–1075
Ashkir Z, Myerson S, Neubauer S, Carlhäll CJ, Ebbers T, Raman B (2022) Four-dimensional flow cardiac magnetic resonance assessment of left ventricular diastolic function. Front Cardiovasc Med 9:866131
Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD et al (2010) Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 3:155–164
Backhaus SJ, Lange T, George EF, Hellenkamp K, Gertz RJ, Billing M et al (2021) Exercise stress real-time cardiac magnetic resonance imaging for noninvasive characterization of heart failure with preserved ejection fraction: the HFpEF-Stress Trial. Circulation 143:1484–1498
Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A et al (2016) Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging 9:e003754
Aqueti VR, Shah AM, Everett BM, Pradhan AD, Piazza G, Bibbo C et al (2023) Coronary flow reserve, inflammation, and myocardial strain: the CIRT-CFR Trial. JACC Basic Transl Sci 8:141–151
Wechalekar AD, Fontana M, Quarta CC, Liedtke M (2022) AL amyloidosis for cardiologists: awareness, diagnosis, and future prospects: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 15:427–441
Griffin JM, Rosenthal JL, Grodin JL, Maurer MS, Grogan M, Cheng RK (2021) ATTR amyloidosis: current and emerging management strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 3:488–505
Popoiu TA, Dudek J, Maack C, Bertero E (2023) Cardiac involvement in mitochondrial disorders. Curr Heart Fail Rep 20:76–87
Kannel WB, McGee DL. (1979) Diabetes and cardiovascular disease. The Framingham study. JAMA. 1079;241:2035–38
van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA (2010) Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care 33:2084–2089
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB (2004) The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27:1879–1884
Sattar N, McMurray J, Borén J, Rawshani A, Omerovic E, Berg N et al (2023) Twenty years of cardiovascular complications and risk factors in patients with type 2 diabetes: a nationwide Swedish cohort study. Circulation 147:1872–1886
Seferović PM, Paulus WJ (2015) Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36(1718–27):27a–27c
Sattar N, McGuire DK (2018) Pathways to cardiorenal complications in type 2 diabetes mellitus: a need to rethink. Circulation 138:7–9
Nichols GA, Hillier TA, Erbey JR, Brown JB (2001) Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care 24:1614–1619
Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr (2004) Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 27:699–703
Dauriz M, Targher G, Laroche C, Temporelli PL, Ferrari R, Anker S et al (2017) Association between diabetes and 1-year adverse clinical outcomes in a multinational cohort of ambulatory patients with chronic heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Diabetes Care 40:671–678
Targher G, Dauriz M, Laroche C, Temporelli PL, Hassanein M, Seferovic PM, Drozdz J et al (2017) In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur J Heart Fail 19:54–65
Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP et al (2017) Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 19:1574–1585
Boonman-de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Liem AH, Rutten GE et al (2012) High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 55:2154–2162
Obokata M, Sorimachi H, Harada T, Kagami K, Saito Y, Ishii H (2023) Epidemiology, pathophysiology, diagnosis, and therapy of heart failure with preserved ejection fraction in Japan. J Card Fail 29:375–388
Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J et al (2015) Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail 8:448–454
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR et al (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371:993–1004
MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB et al (2008) Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 29:1377–1385
Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F et al (2016) European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 18:613–625
Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA et al (2016) Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 133:484–492
Patel RB, Greene SJ, Xu H, Alhanti B, Peterson P, Yancy CW, Piccini J et al (2023) Intersection of atrial fibrillation and heart failure with mildly reduced and preserved ejection fraction in >400 000 participants in the Get With The Guidelines-Heart Failure Registry. Eur J Heart Fail 25:63–73
Butt JH, Kondo T, Jhund PS, Comin-Colet J, de Boer RA, Desai AS et al (2022) Atrial fibrillation and dapagliflozin efficacy in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol 80:1705–1717
Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA et al (2021) Ablation versus drug therapy for atrial fibrillation in heart failure. Results from the CABANA Trial Circulation 143:1377–1390
Löfman ISzummer K, Dahlström U, Jernberg T, Lund LH. (2017) Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail 19:1606–1614
Mc Causland FR (2023) Dapagliflozin and kidney outcomes in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified analysis of the DELIVER randomized clinical trial. JAMA Cardiol 8:56–65
Sharma A, Ferreira JP, Zannad F, Pocock SJ, Filippatos G, Pfarr E et al (2023) Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from the EMPEROR-Preserved trial. Eur J Heart Fail 25:1337–1348
Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL (2014) Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 35:455–469
Butler J, Anker SD, Lund LH, Coats AJS, Filippatos G, Siddiqi TJ et al (2022) Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J 43:4362–4373
Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G et al (2015) Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 17:1050–1056
Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D (2019) Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Open Heart 6:e001012
Cohen-Solal A, Philip JL, Picard F, Delarche N, Taldir G, Gzara H et al (2022) Iron deficiency in heart failure patients: the French CARENFER prospective study. ESC Heart Fail 9:874–884
Rocha BML, Cunha GJL, Menezes Falcão LF (2018) The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol 71:782–793
Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP, Henkens MTHM, Weerts J, Spanjers MHA et al (2021) Iron deficiency impacts prognosis but less exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail 8:1304–1313
von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD (2015) Iron deficiency and cardiovascular disease. Nat Rev Cardiol 12:659–669
López-Vilella R, Lozano-Edo S, Arenas Martín P, Jover-Pastor P, Ezzitouny M, Sorolla Romero J et al (2022) Impact of intravenous ferric carboxymaltose on heart failure with preserved and reduced ejection fraction. ESC Heart Fail 9:133–145
Benfari G, Antoine C, Miller WL, Thapa P, Topilsky Y, Rossi A et al (2019) Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation 140:196–206
Mascherbauer J, Kammerlander AA, Zotter-Tufaro C, Aschauer S, Duca F, Dalos D et al (2017) Presence of ´isolated´ tricuspid regurgitation should prompt the suspicion of heart failure with preserved ejection fraction. PLoS ONE 12:e0171542
Zoghbi WA, Levine RA, Flachskampf F, Grayburn P, Gillam L, Leipsic J et al (2022) Atrial functional mitral regurgitation: a JACC: cardiovascular imaging expert panel viewpoint. J Am Coll Cardiol Img 15:1870–1882
Adamo M, Pagnesi M, Mebazaa A, Davison B, Edwards C, Tomasoni D et al (2023) NT-proBNP and high-intensity care for acute heart failure: the STRONG-HF trial. Eur Heart J 44:2947–2962
Topilsky Y, Inojosa JM, Benfari G, Vaturi O, Maltais S, Michelena H et al (2018) Clinical presentation and outcome of tricuspid regurgitation in patients with systolic dysfunction. Eur Heart J 39:3584–3592
Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J et al (2018) Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging 12:433–442
Taramasso M, Alessandrini H, Latib A, Asami M, Attinger-Toller A, Biasco L et al (2019) Outcomes after current transcatheter tricuspid valve intervention. JACC Cardiovasc Interv 12:155–165
Sorajja P, Whisenant B, Hamid N, Naik H, Makkar R, Tadros P et al (2023) Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med 388:1833–1842
Mangieri A, Pagnesi M, Regazzoli D, Laricchia A, Ho E, Goldberg Y et al (2020) Future perspectives in percutaneous treatment of tricuspid regurgitation. Front Cardiovasc Med 7:581211
Cai S, Bowers N, Dhoot A, Ho EC, Ong G, Eckstein J et al (2020) Natural history of severe tricuspid regurgitation: outcomes after transcatheter tricuspid valve intervention compared to medical therapy. Int J Cardiol 320:49–54
Kresoja KP, Lauten A, Orban M, Rommel KP, Alushi B, Besler C et al (2020) Transcatheter tricuspid valve repair in the setting of heart failure with preserved or reduced left ventricular ejection fraction. Eur J Heart Fail 22:1817–1825
Gröger M, Scheffler JK, Schösser F, Schneider LM, Rottbauer W, Markovic S et al (2021) Percutaneous edge-to-edge mitral valve repair for mitral regurgitation improves heart failure symptoms in heart failure with preserved ejection fraction patients. ESC Heart Failure 8:5010–5021
Bielecka-Dabrowa A, Ebner N, Dos Santos MR, Ishida J, Hasenfuss G, von Haehling S (2020) Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur J Heart Fail 22:2314–2326
von Haehling EN, Dos Santos MR, Springer J, Anker SD (2017) Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 14:323–341
Loncar G, Garfias-Veitl T, Valentova M, Vatic M, Lainscak M, Obradović D, et al. (2023) Bone status in men with heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure. Eur J Heart Fail 25:714–723
von Haehling S (2015) The wasting continuum in heart failure: from sarcopenia to cachexia. Proc Nutr Soc 74:367–377
Chen R, Xu J, Wang Y, Jiang B, Xu X, Lan Y et al (2023) Prevalence of sarcopenia and its association with clinical outcomes in heart failure: an updated meta-analysis and systematic review. Clin Cardiol 46:260–268
Konishi M, Kagiyama N, Kamiya K, Saito H, Saito K, Ogasahara Y et al (2021) Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur J Prev Cardiol 28:1022–1029
von Haehling S, Garfias Macedo T, Valentova M, Anker MS, Ebner N, Bekfani T et al (2020) Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia Sarcopenia Muscle 11:1242–1249
Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L et al (2016) Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 222:41–46
Seiler M, Bowen TS, Rolim N, Dieterlen MT, Werner S, Hoshi T et al (2016) Skeletal muscle alterations are exacerbated in heart failure with reduced compared with preserved ejection fraction: mediated by circulating cytokines? Circ Heart Fail 9:e003027
Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD et al (2019) Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 21:1299–1305
Kinugas Y, Yamamoto K (2017) The challenge of frailty and sarcopenia in heart failure with preserved ejection fraction. Heart 103:184–189
von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N et al (2021) Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail 23:92–113
Agrinier N, Schockmel M, Thilly N, Laborde-Castérot H, Jourdain P, Alla F, Leclercq C, Dany F, Druelle J, Drouet E, Mulak G, Juillière Y, ODIN cohort participants (2018) Effectiveness of a patient education programme in heart failure with preserved ejection fraction: results from the ODIN cohort study using propensity score matching. Arch Cardiovasc Dis 111(1):5–16
von Haehling S, Birner C, Dworatzek E, Frantz S, Hellenkamp K, Israel CW, Kempf T, Klein HH, Knosalla C, Laufs U, Raake P, Wachter R, Hasenfuss G (2022) Travelling with heart failure: risk assessment and practical recommendations. Nat Rev Cardiol 19:302–313
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ et al (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 362:777–781
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR et al (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359:2456–2467
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J et al (2006) The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27:2338–2345
Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K et al (2018) Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 20:1230–1239
McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Rizkala AR, Sabarwal SV, Shah AM, Shah SJ, Shi VC, van Veldhuisen DJ, Zannad F, Zile MR, Cikes M, Goncalvesova E, Katova T, Kosztin A, Lelonek M, Sweitzer N, Vardeny O, Claggett B, Jhund PS, Solomon SD (2020) Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 141:338–351
Vaduganathan M, Mentz RJ, Claggett BL, Miao ZM, Kulac IJ, Ward JH, et al.(2023) Sacubitril/valsartan in heart failure with mildly reduced or pre-served ejection fraction: a pre-specified participant-level pooled analysis of PARAGLIDE-HF and PARAGON-HF. Eur Heart J 44:2982–2993
Green SJ, Fonarow GC (2023) Targeting sacubitril/valsartan for heart failure with mildly reduced or preserved ejection fraction. Eur Heart J 44:2994–2997
Pagnesi M, Metra M, Cohen-Solal A, Edwards C, Adamo M, Tomasoni D et al (2023) Uptitrating treatment after heart failure hospitalization across the spectrum of left ventricular ejection fraction. J Am Coll Cardiol 81:2131–2144
Lund L, Benson L, Dahlström U, Edner M, Friberg L (2014) Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 312:2008–2018
Wernhart S, Papathanasiou M, Rassaf T, Luedike P (2023) The controversial role of beta-blockers in heart failure with preserved ejection fraction. Pharmacol Ther 243:108356
Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J et al (2005) SENOIRS Investigators Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure. Eur Heart J 26:215–225
Silverman DN, Plante TB, Infeld M, Callas PW, Juraschek SP, Dougherty GB et al (2019) Association of β-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction A secondary analysis of the TOPCAT trial. JAMA Network Open 2:916598
Bauersachs J, Jaisser F, Toto R (2015) Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 65:257–263
Bauersachs J, López-Andrés N (2022) Mineralocorticoid receptor in cardiovascular diseases-clinical trials and mechanistic insights. Br J Pharmacol 179:3119–3134
Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK et al (2016) Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 37:455–462
Behnoush AH, Khalaji A, Naderi N, Ashraf H, von Haehling S (2023) ACC/AHA/HFSA 2022 and ESC 2021 guidelines on heart failure comparison. ESC Heart Fail 10:1531–1544
Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N et al (2015) Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 131:34–42
Girerd N, Ferreira JP, Rossignol P, Zannad F (2016) A tentative interpretation of the TOPCAT trial based on randomized evidence from the brain natriuretic peptide stratum analysis. Eur J Heart Fail 18:1411–1414
Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP et al (2021) Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation 143:337–349
Vaduganathan M, Claggett BL, Jhund P, de Boer RA, Hernandez AF, Inzucchi SE et al (2022) Time to clinical benefit of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified secondary analysis of the DELIVER randomized clinical trial. JAMA Cardiol 7:1259–1263
Packer M (2019) Lessons learned from the DAPA-HF trial concerning the mechanisms of benefit of SGLT2 inhibitors on heart failure events in the context of other large-scale trials nearing completion. Cardiovascular Diabetol 18:129
Cunningham JW, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS et al (2022) Dapagliflozin in patients recently hospitalized with heart failure and mildly reduced or preserved ejection fraction. J Am Coll Cardiol 80:1302–1310
Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ et al (2021) Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-Preserved trial. Circulation 144:1284–1294
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G et al (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396:819–829
Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J et al (2021) Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation 143:310–321
McMurray JJV, Docherty KF, Jhund PS (2020) Dapagliflozin in patients with heart failure and reduced ejection fraction. Reply N Engl J Med 382:973
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M et al (2023) 2023 Focused update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 44:3627–3639
Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR et al (2023) Empagliflozin in patients with chronic kidney disease. N Engl J Med 388:117–127
Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T Hou FF et al (2020) Dapagliflozin in patients with chronic kidney disease. New Engl J Med 383:1436–1446
Schulze PC, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S et al (2022) Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation 146:289–298
Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J et al (2022) The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 28:568–574
Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A et al (2020) Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail 22:713–722
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383:1413–1424
Hellenkamp K, Nolte K, von Haehling S (2022) Pharmacological treatment options for heart failure with reduced ejection fraction: a 2022 update. Expert Opin Pharmacother 23:73–680
Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E (2015) Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 38:1730–1735
Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ et al (2016) Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 133:706–716
Hundertmark MJ, Adler A, Antoniades C, Coleman R, Griffin JL, Holman RR et al (2023) Assessment of cardiac energy metabolism, function, and physiology in patients with heart failure taking empagliflozin: the randomized, controlled EMPA-VISION trial. Circulation 147:1654–1669
Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J et al (2018) Resting and exercise haemodynamics in relation to six-minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 20:715–722
Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT et al (2017) Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail 10:e003594
Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B et al (2014) Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 35:3103–3112
Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS et al (2022) Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet 399:1130–1140
Borlaug BA, Blair J, Bergmann MW, Bugger H, Burkhoff D, Bruch L et al (2022) Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation 145:1592–1604
Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S et al (2014) Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 7:590–595
Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F et al (2018) Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 20:738–747
Martens P, Dauw J, Verbrugge FH, Nijst P, Meekers E, Augusto SN Jr et al (2023) Decongestion with acetazolamide in acute decompensated heart failure across the spectrum of left ventricular ejection fraction: a prespecified analysis from the ADVOR trial. Circulation 147:201–211
Sharma K, Vaishnav J, Kalathiya R, Hu JR, Miller J, Shah N et al (2018) Randomized evaluation of heart failure with preserved ejection fraction patients with acute heart failure and dopamine: the ROPA-DOP trial. JACC Heart Failure 6:859–870
Fudim M, Brooksbank J, Giczewska A, Greene SJ, Grodin JL, Martens P et al (2020) Ultrafiltration in acute heart failure: implications of ejection fraction and early response to treatment from CARRESS-HF. J Am Heart Assoc 9:e015752
Cunningham JW, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS et al (2022) Dapagliflozin in patients recently hospitalized with heart failure and mildly reduced or preserved ejection fraction. J Am Coll Cardiol 80:1302–1310
Schulze PC, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S et al (2022) Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation 146:289–298
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK et al (2021) Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 384:117–128
Rosch S, Kresoja KP, Besler C, Fengler K, Schöber AR, von Roeder M et al (2022) Characteristics of heart failure with preserved ejection fraction across the range of left ventricular ejection fraction. Circulation 146:506–518
Popovic D, Alogna A, Omar M, Sorimachi H, Omote K, Reddy YNV et al (2023) Ventricular stiffening and chamber contracture in heart failure with higher ejection fraction. Eur J Heart Fail 25:657–668
Packer M (2023) A reclassification of heart failure based on recognition of heart failure with normal to supernormal ejection fraction, a clinically common form of cardiac contracture, with distinctive pathophysiological and therapeutic features. Eur J Heart Fail 25:669–672
Backhaus SJ, Schuster A, Lange T, Stehning C, Billing M, Lotz J et al (2021) Impact of fully automated assessment on interstudy reproducibility of biventricular volumes and function in cardiac magnetic resonance imaging. Sci Rep 11:11648
Huellebrand M, Ivantsits M, Tautz L, Kelle S, Hennemuth A (2022) A collaborative approach for the development and application of machine learning solutions for CMR-based cardiac disease classification. Front Cardiovasc Med 9:829512
Seetharam K, Raina S, Sengupta pp. (2020) The role of artificial intelligence in echocardiography. Curr Cardiol Rep 22:99
Kutty S (2020) The 21st Annual Feigenbaum Lecture: beyond artificial: echocardiography from elegant images to analytic intelligence. J Am Soc Echocardiogr 33:1163–1171
Kubo T, Kitaoka H (2017) Imaging of left ventricular hypertrophy: a practical utility for differential diagnosis and assessment of disease severity. Curr Cardiol Rep 19:65
Acknowledgements
The present manuscript is the result of a cooperation under the umbrella of the Study Group 10 (heart failure) of the German Cardiac Society. The authors are members of the German Cardiac Society and assembled at the University of Göttingen in January 2023 to critically discuss the current state-of-the-art and gaps in evidence of HFpEF. These meetings were kindly supported by unrestricted grants from AstraZeneca, Bayer, Boehringer Ingelheim, Edwards Lifesciences, Pharmacosmos, and Vifor. Finally, we thank Anja Janssen for excellent secretarial support in writing this article and we thank Eva Meyer-Besting for drawing the graphical abstract and part of the figures.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.v.H. has been a paid consultant for and/or received honoraria payments from Amomed, AstraZeneca, Bayer, Boehringer Ingelheim, BRAHMS, Edwards Lifesciences, MSD, Novo Nordisk, Novartis, Pfizer, Pharmacosmos, Respicardia, Roche, Servier, Sorin, and Vifor. S.v.H. reports research support from Amgen, AstraZeneca, Boehringer Ingelheim, Pharmacosmos, IMI, and the German Center for Cardiovascular Research (DZHK). B.A. has been a paid consultant for and/or received honoraria payments from Abbott, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Edwards Lifesciences, Novartis, Novonordisk, Pfizer, Vifor, and ZOLL. B.A. reports research support from the German Research Foundation (SFB 1213, project B09), and the German Innovations Funds (GBA). T.B. has been a paid consultant for and/or received honoraria payments from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Sanofi, Takeda, and Vifor. F.E. reports speaker and/or consultant honoraria from AstraZeneca, Novartis, Böhringer Ingelheim, Servier, Bayer, Merck, Vifor, Berlin Chemie, and PharmaCosmos. F.E. reports research grants from DFG, BMBF, DZHK, AstraZeneca, and Servier. D.H. has been the recipient of two grants issued by DZHK (Grant Number, 81X3100214 and Grant Number, 81X3100220). D.H. has been paid consultancy and speaking fees by several pharmaceutical companies, namely AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Vifor. D.H. is participant in the BIH Charité Digital Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH). K.H. received speaker and/or consultant honoraria from AstraZeneca, Bayer, BDI, Boehringer Ingelheim, and Novartis. K.H. received travel grants and/or congress fees from Abbott, Bayer, and EuroPCR. K.H. reports research support from AstraZeneca. T.K. has been a paid consultant for and/or received honoraria payments from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Edwards, Endotronix, Norgine, Novartis, Medtronic, Pharmacosmos, Roche Diagnostics, and Vifor. P.R. reports speaker and/or consultant honoraria from Edwards, Medtronic, Abbott, Biotronik, AstraZeneca, Novartis, Bayer, Vifor, Daiichi-Sankyo, CTI GmbH, Diaplan, Elisabeth-KH Essen, Conventus Congressmanagement Jena, BDI, CMI Medizinische Ausstellungs- und Werbegesellschaft Wien, Herzzentrum Leipzig, Leipzig Heart Institute GmbH, Kelcon, and Deutsche Gesellschaft für Kardiologie. P.R. reports research grants from Pfizer and Else-Kröner-Fresenius-Stiftung. K.A.S. has been a paid consultant for and/or received honoraria payments from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, MSD, Novo Nordisk, and Novartis. K.A.S. is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TRR 219; Project-ID 322900939 [C-07]). R.W. has been a paid consultant for and/or received honoraria payments from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, CVRx, Daiichi, Medtronic, Novartis, Pfizer, Pharmacosmos, and Servier. R.W. reports research support from Boehringer Ingelheim, Bundesministerium für Bildung und Forschung, Deutsche Forschungsgemeinschaft, European Union, Medtronic, and the German Center for Cardiovascular Research (DZHK). P.C.S. received honoraria for lectures/consulting from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, BMS, Abiomed, Pharmacosmos, and Amgen not related to this article. P.C.S. reports research support for the department from Boehringer Ingelheim, Edwards, and Abiomed not related to this article. G.H. has been a paid consultant for and/or received honoraria payments from AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Corvia, Impulse Dynamics, Novartis, Pfizer, Servier, Springer, G. Thieme, and Vifor. M.B. is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322 900 939) and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Edwards, Medtronic, Novartis, Recor, Servier, and Vifor. J.B. received honoraria for lectures/consulting from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, CVRx, BMS, Amgen, Corvia, Norgine, and Roche not related to this article; and research support for the department from Zoll, CVRx, Abiomed, Norgine, and Roche, not related to this article. All other authors do not have a conflict of interest relevant to this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Haehling, S., Assmus, B., Bekfani, T. et al. Heart failure with preserved ejection fraction: diagnosis, risk assessment, and treatment. Clin Res Cardiol 113, 1287–1305 (2024). https://doi.org/10.1007/s00392-024-02396-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-024-02396-4