Abstract
Purpose of Review
An emerging subset of dismal sinonasal cancers are those characterized by the loss of a SWItch/Sucrose Non-Fermentable (SWI/SNF) complex unit, such as the SWI/SNF-related Matrix-associated Actin-dependent Regulator of Chromatin (SMARC), which includes two main subtypes: SMARCB1- and SMARCA4-deficient sinonasal carcinomas, ultimately leading to four distinct SWI/SNF-deficient sinonasal tumors. These cancers are rare entities and low treatment responsive malignancies. In fact, they are poorly differentiated and usually detected at a late stage, when invasion of facial and cranial regions had already occurred.
Recent Findings
From a histological standpoint, SWI/SNF-deficient sinonasal carcinomas belong to the group of sinonasal undifferentiated carcinomas (SNUC); however, their distinctive features disclose a special category for these cancers. The identification of biomarkers and signaling pathways has led to the development of emerging therapies, such as immunotherapy and personalized treatments. Finally, we report preliminary findings on 3D in vitro models of sinonasal cancers, as a multidisciplinary tool that could empower the understanding of SWI/SNF-deficient cancer biology.
Summary
Here, we review the current knowledge about histological and molecular features of SWI/SNF-deficient sinonasal cancers, with a focus on treatment options and multidisciplinary research perspectives. The possibility of studying SWI/SNF-deficient sinonasal tumors in-depth would be fostered by the establishment of tumor cell lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although they only make up a minor portion of head and neck cancers, sinonasal tumors include a wide range of epithelial, mesenchymal, and neuroectodermal neoplasms [1]. Even for experienced head and neck pathologists, the intricacy of these malignancies makes their histological diagnosis extremely difficult. In fact, of all head and neck malignant neoplasms, the tumors of the sinonasal region have been found to have the highest proportion of inconsistent diagnosis [2]. As in other malignancies, unique subsets of sinonasal tumors have recently been characterized by their specific molecular modifications because of the advances in molecular and immunohistochemical techniques. The fifth edition of the World Health Organization Classification of the tumors of the nasal cavity and paranasal sinuses includes the following carcinoma types [3]: keratinizing and non-keratinizing squamous cell carcinoma, NUT carcinoma (i.e., a rare, highly aggressive malignancy defined by translocations involving the NUT gene), SWItch/Sucrose Non-Fermentable (SWI/SNF) complex-deficient sinonasal carcinoma, sinonasal lymphoepithelial carcinoma, sinonasal undifferentiated carcinoma, teratocarcinosarcoma, human papilloma virus (HPV)-associated multiphenotypic sinonasal carcinoma, and adenocarcinomas, which are further divided into intestinal-type and non-intestinal type adenocarcinoma.
The SWI/SNF-deficient sinonasal carcinomas are defined as poorly to undifferentiated epithelial malignancies characterized by loss of an SWI/SNF complex subunit. The SWI/SNF-related, matrix-associated actin-dependent regulators of chromatin (SMARC), also called BRG1-associated factors, are components of human SWI/SNF-like chromatin-remodeling protein complexes, and play a key role in reorganization, activation and repression of critical genes, whose loss is involved in tumorigenesis [4]. Sinonasal carcinomas with loss of SMARC subfamily B, member 1 (SMARCB1) and SAMRC subfamily A, member 4 (SMARCA4) have been reported [5]. Histologically, they do not show features that allow a classification into another specific entity [6].
From a clinical point of view, these tumors are highly aggressive, representing much less than 1% of all head and neck malignancies. To date, nearly 200 occurrences of SMARC-deficient sinonasal cancers have been reported [6, 7]. SWI/SNF-deficient sinonasal carcinomas are frequently detected at a locally advanced stage with invasion of orbits and intra-cranium, as well as compression of the nerves, which make them poorly treatable [8]. These tumors occur over a wide age range (i.e., median 52 years) with a slight predilection for male patients; symptoms such as headache, nasal blockage, blurred vision and eye pain being frequently reported [8, 9]. Indeed, since early symptoms are unspecific, more than 80% of patients present a T4 stage disease with invasion of the bone, skull base, or periorbital region, so the diagnosis is established after the disease has already largely progressed [10].
By resulting in a dismal diagnosis and a high mortality, a prompt detection and an appropriate treatment are fundamental steps for any therapeutic options. The best outcomes have currently been obtained with a trimodal therapy management, which includes surgical resection, external beam radiation and systemic chemotherapy [8, 11]. For such rare and mostly intractable diseases, research advancements are urgently needed to address in-depth the biology and progression of this novel type of malignant neoplasm, aiming at developing treatment guidelines and identifying potential therapeutic targets.
Histological Features
Due to the extremely variegated nature of sinonasal cancers, their classification is quite complex and is continuously being updated [12, 13]. Histologically, SWI/SNF-deficient sinonasal carcinomas belong to the group of sinonasal undifferentiated carcinomas (SNUC); in fact, they have been lumped in the group of SNUC, along with other carcinoma subtypes. SWI/SNF complex-deficient sinonasal carcinomas, defined by the loss of a gene of the SWI/SNF complex, include two main subtypes: SMARCB1- and SMARCA4-deficient sinonasal carcinomas, which have been found to account for four distinctive entities: (1) SMARCB1-deficient sinonasal carcinoma; (2) SMARCB1-deficient sinonasal adenocarcinoma; (3) SMARCA4-deficient sinonasal undifferentiated carcinoma; and (4) SMARCA4-deficient subset (~ 80%) of sinonasal teratocarcinosarcoma [14].
SMARCB1-deficient tumors are characterized by mutations, deletions and other somatic alterations in the tumor suppressor gene integrase interactor (INI) 1, referred also as INI1/hSNF5, which encodes a subunit of the SWI/SNF chromatin remodeling complex. This deficiency drives a wide range of malignancies, including many mesenchymal and putatively non-mesenchymal tumors [15]. The histologic and immunophenotypic features of SMARCB1-deficient carcinomas are heterogenous in nature. Among others, malignant rhabdoid tumor, renal medullary carcinomas, epithelioid sarcomas, some epithelioid malignant peripheral nerve sheath tumors, myoepithelial carcinomas and myxoid extraskeletal chondrosarcomas. In most cases, the tumor consists of a relatively uniform population of undifferentiated basaloid cells with round nuclei containing dispersed chromatin and variably prominent nucleoli, organized in solid sheets and nests with peripheral palisading [10]. In addition, interspersed rhabdoid/plasmacytoid cells with abundant eosinophilic cytoplasm and eccentrically placed nuclei can often be identified. In approximately one-third of the cases, the tumor predominantly consists of these rhabdoid/plasmacytoid cells. There is no evidence of squamous differentiation and no signs of dysplasia of the surface epithelium [16]. Cytological smears show groups of cohesive monomorphic polygonal cells with varying amounts of cytoplasm in rhabdoid/plasmacytoid cells, uniform nuclei with tiny nucleoli and delicate chromatin, but neither hyperkeratinization, nor other signs of squamous differentiation can be observed. Mitotic figures and apoptotic bodies are frequently detected, along with frequent background necrosis [17, 18].
SMARCB1-deficient carcinomas are inherently positive for cytokeratins (CKs), including CK5 and CK7, while p63 is expressed in approximately half of the cases [10]. Neoplastic cells lack nuclear expression of SMARCB1 (i.e., INI1), while the immunostaining is retained in fibroblasts, endothelial cells and inflammatory cells. Focal positivity for neuroendocrine markers, including the cluster of differentiation (CD) 56, synaptophysin and chromogranin A, has also been reported [10]. P16 may be occasionally positive; however, high-risk HPV testing has always been negative [10].
A subset of SMARCB1-deficient carcinomas presents glandular differentiation with formation of cribriform structures and tubules containing intracellular and/or intraluminal mucin. Interestingly, foci with yolk sac tumor-like morphology, consisting mainly of microcystic and reticular growth patterns in a myxoid stroma, can also be present [19]. These rare cases have been designated as SMARCB1 (INI1)‑deficient adenocarcinoma [16]. In addition to the loss of INI1 expression, the immunohistochemical profile of these adenocarcinomas includes positivity for CK7, CK20 (focal) and CDX2 (focal), occasionally including yolk sac markers [e.g., glypican-3, alpha fetoprotein (AFP)], as well as germ cell markers [e.g., Spalt-like protein 4 (SALL4)] [20].
Carcinomas with loss of SMARCA4 are extremely rare in the sinonasal tract [21]. These undifferentiated carcinomas consist of nests of monomorphic cells that tend to merge in solid sheets with areas of coagulative necrosis [22]. While in most of the cases neoplastic cells are predominantly large, in some instances the tumor cells resemble those of small cell neuroendocrine carcinoma or tend to be more elongated with neuroepithelial-like elements. These tumors show complete loss of SMARCA4 and retained expression of SMARCB1/INI1, whereas co-loss of SMARCA2 may be present. SMARCA4 carcinomas lack any expression of squamous differentiation markers, but sometimes show expression of neuroendocrine markers [23]. After having excluded other undifferentiated/poorly differentiated malignancies (e.g., sarcomas, melanoma, lymphomas, olfactory neuroblastoma), the differential diagnosis of SWI/SNF carcinomas is mainly against other sinonasal undifferentiated carcinomas and can be performed by using appropriate immunohistochemical markers. NUT carcinoma is positive for epithelial markers (i.e., CKs, p63, p40) and shows a characteristic punctate nuclear immunostaining with the monoclonal NUT antibody [24, 25]. Distinction from SNUC is based on detection of loss of INI1 expression for SMARCB1-deficient carcinoma and adenocarcinoma, or of SMARCA4 (BRG1) for SMARCA4 deficient carcinomas.
Notably, SMARCB1 deficient adenocarcinomas are frequently positive for yolk sac markers including SALL4, alpha fetoprotein, glypican 3 and CDX2. SMARCA4-deficient carcinomas may show positivity for neuroendocrine markers, including synaptophysin, chromogranin and CD56, thus requiring distinction from neuroendocrine carcinomas, which, however, retain SMARCA4 [20]. Finally, a subset of sinonasal teratocarcinosarcomas show loss of SMARCA4, but they can be distinguished from SMARCA4-deficient carcinoma by the presence of sarcomatoid and teratoid tumor elements [26].
The four sinonasal entities described above and imputed uniquely or predominantly to SWI/SNF-deficiency can be identified via histological markers, without the need of gene analysis [14]. In brief, SMARCB1-deficient sinonasal carcinoma lacks gland formation and retains other distinctive traits, whereas SMARCB1-deficient sinonasal adenocarcinoma shows unequivocal glands or yolk sac-like structures; moreover, SMARCA4-deficient sinonasal undifferentiated carcinoma misses glandular or squamous immunophenotypes, whereas SMARCA4-deficient sinonasal teratocarcinosarcoma displays sarcomatoid and teratoid features.
Molecular Features
SMARCB1 is a core subunit of the adenosine triphosphate (ATP)-dependent chromatin remodeling complex SWI/SNF, encoded at chromosome position 22q11.2 and involved in chromatin remodeling and transcriptional regulation [27] (Fig. 1).

© 2022 by the authors. Licensee MDPI, Basel, Switzerland, under the terms and conditions of the Creative Commons Attribution (CC BY) license [27]
SMARCB1 functional domains are shown schematically, along with a list of harmful somatic mutations. The SMARCB1 protein has four functional domains: the highly conserved putative coiled-coil C-terminal helix domain, CTD (aa335-375), two highly conserved imperfect repeat domains, Rpt1 (aa186-248) and Rpt2 (aa259-319) and a winged helix domain DNA-binding domain, DBD (aa10-110). Reproduced under
The SWI/SNF complex localizes to sites marked by acetylation of histone H3 lysine 27 (H3K27ac) and cooperates with transcription factors to establish an open chromatin state, thereby promoting transcription. SMARCB1 regulates cell proliferation and gene transcription by interacting with four signaling pathways: p16INK4a-Rb-E2F pathway, which regulates chromosomal stability and cell cycle progression through regulation of retinoblastoma; Polycomb pathway, through the inhibition of Polycomb Repressive Complex 2 (PRC2) and its catalytic subunit Enhancer of Zeste Homologue 2 (EZH2), which mediates gene silencing by catalyzing the trimethylation of histone H3 at lysine 27 (H3K27me3) at the promoter regions of target genes; Sonic Hedgehog (Shh) signaling pathway, which is a major regulator of cell differentiation and cell proliferation, via regulation of GLI1; canonical WNT signaling pathway, which regulates cell fate, proliferation and survival. Thus, SMARCB1/INI1 deficiency causes cellular proliferation through the over-expression of cyclin D1, an increase in EZH2 activity, overexpression of GLI1 and aberrant activation of the WNT signaling pathway, resulting in WNT/β-catenin overexpression [28, 29•].
Fluorescence in situ hybridization (FISH) shows that SMARCB1 protein deficiency is mainly due to biallelic deletions involving the SMARCB1 gene locus. Monoallelic deletions, missense mutations and in frame deletions have also been reported [10, 30]. In several cases, FISH revealed chromosome 22q loss and deletions of genes close to SMARCB1 on chromosome 22q, including NF2 and CHEK2 [31]. Recently, a comprehensive genomic profiling using next-generation sequencing (NGS) has been performed; however, apart from SMARCB1 loss, no additional highly recurrent chromosomal alterations have been observed so far [32]. Gene sequencing also revealed that this type of cancer has a stable microsatellite status and a low tumor mutation burden [8]. Moreover, neither EBV nor HPV DNA has been detected.
Furthermore, a significantly higher methylation of RASSF1 gene in these neoplasms has been reported by an extensive analysis of DNA methylation [7]. These authors investigated selected microRNA expression, finding upregulation of miR-9 and miR-21 expression and downregulation of miR-45 expression, even if these differences in the obtained results were not statistically significant [33]. The identification of biomarkers and signaling pathways has led to the development of emerging therapies and personalized treatments. Between these, the EZH2 inhibitor Tazemetostat has emerged as a promising therapy, currently tested in several phase I–II trials, including diffuse large B cell lymphoma or SMARCB1 negative or SMARCA4 negative solid tumors [34].
Current Therapies
To date, less than 200 cases of SMARCB1-deficient carcinomas have been reported. Combined surgery and radiotherapy or chemoradiation are the current mainstay of treatment for these highly aggressive tumors. However, a universally accepted treatment guideline is still lacking for these cancers. Surgical removal with wide margins is the standard of care, although this is not always possible depending on the anatomic site of the tumor mass. Chemotherapy with docetaxel, cisplatin, 5-fluorouracil (TPF) and etoposide are used as an adjuvant treatment, as well as charged-particle therapy (e.g., proton therapy and carbon ion therapy). The optimal sequence of different treatments has not been determined yet and can vary from patient to patient [35]. Despite this aggressive treatment, local recurrence or metastasis to regional lymph nodes is found to occur in approximately one-third of patients, while nearly one-third is reported to develop distant metastasis to the liver, bone, lung and serous membranes [36].
Regardless of the type of treatment used, the prognosis of patients with sinus cancer is dismal, with an overall 5-year survival rate of 30%–50%. Local recurrence often occurs within 2 years of follow-up and is the leading cause of mortality [8, 37]. Significant progress has been obtained in recent years in understanding the molecular function of SMARCB1. These new insights have led to the discovery of numerous potential therapeutic vulnerabilities in SMARCB1-deficient cancers (Fig. 2).

© 2022 by the authors. Licensee MDPI, Basel, Switzerland, under the terms and conditions of the Creative Commons Attribution (CC BY) license [38]
Different sites for the action of new drugs. Therapy depends on what type of SMARCB1-deficient carcinoma is detected at diagnosis. Reproduced under
Among them, the EZH2 inhibitor Tazemetostat has recently received Food and Drug Administration (FDA) approval for the treatment of these malignancies. In addition, immune checkpoint inhibitors are gaining acceptance for the treatment of these carcinomas and are currently being studied in several clinical trials. The immune checkpoint inhibitors Nivolumab, Atezolizumab and Pembrolizumab, targeting the PD-1/PD-L1 and CTLA-4/CD80/86 checkpoints, are the immune therapies mostly evaluated in the clinics for these diseases so far [37]. To enhance the efficacy of immune checkpoint inhibitors and improve their antitumor immune response, several combinatorial strategies have also been proposed, which include combination with other checkpoint inhibitors, targeted therapies, or anti-angiogenic agents [27, 38].
Moreover, further studies are still needed to confirm other viable therapeutic targets for SMARCB1-deficient tumors, such as tyrosine kinase inhibitors, MYC inhibitors, MDM2/4 inhibitors and proteasomal inhibitors, considering the relevance of heterogeneous tumor microenvironments (TME) in the clinic [39].
A recent study analyzed the clinicopathological features and prognoses of the new molecularly defined entities, as reported in the latest edition of the World Health Organization (WHO) classification of sinonasal carcinomas [6]. The authors compared SMARCB1-deficient (n = 139) versus SMARCA4-deficient (n = 45) sinonasal carcinomas. The latter was found at a significantly younger age with respect to SMARCB1-deficient (median, 42.0 versus 53.0), but associated to reduced rate of administered radiotherapy. Anyway, the disease-specific survival was not different between the two SWI/SNF-deficiencies.
Multidisciplinary Research Perspectives
The treatment of patients with these cancers is complex and has gone considerable transformation, leading to some improved outcomes. One of the main obstacles to the creation of efficient, patient-specific therapies is heterogeneity in cancer, which is particularly evident in sinonasal cancers [39, 40].
In addition, the rarity of these tumor entities strongly contributes to lesser advancements in successful treatments. In fact, clinical trials availing themselves of sufficiently large patient groups are really challenging in these unfortunate cases, considering that certain therapeutic pathways might not work against tumors with different origins and features [40]. Beyond heterogeneity, other less-studied microenvironmental characteristics, including non-neoplastic cells, niche-relevant soluble molecules and the altered extracellular matrix (ECM), have also been linked to tumor diversification and response to therapy [41, 42]. Specifically, the cells that make up the TME, such as fibroblasts, immune cells, endothelial cells, among others, exhibit extreme biological diversity [43]. These cells can both directly and indirectly affect the phenotype of neoplastic cells through intricate reciprocal signaling mechanisms mediated by soluble cues, cell–cell contact and ECM remodeling.
With the aid of a variety of cancer models, spanning from conventional in vitro [i.e., bidimensional (2D) cell monolayer] cultures to in vivo (i.e., animal) models, which represent crucial elements in the effective development of novel therapeutics in the laboratories around the world, the field of cancer biology has gained tremendous research advancement over the past few decades. In recent years, three-dimensional (3D) cell cultures have provided novel research opportunities with respect to traditional methods, by enabling the investigation of cancer in a realistic (i.e., higher complexity) environment, with tumor cells developing as seen in vivo [44]. For quick drug screening, fundamental cancer research and cancer diagnostics, 3D cell culture models function as less expensive and for many aspects better reliable platforms than animal models [45]. In time, many 3D culture systems are getting available, encompassing the simplest spheroids (i.e., cellular aggregates), tumor organoids or tumoroids (i.e., from patient’s cells), 3D organs-of-chip (i.e., using microfluidics), up to tissue engineered cancer constructs (i.e., using a biomaterial scaffold for cancer cell growth) [44]. The latter in vitro models take advantage of the multi- and inter-disciplinary knowledge built by the tissue engineering field for normal tissue/organ regeneration over the last 3 decades and can be built with an ideally infinite complexity by including different cell types, including immune cells, and ECM features, such as architectural, biochemical and mechanical cues [45]. These 3D platforms thus demonstrate versatility and customizability to reproduce the complexity and heterogeneity of TMEs in different cancers, which could be applied to sinonasal settings (Fig. 3).
Schematic representing the process of obtaining primary cells line from a patient for personalized medicine to allow the search for new molecular targets or the study of new drugs. Tumor tissue can be obtained from various clinical situations including surgery, endoscopy or needle aspiration. Primary culture should be isolated using specific protocols and monitoring what is isolated. The use of 3D models, in which the primary cultures are seeded, reproduces the TME, thus providing optimal conditions. This can be used for customized therapy or research into new targets. [Image sketched by the authors]
In a tissue engineering approach, these three key elements (i.e., known as the tissue engineering triad) are all necessary and must be properly designed: the cells, the scaffold and the stimuli. Such a fundamental paradigm must be applied to cancer tissue engineering. Primary human cell cultures, derived from the patient, are expected to better retain the morphological characteristics and the phenotypic traits of the original tumor; therefore, although difficult to be established especially in the case of rare tumors, they offer a great advantage in 3D in vitro model-based research [46•].
Patient-derived primary cells and immortalized cell lines differ significantly on several levels, which later reverberates in the outcomes of their manipulation. The key distinction between the two cell models is their genetic makeup. The foundation of personalized treatment is in assessing and comprehending genetic variety. The high genetic variability of cell lines, on the other hand, is a result of mutation accumulation brought on by their immortalization and numerous passages, whereas cells isolated from patients preserve the genetic and molecular background of the individuals and thereby become an accurate representation of the disease condition. However, because of the changes in cell physiology and the outcomes produced, this form of variability is not pertinent for the objectives of personalized medicine and, on the contrary, may generate biased results. In this instance, the progress of precision medicine is significantly hampered, rather than driven, by the mutations that result from unrestricted in vitro cultivation. The sensitive preservation and management of primary cells in culture presents a real challenge when working with them [46•]. Non-immortalized cells are also difficult to be maintained in vitro and frequently need specialized culture procedures and supplements. However, patient-derived primary cells continue to be the most accurate in vitro model for comprehending disease mechanisms and evaluating new medications, despite their short lifespan, ethical and culture issues.
The second tissue engineering pillar relies on porous biomaterials, mostly polymer-based, known as “scaffolds”, which requires complementary knowledge in Biomaterials Science and Engineering [47]. Different scaffold types can be fabricated, including hydrogels, sponges, micro- and nano-fiber meshes and 3D printed shapes, aimed at resembling the morphology, as well as the physico-chemical and mechanical properties of the ECM, thus enabling the mimicry of soft and hard tumors, which are also found in sinonasal cancers [48]. Different scaffolds have shown diverse response by primary cancer cells; therefore, the scaffold properties should match as much as possible those of the tissue of interest, which could be, depending on the specific object of the study, the native tissue ECM where the cancer originates, the tumor ECM where the cancer already established, or the spare organ ECM housing cancer metastases [48,49,50,51]. In our experience, highly hydrophilic spongy scaffolds are an optimal platform for the culture of many nasal tissues, including mucosal tissues where sinonasal tissues, such as mucous melanoma and intestinal-type adenocarcinoma (ITAC) develop [48, 52].
The third pillar regards the microenvironmental stimuli, which cover a wide and still mostly unknown field of study. In fact, as the scaffold mimics the ECM, such stimuli can be given by its inner architecture (e.g., pore size and shape) [47,48,49], and/or material composition and added biomolecules [47,48,49, 51], mechanical, electrical and other properties, molecules secreted by TME cells, and so on.
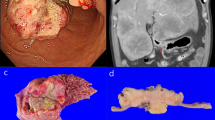
Noticeably, in regenerative tissue engineering, the biology of the target (i.e., healthy and functional tissue/organ) is much better known than in cancer tissue engineering, where tumor biology itself is a still quite unveiled subject of study. As such, the comparison with the molecular and histological features of the tumor specimens remains the only route for any meaningful investigation [44, 46•, 49]. The limiting factors hampering 3D in vitro models of sinonasal cancers to come into action are the small number of primary cell lines that have been isolated, along with the rarity and heterogeneity of sinonasal tumors. In the last decade, researcher groups have put efforts to synergize and obtain primary cell lines of various rare sinonasal tumors of the nose, by making an ITAC cell line available [53]. However, to the best of our knowledge, none long-lasting cell line of SMARCB1-deficient sinonasal carcinoma has been established so far. We have recently succeeded in isolating a cell line from a patient diagnosed with SNUC, located in the right maxillary sinus with negative INI-1 immunohistochemical determination (i.e., SMARCB1-deficient carcinoma) [54]. Isolation was performed using a new, optimized protocol and several steps were necessary leading to a cell culture, in which the phenotypic characteristics of the original tumor were found (Fig. 4).
Isolation of the primary culture of a sinonasal tumor from a patient diagnosed with SNUC, located in the right maxillary sinus with negative INI-1 immunohistochemical determination (i.e., SMARCB1-deficient carcinoma) [54]. The study was approved by CEAVNO Regional ethics committee, Pisa, Italy; approval number 17455 of June 25, 2020. A, B Histologic features of the tumor at diagnosis: A Hematoxylin and Eosin, and B immunohistochemistry of INI-1, where negativity is largely observed. C Primary cell culture under inverted light microscopy at passage 20. D, E Immunofluorescence characterization of the cell line, nuclei in blue with 4′,6-diamidino-2-phenylindole (DAPI): D Pan-CKs in yellow; E EZH2 protein in green and INI-1 (absent) in red. [Original images of the authors]
The culture was stable, delicate with a good growth rate, until passage 22, when it started declining. Succeeding in establishing SWI/SNF-deficient sinonasal cancer cell lines would be a turning point for building new 3D in vitro models of sinonasal cancers and studying such a sinonasal pathology at an improved level.
Conclusion
Sinonasal cancers are a very heterogeneous group of tumors. They share similar problems common to rare diseases: uncertainty of diagnosis, lack of therapies, poor research opportunities, difficulties in clinical trials, lack of expertise and of reference centers. Among sinonasal cancers, SWI/SNF-deficient sinonasal tumors are utmost rare entities, poorly differentiated and in many cases unsuccessfully treated, with a 5-year survival rate of 30%–50%. The current knowledge about histological and molecular features of SWI/SNF-deficient sinonasal cancers has elucidated distinctive characters, but the poor understanding of the biology of these cancers has prevented relevant research advancements in this field up to now. If SMARCB1- and SMARC4-deficient cell lines will be established, novel tools to study these pathologies could be made available, which are founded on multidisciplinary research. The identification of biomarkers and signaling pathways has led to the development of emerging therapies, such as immunotherapy and personalized treatments, which in a future scenario could be screened on 3D in vitro models able to mimic the TMEs of these tumor entities.
Data Availability
Data will be made available upon a reasonable request to the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Virk JS, Chan J, Dimitrov L, Williamson A, Sandison A, Weir J, Clarke P. Sinonasal cancer: an overview of the emerging subtypes. J Laryngol Otol. 2020;134(3):191–6. https://doi.org/10.1017/S0022215120000146.
Choi KY, Amit M, Tam S, Bell D, Phan J, Garden AS, Williams MD, Ferrarotto R, Adel KE-N, Raza SM, DeMonte F, Kupferman ME, Hanna EY, Su SY. Clinical implication of diagnostic and histopathologic discrepancies in sinonasal malignancies. Laryngoscope. 2021;131(5):E1468–75. https://doi.org/10.1002/lary.29102.
Thompson LDR, Bishop JA. Update from the 5th edition of the World Health Organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Head Neck Pathol. 2022;16:1–18.
Krishnamurthy N, Kato S, Lippman S, Kurzrock R. Chromatin remodeling (SWI/SNF) complexes, cancer, and response to immunotherapy. J Immunother Cancer. 2022;10(9):e004669. https://doi.org/10.1136/jitc-2022-004669.
Bell D, Hanna EY, Agaimy A, Weissferdt A. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: a single-institution experience. Virchows Arch. 2015;467:649–56.
Vuong HG, Le T, Le TTB, Le HT, El-Rassi ET, McKinney KA, Dunn IF. Clinicopathological features and prognostic outcomes of molecularly defined entities in the new edition of the WHO classification of sinonasal carcinoma. Front Oncol. 2023;13:1117865. https://doi.org/10.3389/fonc.2023.1117865.
Laco J, Chmelařová M, Vošmiková H, Sieglová K, Bubancová I, Dundr P, Němejcová K, Michálek J, Čelakovský P, Mottl R, Sirák I, Vošmik M, Ryška A. SMARCB1/INI1-deficient sinonasal carcinoma shows methylation of RASSF1 gene: a clinicopathological, immunohistochemical and molecular genetic study of a recently described entity. Pathol Res Pract. 2017;213(2):133–42. https://doi.org/10.1016/j.prp.2016.10.012.
Lee VH, Tsang RKY, Lo AWI, Chan SY, Chung JCK, Tong CC, et al. SMARCB1 (INI-1)-deficient sinonasal carcinoma – a systematic review and pooled analysis of treatment outcomes. Cancers (Basel). 2022;14(13):3285. https://doi.org/10.3390/cancers14133285.
Parsel SM, Jawad BA, McCoul ED. SMARCB1-deficient sinonasal carcinoma: systematic review and case report. World Neurosurg. 2020;136:305–10.
Agaimy A, Hartmann A, Antonescu CR, Chiosea SI, El-Mofty SK, Geddert H, et al. SMARCB1 (INI-1)-deficient sinonasal carcinoma: a series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol. 2017;41:458–71.
Mourad WF, Hauerstock D, Shourbaji RA, Hu KS, Culliney B, Li Z, Jacobson A, Tran T, Manolidis S, Schantz S, Urken M, Persky M, Harrison LB. Trimodality management of sinonasal undifferentiated carcinoma and review of the literature. Am J Clin Oncol. 2013;36(6):584–8. https://doi.org/10.1097/COC.0b013e31825eb3a5.
Hermsen MA, Bossi P, Franchi A, Lechner M. Sinonasal cancer: improving classification, stratification and therapeutic options. Cancers. 2023;15(6):1675. https://doi.org/10.3390/cancers15061675.
Agaimy A, Franchi A. Special mesenchymal neoplasms of the head and neck: update from the 2022 WHO | Spezielle mesenchymale Tumoren der Kopf-Hals-Region: Neues aus der WHO 2022. Pathologie. 2023;44(4):250–8. https://doi.org/10.1007/s00292-023-01203-7.
Agaimy A. SWI/SNF-deficient sinonasal carcinomas. Adv Anat Pathol. 2023;30(2):95–103. https://doi.org/10.1097/PAP.0000000000000372.
Dermawan JK, Singer S, Tap WD, Nacev BA, Chi P, Wexler LH, Ortiz MV, Gounder M, Antonescu CR. The genetic landscape of SMARCB1 alterations in SMARCB1-deficient spectrum of mesenchymal neoplasms. Mod Pathol. 2022;35(12):1900–9. https://doi.org/10.1038/s41379-022-01148-x.
Stelow EB, Jo VY, Mills SE, Carlson DL. A histologic and immunohistochemical study describing the diversity of tumors classified as sinonasal high-grade nonintestinal adenocarcinomas. Am J Surg Pathol. 2011;35.
Kakkar A, Antony VM, Pramanik R, Sakthivel P, Singh CA, Jain D. SMARCB1 (INI1)–deficient sinonasal carcinoma: a series of 13 cases with assessment of histologic patterns. Hum Pathol. 2019;83:59–67.
Allison DB, Bishop JA, Ali SZ. Cytopathologic characteristics of SMARCB1 (INI-1) deficient sinonasal carcinoma: a potential diagnostic pitfall. Diagn Cytopathol. 2016;44:700–3.
Shah AA, Jain D, Ababneh E, Agaimy A, Hoschar AP, Griffith CC, et al. SMARCB1 (INI-1)-deficient adenocarcinoma of the sinonasal tract: a potentially under-recognized form of sinonasal adenocarcinoma with occasional yolk sac tumor-like features. Head Neck Pathol. 2020;14:465–72.
Alsayed AM, Aljufairi EA, Alshammari AO, Alsindi KA, Sabra OA. INI-1-deficient sinonasal carcinoma: case report with emphasis on differential diagnosis. Case Rep Pathol. 2022;30(2022):5629984. https://doi.org/10.1155/2022/5629984.
Agaimy A, Weichert W. SMARCA4-deficient sinonasal carcinoma. Head Neck Pathol. 2017;11(4):541–5. https://doi.org/10.1007/s12105-017-0783-4.
Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient sinonasal carcinoma: a series of 10 cases expanding the genetic spectrum of SWI/SNF-driven sinonasal malignancies. Am J Surg Pathol. 2020;44.
Agaimy A. Proceedings of the North American Society of Head and Neck Pathology, Los Angeles, CA, March 20, 2022: SWI/SNF-deficient sinonasal neoplasms: an overview. Head Neck Pathol. 2022;16(1):168–78. https://doi.org/10.1007/s12105-022-01416-x.
Vakani PN, Maheshwari J, Maheshwari M, Shah B. Sinonasal NUT midline carcinoma: a new histological entity. Indian J Pathol Microbiol. 2020;63(1):103–5.
Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–91.
Rooper LM, Uddin N, Gagan J, Brosens LAA, Magliocca KR, Edgar MA, Thompson LDR, Agaimy A, Bishop JA. Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol. 2020;44(10):1331–9. https://doi.org/10.1097/PAS.0000000000001508.
Ngo C, Postel-Vinay S. Immunotherapy for SMARCB1-deficient sarcomas: current evidence and future developments. Biomedicines. 2022;10:650.
Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017;108:547–52.
• Hermsen MA, Riobello C, García-Marín R, Cabal VN, Suárez-Fernández L, López F, et al. Translational genomics of sinonasal cancers. Semin Cancer Biol. 2020;61:101–9. This study shows that genetic characterization of sinonasal cancers via Next Generation Sequencing is useful in tumor subtype classification, as well as in molecular target identification for treatment with specific inhibitors.
Dogan S, Cotzia P, Ptashkin RN, Nanjangud GJ, Xu B, Momeni Boroujeni A, et al. Genetic basis of SMARCB1 protein loss in 22 sinonasal carcinomas. Hum Pathol. 2020;104:105–16.
Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. https://doi.org/10.1016/j.pharmthera.2020.107753. Epub 2020 Nov 28. PMID: 33259885; PMCID: PMC8084948.
Jurmeister P, Glöß S, Roller R, Leitheiser M, Schmid S, Mochmann LH, et al. DNA methylation-based classification of sinonasal tumors. Nat Commun. 2022;13:7148.
Laco J, Kovaříková H, Chmelařová M, Vošmiková H, Sieglová K, Baranová I, et al. MicroRNA expression in SMARCB1/INI1-deficient sinonasal carcinoma: a clinicopathological and molecular genetic study. Virchows Arch. 2018;472:871–5.
Mittal P, Roberts CWM. The SWI/SNF complex in cancer — biology, biomarkers and therapy. Nat Rev Clin Oncol. 2020;17:435–48.
Amit M, Abdelmeguid AS, Watcherporn T, Takahashi H, Tam S, Bell D, et al. Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. JCO. 2019;37:504–12.
Rooper LM, Westra WH. A protein lost, a diagnosis gained: a review of SMARCB1-deficient sinonasal carcinomas. AJSP Rev Rep. 2018;23.
Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014;11:460–72.
Cooper GW, Hong AL. SMARCB1-deficient cancers: novel molecular insights and therapeutic vulnerabilities. Cancers. 2022;14:3645.
Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–64.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94.
Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213.
Tiwari A, Trivedi R, Lin SY. Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J Biomed Sci. 2022;29(1):83. https://doi.org/10.1186/s12929-022-00866-3.
Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921–5. https://doi.org/10.1016/j.cub.2020.06.081.
Contessi Negrini N, Franchi A, Danti S. Biomaterial-assisted 3D in vitro tumor models: from organoid towards cancer tissue engineering approaches. Cancers. 2023;15(4):1201. https://doi.org/10.3390/cancers15041201.
Ricci C, Moroni L, Danti S. Cancer tissue engineering-new perspectives in understanding the biology of solid tumours - a critical review. OA Tissue Eng. 2013;1(4):1–4.
• Hasbum A, Karabulut O, Reyes RE, Ricci C, Franchi A, Danti S, et al. Combined application of patient-derived cells and biomaterials as 3D in vitro tumor models. Cancers. 2022;14:2503. This report highlights that 3D in vitro models obtained by culturing primary tumor cells on porous biomaterials allow reliable cancer microenvironments to be obtained for personalized screening of therapies, which can be useful in sinonasal cancers.
Lazzeri L, Cascone MG, Danti S, Moscato S, Bernardini N. Gelatine/PLLA sponge-like scaffolds: morphological and biological characterization. J Mater Sci - Mater Med. 2007;18(7):1399–405. https://doi.org/10.1007/s10856-007-0127-0.
Danti S, Ricci C, Macchi T, Parchi P, Franchi A. 3D in vitro models of hard and soft tumours. Proceedings of 3rd Biannual Conference of Biomaterials and Novel Technologies for Healthcare (BioMaH), 18–21October 2022, Rome, Italy. ISBN 978 88 8080 500 7. https://biomah.ism.cnr.it/?page_id=665. Accessed 02 Sept 2023.
Ricci C, Mota C, Moscato S, D’Alessandro D, Ugel S, Sartoris S, et al. Interfacing polymeric scaffolds with primary pancreatic ductal adenocarcinoma cells to develop 3D cancer models. Biomatter. 2014;4:e955386.
Angeloni V, Contessi N, De Marco C, Bertoldi S, Tanzi MC, Daidone MG, Farè S. Polyurethane foam scaffold as in vitro model for breast cancer bone metastasis. Acta Biomater. 2017;63:306–16. https://doi.org/10.1016/j.actbio.2017.09.017.
Contessi Negrini N, Ricci C, Bongiorni F, Trombi L, D’Alessandro D, Danti S, et al. An Osteosarcoma model by 3D printed polyurethane scaffold and in vitro generated bone extracellular matrix. Cancers. 2022;14:2003.
D’Alessandro D, Moscato S, Fusco A, De la Ossa JG, D’Acunto M, Trombi L, Feula M, Serino LP, Donnarumma G, Petrini M, Berrettini S, Danti S. Poly(vinyl alcohol)/gelatin scaffolds allow regeneration of nasal tissues. Appl Sci. 2021;11(8):3651.
Pérez-Escuredo J, García Martínez J, García-Inclán C, Vivanco B, Costales M, Álvarez Marcos C, Llorente JL, Hermsen MA. Establishment and genetic characterization of an immortal tumor cell line derived from intestinal-type sinonasal adenocarcinoma. Cell Oncol (Dordr). 2011;34(1):23–31. https://doi.org/10.1007/s13402-010-0002-8.
Macchi T. Messa a punto di modelli cellulari 2D e 3D per lo studio di neoplasie maligne del tratto naso-sinusale. Master’s Degree Thesis in Molecular Biotechnologies, University of Pisa, Italy, academic year 2022–2023. Thesis file number etd-09052022-130352. Consultable at https://etd.adm.unipi.it/ETD-db/ETD-browse/send_author_email?URN=etd-09052022-130352. Accessed 02 Sept 2023.
Acknowledgements
The authors thank Dr. Niccola Funel (Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy) for his technical support to immunofluorescence staining of SMARCB1-deficient sinonasal tumor cell line.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This research was funded by Tuscany Region under Health Program 2018, project name “Sinonasal cancer: In depth genetic analysis of patients for personalized treatment and disease monitoring (acronym: ADAPTA)”, grant number CUP I58D20000540002.
Author information
Authors and Affiliations
Contributions
S.D., C.R. and T.M. wrote the main manuscript text; S.D. and C.R. prepared the figures; I.D., S.B. and A.F. performed investigation and supervision; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Danti, S., Ricci, C., Macchi, T. et al. SWI/SNF-Deficient Sinonasal Carcinomas: Multidisciplinary Research Perspectives. Curr Otorhinolaryngol Rep 11, 472–481 (2023). https://doi.org/10.1007/s40136-023-00495-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-023-00495-w