Abstract
One of the most common musculoskeletal disorders, osteoarthritis (OA), causes worldwide disability, morbidity, and poor quality of life by degenerating articular cartilage, modifying subchondral bone, and inflaming synovial membranes. OA pathogenesis pathways must be understood to generate new preventative and disease-modifying therapies. In recent years, it has been acknowledged that gut microbiota (GM) can significantly contribute to the development of OA. Dysbiosis of GM can disrupt the “symphony” between the host and the GM, leading to a host immunological response that activates the “gut–joint” axis, ultimately worsening OA. This narrative review summarizes research supporting the “gut–joint axis” hypothesis, focusing on the interactions between GM and the immune system in its two main components, innate and adaptive immunity. Furthermore, the pathophysiological sequence of events that link GM imbalance to OA and OA-related pain is broken down and further investigated. We also suggest that diet and prebiotics, probiotics, nutraceuticals, exercise, and fecal microbiota transplantation could improve OA management and represent a new potential therapeutic tool in the light of the scarce panorama of disease-modifying osteoarthritis drugs (DMOADs). Future research is needed to elucidate these complex interactions, prioritizing how a particular change in GM, i.e., a rise or a drop of a specific bacterial strain, correlates with a certain OA subset to pinpoint the associated signaling pathway that leads to OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Osteoarthritis (OA) and gut microbiota (GM) have been linked because GM dysbiosis can disrupt the gut barrier, activate the “gut–joint axis,” and modulate innate and adaptive immunity through various mediators. |
The gut–joint axis mediators include short-chain fatty acids, lipopolysaccharide, lipoteichoic acid, tryptophan metabolites, serotonin, gamma-aminobutyric acid, bile acids, flagellin, and β-glucans. |
GM can alter chronic pain’s central and peripheral sensitization pathways in many ways where metabolites, neurotransmitters, neuromodulators, and microbial byproducts are implicated. |
Targeting the GM could be a new way of treating and preventing OA and its related pain. Interventions that have been suggested include dietary changes, prebiotics, probiotics, nutraceuticals, exercise, and fecal microbiota transplantation as major GM modulators. |
This review aims to explore the complex relationship between GM and OA, focusing on how the immune system shapes this bridge. |
Introduction
Osteoarthritis (OA) is a chronic degenerative disorder characterized by the progressive degradation of the structures of the joints, such as the articular cartilage, the subchondral bone, the ligaments, the capsule, and the synovium [1]. To put it another way, it is the most widespread kind of arthritis and a significant contributor to both pain and disability in people of middle age and older [2].
A key characteristic of OA is the deterioration of the structure and function of articular cartilage. Additionally, OA is identified by changes in bone structure and synovitis, which leads to clinical complaints such as joint pain and limitations in mobility, social participation, and quality of life [3].
OA has long been attributed to biomechanical factors [4], ageing [5], and genetic predisposition [6]. However, a paradigm shift in our understanding of OA etiology is underway, with a growing body of evidence implicating the gut microbiota (GM) as a critical player in the intricate dance of joint health.
The GM is the resident population of the human gut. It is believed to consist of over 1014 microorganisms, encompassing the three primary categories of life: Bacteria, Eukarya, and Archaea. The bacterial domain has the highest representation [7,8,9,10]. Currently, six distinct phyla of bacteria have been acknowledged. The Firmicutes and Bacteroidetes are the most abundant, with Actinobacteria and Proteobacteria following closely after [11]. The composition of the GM is unmatched. The variation in an individual’s characteristics is subject to ongoing and dynamic changes throughout their life, influenced by both intrinsic elements like hereditary patterns and gastric acid secretions and extrinsic factors like nutrition, medical conditions, and environmental influences [12, 13]. Indeed, proton pump inhibitors can modify the GM’s composition by decreasing the stomach acid levels. This reduction in acid can result in an excessive proliferation of specific bacterial species in the upper gastrointestinal tract [14]. The influence of oral health on oral microbiota and GM must also be underlined [10, 13, 15]. The dissemination of oral bacteria (OB) throughout the body, including the gut, has been linked to many systemic disorders. Research on animals and humans has demonstrated that OB can migrate to the gut and alter its microbiota, potentially affecting the immune defense system. Ectopic displacement of OB primarily happens in individuals with severe systemic disorders, as well as those with “chronic” periodontitis. For instance, Porphyromonas gingivalis can disrupt the balance of microorganisms in the subgingival microbiota and weaken the immune system, possibly leading to dysregulation in the gut [16].
A strong relationship between OA and GM has been emerging in recent years since GM dysbiosis (an alteration in the amount, composition and diversity of microbiota [17]) can disrupt the gut barrier, activate the “gut–joint axis”, modulate innate and adaptive immunity through LPS release, short-chain fatty acid modification, macrophage activation, cytokine liberation, regulation of T-cell responses and B cells differentiation [18, 19]. Moreover, GM can influence OA progression by interacting with other OA risk factors such as age, sex, obesity, inflammation, and mechanical load [20].
As OA is a condition affecting people of middle age and older [2], a further link with GM can be found in the fact that the elderly have an altered GM [21]. Indeed, a 2017 study showed that the GM of old mice transferred to germ-free young mice could promote “inflammaging”, that is, chronic low-grade inflammation at both the gut and systemic levels [22]. GM dysbiosis driven by age could therefore be another key player involved in OA pathogenesis.
The gut–joint axis is, in reality, a gut–immune–joint axis, as the immune system serves as the conduit through which the GM may impact the articular environment. Research has observed that the GM and its associated components and metabolites interact with OA by triggering both local and systemic innate immune responses [23]. The imbalance of microorganisms in the gut can influence the development of early CD4 + T cells into either effector T cells or Treg cells, which is essential for maintaining a balanced immune system and preventing joint inflammation [24]. Therefore, the GM, as a whole, can influence the development of OA by impacting the immune system. Nevertheless, the specific mechanisms underlying the connection between the innate/adaptive immune system and the GM in osteoarthritis remain unknown.
This review aims to unravel the multifaceted connection between GM and OA, delving into the intricate web of interactions with a particular emphasis on their impact on the immune system. By illuminating these intricate connections, we hope to pave the way for targeted therapeutic interventions that harness the potential of the GM to modulate immune responses and, consequently, mitigate the impact of OA and associated pain on joint health.
The PubMed, Web of Science, Google Scholar, and Scopus databases were examined for articles in line with the review topic from January 2005 to January 2024, with an English language limitation. The search strategy was created by combining terms relevant to the aim of this review. The following Boolean keywords were applied: (“gut” AND “microbio*” AND “osteoarthritis”);(“gut AND “microbio*” AND “Immune system”); (“gut” AND “microbio*” AND “chronic pain”).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Gut–Immune System Cross-talk: How the Gut Modulates the Immune System
Through the interaction of several cell types throughout the mucosa, including B cells, T cells, monocytes, macrophages, NK cells, and dendritic cells (DCs), GM may have an impact on the innate and adaptive immune system, resulting in a putative immunomodulator [25, 26]. It has been demonstrated that cell wall elements, such as peptidoglycan [27], can bind to receptors on the surface of monocytes and macrophages, thereby indirectly inducing the production of cytokines by immune cells [28]. The disruption of a harmonic balance of the cross-talk gut–immune system can lead to increased LPS, cytokine release, and systemic inflammation, ultimately contributing to the pathogenesis of OA (Fig. 1) [29]. In the next paragraphs, an overview of the interaction between the GM and the immune system will be given mainly in the light of OA pathogenesis (Fig. 1).
Microbial Symphony: the gut microbiota (GM) is a meticulous orchestra director through his intricate interactions with the immune System. When impaired, i.e., dysbiosis, an outburst of cytokine release with increased LPS translocation in the bloodstream and, therefore, a systemic inflammation, which starts as a low-grade inflammation, contributes to the pathogenesis of osteoarthritisFig. 1
Microbial Symphony: the gut microbiota (GM) is a meticulous orchestra director through his intricate interactions with the immune System. When impaired, i.e., dysbiosis, an outburst of cytokine release with increased LPS translocation in the bloodstream and, therefore, a systemic inflammation, which starts as a low-grade inflammation, contributes to the pathogenesis of osteoarthritisFig. 1
Microbial Symphony: the gut microbiota (GM) is a meticulous orchestra director through his intricate interactions with the immune System. When impaired, i.e., dysbiosis, an outburst of cytokine release with increased LPS translocation in the bloodstream and, therefore, a systemic inflammation, which starts as a low-grade inflammation, contributes to the pathogenesis of osteoarthritis
Microbial symphony: the gut microbiota (GM) is a meticulous orchestra director through intricate interactions with the immune system. When impaired, i.e., through dysbiosis, an outburst of cytokine release with increased LPS translocation in the bloodstream and therefore a systemic inflammation, which starts as a low-grade inflammation, contributes to the pathogenesis of osteoarthritis
Gut Microbiota and Innate Immunity
The first line of defense, innate immunity, employs proteins encoded in the germline to identify pathogens and stimulate immune responses. Upon encountering a pathogen, the innate immune cell either eliminates it or activates the adaptive immune response to combat it. OA pathogenesis and progression are significantly influenced by the activation of the innate immune system, which identifies damage-associated molecular patterns (DAMPs) via interactions with pattern-recognition receptors (PRRs) [30]. DAMPs are molecules released from injured or dying cells as a part of the innate immune response due to trauma or a pathogen infection [31]. DAMPs resemble microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs), the main source of which is the GM [32]. PRRs are cytosolic and endosomal receptors found on the cell surface. They include Toll-like receptors (TLRs), NOD-like receptors, and others [33]. PRRs, which are abundantly present on the outer membrane of macrophages and other immune cells, can discern a vast array of danger signals; this is comparable to how GM metabolites influence the innate immune system. When PRRs detect danger signals, the inflammatory signaling pathway is subsequently activated [34].
Concurrently identifying microbial DNA and the intestinal microbiome in the knees of patients with OA, it was hypothesized that enteric dysbacteriosis could accelerate the progression of OA by stimulating the innate immune system [35]. Furthermore, Liu et al. [23] demonstrated that the GM, components associated with GM, and their corresponding metabolites affected OA by stimulating innate immune responses at both the local and systemic levels. The following events in sequence represent the mechanism by which the innate immune system influences OA:
-
(1)
Synovial joint immune cells are stimulated and produce DAMPs through interactions with constant PRRs [36].
-
(2)
The innate immune response is triggered by host reactions to DAMPs [36].
-
(3)
Prompt-onset inflammatory responses are initiated [36].
In addition to macrophages, neutrophils, dendritic cells (DCs), natural killer (NK) cells, and mast cells comprise the innate immune cells.
Macrophages play a key role in breaking down each component of innate immunity, mediating the immune response between OA and GM. It has been shown that in the OA synovial membrane and cartilage, macrophages and their mediators were highly connected to inflammatory alterations and devasting reactions [37].
The so-called “two-hit theory” has described the involvement of LPS in knee OA patients’ joint space size, pain intensity, and pathogenesis and severity of osteophytes [38]. This theory additionally provides potential mechanisms by which LPS contributes to the progression of OA. The first hit takes place when lipopolysaccharide (LPS) stimulates joint macrophages via CD14–TLR4–MD-2 complexes. Subsequently, LPS triggers a comprehensive inflammatory response and joint structure degradation via coexistence and complementarity mechanisms, including the inflammasome pathway or DAMPs [39]. Furthermore, fibroblast-like synoviocyte pyroptosis can be induced by LPS through the action of either nod-like receptor protein (NLRP) 1 or NLRP3 inflammasomes. This process is known to contribute to the advancement of OA [40]. LPS is essential for the pathogenesis of osteoarthritis and macrophage-associated inflammatory responses, both of which are critical components of innate immunity.
Furthermore, fecal microbiota transplantation (FMT) from subjects with OA and metabolic syndrome accelerates OA in mice, which activates TGF-b signaling pathways to regulate multiple immune cells, such as macrophages, NK cells, DCs, T cells, and B cells [41].
Neutrophils are among the most abundant cells of innate immunity. It has been demonstrated that the number of neutrophils was greatest in knee synovial fluid with increased levels of TGF-b and elastase, which are strongly linked to the severity of radiographic knee OA. Moreover, neutrophils and macrophages in knee OA joints have a mutually beneficial interaction in both the progression and worsening of OA [42].
DCs are antigen-presenting cells that come from monocytes. They link the innate and adaptive immune systems by recognizing and responding to PAMP and DAMP [43]. In normal conditions, certain subsets of DCs reside in the intestinal mucosa. Upon encountering microorganisms, they undergo a transformation into an inflammatory state through the suppression of disabled homolog 2 (DAB2) gene expression. This process is hindered by the interactions between TLR ligands TRIF and MyD88 [44]. Butyrate and propionate, which are short-chain fatty acids (SCFA) GM metabolites, may inhibit the growth of DCs by blocking the Na(+)-coupled monocarboxylate transporter (Slc5a8) through the inhibition of HDACs [45]. Moreover, propionate can safeguard against allergic inflammation by regulating DCs. Another study demonstrated that the surface components and metabolites of Lactobacillus reuteri can stimulate the maturation of immature DCs and increase the production of the anti-inflammatory cytokine IL-10 by DCs [46]. If we shift to the joints where OA develops, DCs may initiate and accelerate the advancement of OA through the TLR family, particularly TLR4, as it has been proven in mice with OA where Nie et al. demonstrated a notable increase in the expression of TLR 1–8 in DCs [44, 47].
NK cells are a subset of lymphocytes that comprise around 15% of all lymphocytes in the bloodstream. They are characterized by the presence of CD56 and the absence of CD3. NK cells play a crucial role in the innate immune system by eliminating pathogens using death-inducing receptors or by releasing soluble chemicals such as perforin and granulysin [48].
It has been found that NK cells constituted over 30% of the CD45 + lymphocytes in the synovium of individuals with OA with the presence of the chemoattractant receptors CCR5 and CXCR3 [49]. Compared to NK cells found in the blood, NK cells in the synovium of patients with OA have a silent phenotype indicative of post-activated exhaustion [50]. However, the production of IFN-g by these NK cells can stimulate neutrophils, macrophages, and DCs. Furthermore, the expression of NKG2D by NK cells can stimulate the activation of T lymphocytes [51].
Mast cells are watchful agents of the innate immune system and promptly react to both internal danger signals and external infections. Mast cell degranulation, which involves the release of preformed mediators such as pro-inflammatory lipids, tryptases, histamine, chemokines, and cytokines, can be induced by several factors, including the IgE receptor FcεRI, IL-33, and complement receptor C5aR [52]. When we focus on GM, butyrate inhibits the production of cytokines, specifically IL-6 and TNF-α, in mast cells generated from murine bone marrow. This suppression is achieved by suppressing HDAC activity in the cells [53]. Moreover, propionate and butyrate can prevent the release of granules from human or mouse mast cells, whether or not IgE is involved. This suppression is linked to the inhibition of HDAC [54].
LPS can stimulate fully developed mast cells to generate tryptase, chymase, and carboxypeptidase [55]. If we look at the joint side, it has been observed that mast cells are dispersed in varying patterns inside osteophytes and knee synovial fluid, potentially exacerbating the inflammatory pathophysiology of osteoarthritis [56]. This led researchers to introduce a novel classification system for the synovial tissue in patients with OA based on the levels of mast cells (low, medium, and high). They identified specific markers for mast cells in a mouse model and found that blocking histamine activity can decrease the severity of OA symptoms and the release of substances associated with OA [57].
Gut Microbiota and Adaptive Immunity
Recent studies have specifically focused on the impact of adaptive immunity on the GM and osteoarthritis OA. An investigation of immune cells, their cytokines, and synovial inflammation in OA found that the predominant immune cells present in OA synovial tissues were mast cells, macrophages, and T cells. B, NK, and plasma cells were also detected in smaller quantities. Furthermore, they observed a high abundance of cytokines associated with T cells or macrophages in OA synovial tissues, suggesting that T cells and macrophages were activated in these tissues [58]. Another study discovered that T cells and macrophages were responsible for producing most pro-inflammatory cytokines, even without additional stimulation. Notably, CD4 + and CD69 + T cells were highly abundant [59]. Furthermore, there was a significant correlation between the quantity of CD4 + T cells in synovial tissues and the severity of pain, as measured by the visual analog scale (VAS) [59].
Currently, there is a consensus that there are notable changes in Th17 cells, Th9 cells, T memory cells, cytotoxic T cells, regulatory T (Treg) cells, and Th1 cells in the synovial fluid, synovial tissues, and peripheral blood of individuals with OA [60].
The GM has been suggested as a potential risk factor for OA and may regulate the T-cell response, particularly for Th17 cells [24]. Th17 cells, the first form of CD4 + T cells, are distinguished by their ability to produce pro-inflammatory cytokines, including IL-22, IL-21, and IL-17. The induction of Th17 cells can be triggered by pro-inflammatory cytokines such as IL-21, IL-6, and TGF-b [61]. TGF-b plays a two-fold role in immune regulation. Small quantities of TGF-b promote the development of Th17 cells, whereas large quantities of TGF-b stimulate the formation of Treg cells, which suppress inflammation [61]. Disruptions in the composition of microorganisms in the intestines can influence the transformation of CD4 + T cells into either regulatory T cells or effector T cells. This process is essential for maintaining a balanced immune system and preventing joint inflammation [24]. How do perturbations in GM lead to activation of Th17 cells? On the one hand, segmented filamentous bacteria can cause the buildup of Th17 cells in the synovial fluids and synovium of individuals with OA. This is achieved by increasing the synthesis of local serum amyloid A, which in turn stimulates DCs in the lamina propria to induce the formation of Th17 cells [24].
On the other hand, the concurrent administration of L. acidophilus, vitamin B, and curcumin in a rat model of OA resulted in pain relief, preservation of cartilage, modulation of the anabolic/catabolic equilibrium, and reduction of pro-inflammatory cytokines, such as MCP-1, TNF-α, IL17, and IL-1b. Moreover, T follicular helper (TFH) cells can control the activation of B cells to produce immunoglobulins by releasing IL-21. Butyrate is essential for maintaining the balance of the GM as It can stimulate the production of inducible Treg (iTreg) cells by increasing histone acetylation, which promotes gene expression by blocking HDAC activity [62].
Regarding B cells, they are responsible for producing immunoglobulins and modulating immune responses. Regulatory B cells (Bregs) function as immunosuppressive cells by upholding immunological tolerance through the secretion of IL-10, IL-35, and TGFb1, which in turn suppress the activation of Th1 cells, Th17 cells, and CD8 + T cells. They also play a role in controlling the differentiation of macrophages and DCs. Additionally, Bregs promote the development of regulatory T cells (Tregs) [63, 64]. An example of the link between GM and B cells is that the administration of butyrate as a supplement enhanced the activation of AhR in Bregs, leading to the alleviation of arthritis [65]. A study reported that individuals with RA had lower levels of SCFAs, such as valerate, butyrate, propionate, and acetate, and it was also observed that the amounts of these SCFAs were positively associated with the frequency of Bregs in the peripheral blood, rather than Tregs [66]. Administration of SCFAs alleviated arthritic symptoms, enhanced the occurrence of Bregs, and decreased the occurrence of transitional B and follicular B cells in collagen-induced mice arthritis by activating the FFA2 receptor [66]. In another study, SCFAs administration led to an increase in the expression of Bregs and an improvement in the clinical scores of arthritis in mice with collagen-induced arthritis. This effect was observed to rely on the inhibitory activity of HDAC [67]. These data suggest that the GM may have a similar regulatory influence on Bregs in OA.
The Gut–Joint Axis: How Dysbiosis Leads to Osteoarthritis
GM may be pivotal in the pathogenesis of OA itself and the perception of the pain that accompanies this condition [68].
Dysbiosis refers to an imbalance or disruption in the GM, significantly affecting gut permeability. It can appear in different forms, such as decreasing beneficial microorganisms and increasing dangerous or harmful species. Dysbiosis can arise due to various variables, including nutrition, antibiotic usage, stress levels, infections, lifestyle habits (such as smoking and excessive alcohol consumption), obesity, metabolic syndrome, age, and genetic predisposition [17].
Dysbiosis can result in a range of adverse effects on human health, such as:
-
(1)
Inflammation: dysbiosis can result in the secretion of pro-inflammatory cytokines and the generation of inflammatory compounds by the GM. Persistent inflammation is linked to various health issues, such as inflammatory bowel diseases (IBD), obesity, and autoimmune diseases [69].
-
(2)
Metabolic diseases, such as obesity and type 2 diabetes [70].
-
(3)
Impaired immune function: dysbiosis can harm the immune system, perhaps rendering patients more vulnerable to infections and autoimmune disorders [71].
Another consequence of dysbiosis that has been recently unraveled is the disruption of the “gut–joint axis”. GM is closely associated with various factors related to OA, including ageing, obesity, food, activity, joint abnormalities, and trauma. The complex connection between the gut and joints has led to the development of the gut–joint axis idea, which proposes that the GM may be crucial in understanding the development of OA [20].
This interplay is shaped by GM itself and its metabolites. SCFAs have been identified as potential contributors to osteoarthritis OA across several microbial communities. They are thought to regulate the balance of bone, decrease inflammation, and hinder bone breakdown by directly influencing osteoclasts’ activity [72]. Furthermore, a damaged intestinal barrier, commonly known as “leaky gut syndrome,” can result in the movement of microbial products into the bloodstream, leading to endotoxemia and an inflammatory condition. Performing fecal microbiota transplantation (FMT) from donors with metabolic issues in animal experiments accelerated the progression of OA, establishing a distinct correlation between GM and the initiation of OA [73].
How Pain is Generated at the Joint Level
A few pathophysiological mechanisms may occur in OA-related pain. An intense nociceptive drive from joint capsule, ligaments, periosteum, menisci, subchondral bone, and synovium has historically been used to characterize pain perception in OA as a peripheral joint condition [74]. Subgroups of individuals with OA may also exhibit characteristics of neuropathic pain, such as electric shocks and searing pain, according to recent research [75].
An intense and continuous intra-articular nociceptor activation can lead to pain chronification through neurogenic inflammation involving nociceptors and non-neuronal cells such as mast cells, basophils, platelets, macrophages, neutrophils, endothelial cells, keratinocytes, and fibroblasts, that in turn can amplify this damaging inflammation [76]. This persistent inflammatory state comprises increased vascularity, fibrin deposits, fibroblast-like synoviocytes hyperplasia/hypertrophy, and the infiltration of inflammatory cells such as lymphocytes and macrophages, leading to cartilage breakdown, subchondral bone remodeling synovitis and fibrosis [77]. All of these conditions could be caused by or made worse by microbial dysbiosis [78].
As a result of the inflammatory state, a decrease in the threshold and/or an increase in the amplitude of responsiveness at the sensory nerve fibers’ peripheral ends can occur (i.e., peripheral sensitization).
Also, in the dorsal root ganglions (DRGs), neuronal cell bodies coexist with small satellite glial cells and macrophages, and their interactions may facilitate the shift from acute to chronic pain [79].
Central sensitization can also occur in OA-related pain [80]. The “train” of joint nociceptor inputs results in an excessive CNS activation with functional and structural CNS changes. Central sensitization may lead to a combination of three phenomena: hyperalgesia, allodynia, and global sensory hyperresponsiveness [81] in response to any sensory input coming from the body’s periphery or external environment. Finally, central sensitization can explain spontaneous joint pain without sensory input from the periphery [81].
The Link Between GM and OA-Related Pain
GM has the potential to affect the central and peripheral sensitization mechanisms that contribute to chronic pain in a variety of ways. Several GM-produced substances are implicated, including metabolites, neurotransmitters, neuromodulators, and microbial byproducts [82]. The GM can adjust the sensitivity of neurons in the DRG and control the inflammation of nerves in both the peripheral and CNS in cases of chronic pain. Directly acting on primary sensory neurons in the DRGs, PAMPs cause immune cells to release chemokines and cytokines that promote inflammation. Conversely, PAMPs can directly activate or sensitize primary sensory neurons in DRGs. LPS may bind to TLR4 to trigger the activation and increased sensitivity of pain-sensing neurons in the DRGs, partly through a mechanism involving the Transient receptor potential vanilloid 1 (TRPV1) receptor [83]. Further, a mechanism independent of TLR4 is responsible for LPS’s direct stimulation of the transient receptor potential ankyrin 1 (TRPA1) channel, which in turn triggers the release of the calcitonin gene-related peptide (CGRP), calcium flow, and action potentials in sensory neurons that detect pain [84]. Thus, PAMPs originating from GM can potentially enhance peripheral sensitization by directly affecting primary nociceptive neurons or indirectly influencing immune cells to cause heightened neuronal excitability, resulting in peripheral sensitization.
Regarding central sensitization, the activation of glial cells, specifically microglia and astrocytes, can generate pro-inflammatory molecules known as cytokines or chemokines, including TNF-α, IL-1β, and CXCL1. This activation can lead to an increase in glutamatergic synaptic neurotransmission, a decrease in GABAergic synaptic neurotransmission, or both [85, 86]. Both effects contribute to the formation of central sensitization [87]. The link between GM and central sensitization is that GM significantly influences microglia’s maturation, morphology, and immunological function [88].
Furthermore, both parasympathetic and sympathetic systems have a major influence on GM and vice versa, therefore possibly affecting OA-related pain. It was shown that removing both superior cervical ganglia substantially impacted the composition of GM, suggesting that the sympathetic pathway plays a function in controlling GM [89]. Similarly, the vagus nerve and the enteric nervous system are critical links between GM and the peripheral nervous system [68]. Specifically, the vagus nerve has a dual purpose: it establishes a direct link between the gastrointestinal system and the brain, functioning as a conduit for all the GM mediators. On the other hand, vagal signaling plays a vital role in controlling immunological responses in the body’s periphery, as evidenced by studies on patients who have undergone vagotomy [90]. We can speculate that the implications of GM on autonomic dysfunctions may mimic or resemble some of the pathways implicated in other forms of chronic pain associated with autonomic nervous system derangement, such as fibromyalgia [91].
In a review exploring the GM-OA interplay by Favazzo et al., essential findings were identified: (a) there is a connection between the levels of bacterial metabolites in the bloodstream and joint degeneration; (b) antibiotics, a germ-free environment and a high-fat diet can cause changes in the microbial community in the gut; (c) dietary supplementation with joint-protective nutraceuticals may exert their effects by altering the composition of the GM [92].
If we look at experimental studies, three investigations [93,94,95] have discovered a direct association between the amounts of certain types of microorganisms or microbial products, notably LPS, and the inflammatory condition and intensity of symptoms related to osteoarthritis, including knee WOMAC discomfort. The primary microbial groups believed to be implicated are Clostridium and Streptococcus species. Clostridium has been demonstrated to stimulate Th17 cells and contribute to the development of arthritis. On the other hand, it is suggested that Streptococcus may induce heightened knee pain by activating macrophages either locally or systemically [94]. The assumptions above align with previous findings that establish a correlation between LPS and LPS-binding protein in patients with OA’s serum and synovial fluid and the presence of activated macrophages in the knee. Furthermore, it correlates these factors with the severity of OA and its primary symptom, pain [95]. In another study, a distinct DNA pattern of microorganisms was discovered in the knee and hip cartilage samples taken from patients with OA [96]. The study also showed increased Gram-negative components, such as LPS, in human OA cartilage. It is plausible to hypothesize that gut dysbiosis contributes to the development of inflammation in both local and systemic areas. This is likely due to the release of microbial products or metabolites through a compromised epithelial barrier, ultimately leading to the onset or worsening of pain associated with OA (Fig. 2).
Effects of GM on OA-related pain through modulation of peripheral sensitization, DRG modulation, and central sensitization. Different GM mediators are implicated in this interplay. Some may have a protective effect (for example, SCFAs), hence slowing down OA progression and dulling its pain, while others (for instance, LPS) may be harmful and speed up OA development. BA bile acids, LPS lipopolysaccharide, SCFAs short-chain fatty acids, LTA lipoteichoic acid, 5-HT 5-hydroxytryptamine, GABA γ-Aminobutyric acid
GUT Microbiota Modulation Strategies
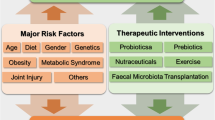
Harnessing the GM may represent a novel strategy to prevent and treat OA. Among the main GM modulators, the interventions that have been proposed are Diet and prebiotics, probiotics, nutraceuticals, exercise, and FMT (Fig. 3).
Diet and Prebiotics
Prebiotics are defined as a substance specifically used by microorganisms in the host’s body, resulting in a positive impact on health [97]. To be classified as prebiotic, the substance must possess the following characteristics: resistance to the acidic pH of the stomach, immunity to hydrolysis and absorption in the gastrointestinal tract, ability to undergo fermentation by GM, and the capacity to selectively promote the growth and activity of the GM with beneficial effects on the host [98].
Various categories of prebiotics exist, the majority of them being oligosaccharides, which are carbohydrates. The carbohydrate groups consist of galactooligosaccharides, fructans, and other oligosaccharides derived from starch, pectin, and glucose [99]. However, prebiotics are not exclusively restricted to carbohydrates. For instance, there are cocoa-derived flavanols, which, as indicated by in vivo and in vitro investigations, have stimulating effects on lactic acid bacteria [100]. Each prebiotic has a peculiar mechanism of action. For instance, fructans specifically act on lactic acid bacteria, while galactooligosaccharides target Bifidobacteria, Lactobacilli, Enterobacteria, Bacteroidetes, and Firmicutes [99]. Moreover, starch and glucose-derived oligosaccharides raise butyrate production by targeting Bifidobacteria [99].
There are many ways by which we can administer prebiotics. Oral supplementation may be an option, but the cheapest would be changing the patient’s lifestyle by focusing on their eating habits and motivating them to incorporate more GM-friendly foods into their daily routine. The influence of nutrition on GM’s composition, variety, and richness is significant throughout life. A diet that includes a wide range of fruits, vegetables, and fibers is linked to increased abundance and diversity of gut microorganisms [101]. For example, a study on mice showed that the consumption of fruits and vegetables not only increased the number of Firmicutes but also decreased the number of Bacteroidetes, resulting in a greater gut diversity [102]. Fresh salad and herbs should be prioritized for their high microbial load [103]. An example of foods that contain a high concentration of prebiotics is sugar beet, garlic, asparagus chicory, onion, Jerusalem artichoke, wheat, honey, banana, barley, tomato, rye, soybean, human’s and cow’s milk, etc. [99].
A few pre-clinical studies [104, 105] that explore the potential of oligofructose have been conducted. In a study, the administration of oligofructose corrected the imbalance in the intestinal microflora of obese mice. This was achieved by increasing the presence of important commensal microflora, specifically beneficial Bifidobacteria. As a result, it suppressed the subsequent inflammatory responses in both the local and systemic circulation, ultimately reducing joint cartilage injury associated with osteoarthritis [105]. Supplementing with oligofructose can prompt Bifidobacteria to reduce gut permeability, enhance the production of tight junction proteins, and suppress inflammation. Furthermore, oligofructose decreased the levels of pro-inflammatory cytokines, specifically MCP-1 and IL-12, while simultaneously increasing the levels of the anti-inflammatory cytokine IL-10 in obese mice. In addition, oligofructose inhibited the shift of macrophages associated with obesity to the joint synovium and decreased the levels of the pro-inflammatory cytokine MCP-1 in the joints of obese mice. This may suggest that oligofructose plays a role in regulating macrophage migration in the development of OA [105].
Another study found that early oligofructose supplementation could counteract the adverse effects of a high-fat/high-sucrose diet on joint damage in a rat model of OA. Additionally, this supplementation greatly improved insulin resistance, restored GM imbalances, and reduced endotoxins in the body [104]. The changes in the GM composition showed an increase in the populations of Bifidobacterium, Bacteroides/Prevotella, and Roseburia. At the same time, the levels of Akkermansia muciniphila, Methanobrevibacter, Faecalibacterium prausnitzii, Clostridium cluster I, and Clostridium cluster IV dropped. Bifidobacterium, Bacteroides/Prevotella, and Roseburia had a positive correlation with the preservation of cartilage, whereas Akkermansia muciniphila, Faecalibacterium prausnitzii, and Clostridium cluster IV displayed a positive association with cartilage deterioration. Regardless, the rats that were exposed to a high-fat/high-sucrose diet for 12 weeks were unable to recover from the existing knee damage even when given prebiotics and subjected to exercise [104], thus underlining the potential role of oligofructose as a prophylactic measure but not as a treatment.
Probiotics
Probiotics are live microorganisms that benefit the host when supplemented in sufficient quantities [106]. Most data available comes from pre-clinical studies, but some trials on patients have been completed, and some are ongoing. The main probiotics strains that have been investigated are Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus plantarum, and Streptococcus thermophilus (TCI633).
In a study on rats, Lactobacillus casei has been shown to alleviate inflammatory joint damage in collagen-induced arthritis [107]. Interestingly, Lactobacillus casei has also been investigated in association with type II collagen and glucosamine, where it has shown a synergic action in reducing pain cartilage breakdown and lymphocyte infiltration compared to glucosamine or probiotics alone [108]. At the molecular level, this synergistic action developed by decreasing the expression of the COX-2 enzyme, several pro-inflammatory cytokines (TNF-α, IL-1β, IL-2, and IFN-γ), and matrix metalloproteinases (MMP1, MMP3, and MMP13), while increasing anti-inflammatory cytokines (IL-4 and IL-10) in both synovial fibroblasts and chondrocytes [108]. A randomised, double-blind clinical investigation suggested that Lactobacillus casei Shirota had advantageous benefits on the treatment outcomes of knee joints affected by OA. This study examined 537 patients with knee OA and reported that taking a daily supplement of the probiotic Lactobacillus casei Shirota for 6 months resulted in significant improvements in the western Ontario and McMaster Universities Arthritis Index (WOMAC) functional scale, pain VAS. Moreover, compared to a placebo group, a reduction in systemic inflammation was noted as measured by hs-CRP levels [109]. CRP levels in OA patients, as demonstrated by Jin et al., are strongly correlated with pain and a decline in physical abilities [110].
Lactobacillus rhamnosus, a butyrate producer strain, showed pre-clinically in a rat model of OA an improvement in joint pain and inflammation [111]. The molecular pathway implicated in the rats that received the probiotic is an increased expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) and γ-aminobutyric acid (GABA), which are known to regulate pain. It has been shown that PPAR-γ ligands effectively hinder significant inflammatory signaling pathways in individuals with OA. They also reduce the COX-2/PGE2 pathway, MMP-1, and IL-6 production in human synovial fibroblasts [112].
Lactobacillus rhamnosus has also been investigated in a trial on a single individual, a 67-year-old female with OA In her lower back and right ankle [113]. The trial was organized in three blocks, each lasting 10 weeks. Probiotic supplementation consisted of a daily dosage of two capsules containing a combination of Lactobacillus rhamnosus, Saccharomyces cerevisiae, and Bifidobacterium animalis ssp. Lactis. The interventional group resulted in a modest yet medically meaningful decrease in pain score for the patient (VAS score 4.9 ± 2.2 in the placebo group vs 4.0 ± 1.7 in the probiotic group) [113].
Lactobacillus acidophilus, a probiotic generally used in yoghurt and other fermented dairy products, has been showing pre-clinically properties in relieving OA-associated pain by suppressing the production of pro-inflammatory cytokines and minimizing damage to the cartilage [114]. Furthermore, it was noted that the levels of TRPV1 and CGRP in the DRG were elevated in OA rats but were reduced by treatment with the probiotic. TRPV1 and CGRP have a potential role in mediating the pain phenotype in OA rats [115].
In another experimental murine model, Lactobacillus acidophilus was supplemented twice a week orally to mice with OA produced by partial medial meniscectomy [116]. The treatment resulted in a notable decrease in pain, the expression of TRPV1 in the DRG, and pro-inflammatory markers such as TNF-α and NF-κB in the knee. The study demonstrated that the administration of Lactobacillus acidophilus effectively alleviated knee joint pain caused by inflammation and halted the progression of OA when treatment was initiated during the inflammatory stage of joint pain [116]. In addition, the administration of Lactobacillus acidophilus resulted in a large increase in the abundance of the Akkermansia genus and bacteria belonging to the Lachnospiraceae family, which are responsible for SCFAs production [117].
Lactobacillus plantarum is a lactic acid bacterium found in the human stomach, possessing some immunoregulative proprieties by reducing the concentration of anti-inflammatory cytokines. In a pre-clinical study [118], this probiotic was evaluated In a rat model of knee OA produced by anterior cruciate ligament transection (ACLT). After 6 weeks, the intervention resulted in a notable decrease in pain-related behavior. The group treated with Lactobacillus plantarum showed low cartilage degradation and reduced levels of TNF-α and IL-1β, indicators of synovial inflammation generated by ACLT.
Streptococcus thermophilus (TCII633) is a new strain that has been identified in human breast milk, which possesses the ability to synthesize hyaluronic acid (HA) [119]. In a rat OA model with ACLT, the administration of TCI633 (at a dosage of 5 × 1010 or 5 × 1011 CFU/kg/day) and glucosamine led to pain behavior reduction, joint swelling reduction, and synovial tissue inflammation reduction in an osteoarthritis rat model. Additionally, an increase in the expression of type II collagen in the cartilage was noted [120]. HA has been recognized, together with physical and rehabilitative interventions, as an effective agent in improving disability, pain, and quality of life in subjects with knee and ankle OA [121].
Streptococcus thermophilus has also been investigated in a clinical trial on humans lasting 12 weeks, with 80 participants [122]. The treatment showed a significant improvement of 41.58% in serum collagen type II C-telopeptide (sCTX-II) and 39.58% in serum CRP. Despite these positive laboratory results, the pain, stiffness, and function values at 0, 4, 8, and 12 weeks in the probiotic group exhibited comparable levels without any significant statistical disparity among them. Moreover, the study found that the absence of any change in WOMAC scores demonstrated that the intervention effectively slowed down the progression and development of osteoarthritis by the end of the experiment [122].
A few trials exploring probiotics in OA patients have been completed, but the results have not been published yet [123,124,125]. It can be summarized that probiotics may alleviate pain associated with OA by decreasing the expression of MCP-1, CCR2, TRPV1, and CGRP in the DRG, by suppressing the expression of MMP, COX-2, MCP-1, CCR2, and pro-inflammatory cytokines in the tissues of the joints and by increasing the quantity of SCFA or type II collagen [126].
Nutraceuticals
Chondroitin and glucosamine are nutraceuticals commonly used in clinical practice for OA patients for their analgesic and chondroprotective effects [127].
The connection of these substances with GM is duplex: on one hand, they can regulate GM, and by doing so, they affect the GM–joint axis, potentially alleviating OA symptoms. On the other hand, the GM itself can also influence the effectiveness of nutraceuticals in the gut because they impact the metabolism of glucosamine sulfate and chondroitin metabolism, thereby restricting the efficacy of administering these substances orally. For instance, the degradation products of CSA, the main component of chondroitin sulfate, have been investigated in the GM of six healthy humans. It has been found that each subject’s GM showed different degrading activities, but all the end products contained DUAGalNAc4S. This suggests that chondroitin sulfate can be easily broken down to different extents by various microbial communities. The degradation may contribute to the low bioavailability and varying effects of chondroitin sulfate in managing patients with OA [128].
A pre-clinical study on mice discovered that chondroitin sulfate disaccharides could decrease blood LPS levels and enhance the levels of fecal total SCFAs, particularly butyrate. Furthermore, the addition of chondroitin sulfate resulted in a decrease in the presence of Proteobacteria and an increase in the presence of Bacteroidetes in the gastrointestinal tract [129]. This indicates that chondroitin sulfate is a bioactive nutraceutical with anti-inflammatory properties and protects the gut and its resident microbiota. In another pre-clinical study that compared the effectiveness of Chondroitin sulfate, chicken cartilage powder and collagen peptides in treating OA, it was found that chondroitin sulfate had the most effective therapeutic effect by significantly improving the structure of joint cartilage and reducing OA scores through a decrease in the levels of inflammatory cytokines, such as prostaglandin E2, TNF-α, IL-1b, IL-6, and IL-17, in the serum or synovial fluid. Additionally, chondroitin sulfate increased the presence of Bacteroidetes, a type of bacteria associated with beneficial effects [130].
Regarding glucosamine, a study found that supplementing glucosamine sulfate or green-lipped mussel extract could alleviate symptoms of OA by regulating the GM [93]. A systematic review identified eight studies that examined how glucosamine sulfate or chondroitin sulfate affected the composition of the GM in either adult people or animals [131]. The authors reported that chondroitin sulfate enhanced the presence of Bacteroides in the intestines of both people and mice, hinting at its potential role in alleviating OA. However, there was minimal data about the impact of glucosamine sulfate on the GM [131]. Further research is needed on nutraceuticals to study the potential effect of OA treatment by GM modulation.
Exercise
Data from pre-clinical and clinical studies demonstrated that exercise is a cornerstone of GM modulation. For instance, since 2008, running exercises in mice have been known to change GM, leading to an increase in butyrate levels, an SCFA that is pivotal as it is the primary fuel utilized by colon cells [132]. Consequently, the more butyrate is available, the higher the growth of cells in the colon lining, with an improvement in the gut barrier and the modulation of the host’s immune system and gene expression [133]. The same dynamic can be seen in humans, as a study showed that rugby players had a more diverse and abundant GM compared to inactive slim individuals. The athletes had higher levels of 40 different types of bacteria and exhibited reduced levels of Bacteroides and Lactobacillus species [134]. In these studies, exercise is not the only variable in the equation. The diet differed between the two comparative groups, with athletes consuming more proteins than the other group [134]. Similarly, another study found that physically active women who engaged in at least 3 h of exercise per week had higher amounts of Faecalibacterium prausnitzii, Roseburia hominis, and Akkermansia muciniphila compared to inactive individuals. These strains are key players in GM eubiosis as the first two strains produce butyrate while the latter is involved in enhanced metabolic health [135]. A possible explanation of this GM modulation by physical activity could be found in the ability of exercise to modify the gene expression of intraepithelial lymphocytes. This, in turn, reduces the production of pro-inflammatory cytokines and increases the secretion of anti-inflammatory cytokines and antioxidant compounds [136]. Moreover, exercise can impact the integrity of the mucus layer along the gastrointestinal mucosa, exerting a double action by hindering the attachment of microorganisms to the lining of the mucosa and by acting as a source of nourishment for particular microorganisms such as Akkermansia muciniphila [137].
Lastly, subjects engaged in more physical activities have lower resting levels of circulating bacterial endotoxin LPS and a stronger heat-shock protein (HSP) response to heat stress than sedentary individuals [138]. How is this more robust HSP response going to help? By hindering the deterioration of tight junction proteins that connect epithelial cells, thus reinforcing the gut barrier [139].
A possible mechanism behind these data may be found in the connection between gut lymphatic vessels and GM. The lymph production and propulsion process is essential for maintaining the proper balance of fluid in the interstitial tissue and serosal spaces [140]. Exercise may contribute to lymph propulsion by an extrinsic mechanism, which usually affects blood vessels in parts of the body that undergo regular motions, such as the heart or skeletal muscles and lymphatic vessels affected by heart activity or breathing, intestinal movement, and external pressure [141]. For this propulsion to happen, it is pivotal to maintain the integrity of the lacteals, i.e., the lymph vessels located in the villi, whose integrity depends on GM eubiosis [142]. Lymph propulsion may allow several GM mediators discussed above to reach their target, such as the joint or the brain along the gut–joint and gut-brain axis (Fig. 4).
Established that exercise strongly impacts GM, this “positive” perturbation has to be linked to a positive change in OA pathogenesis.
Therapies involving aerobic exercise and prebiotic fiber, individually or in combination, effectively shielded knee joints from harm in rat models of OA. This indicates that exercise may impact the advancement of OA in conjunction with GM [104] by influencing apoptotic, pro-inflammatory, and anti-inflammatory signals. Similarly, physical activity in rats with an OA model enhances the variety of microorganisms in the GM, decreases the levels of LPS in synovial fluids and blood, reduces the expression of MMP-13 and TLR4, and improves cartilage damage [143].
Whole body vibration (WBV) is an innovative neuromuscular approach that utilizes the vibration produced by a vibration platform to enhance the bioactivity of muscle groups [144]. This therapy has demonstrated in rats that it leads to a notable rise in the numbers of CD4 and CD25 positive lymphocytes, as well as an improvement in the differentiation of Treg cells with a significant rise in the abundance of Lactobacillus animalis [145]. The changes in the composition of GM, specifically concerning Lactobacillus spp., caused by WBV were found to be associated with the development of T reg cells in mice. These cells have a crucial function in the human intestine by producing IL-10, which is necessary for maintaining the balance and stability of the intestinal environment and the GM [145]. Thus, WBW may affect OA pathogenesis by modulating GM, which in turn affects the immune system, dulling the low-grade inflammation, which is the light motive of OA pathogenesis [146].
Fecal Microbiota Transplantation
FMT is a medical procedure whereby a dysbiotic patient receives stools from a “healthy” donor to restore eubiosis [147]. Every bacteria that live in the gut normally, along with all its byproducts, is present in an FMT sample. Because of this, this therapy might be more effective than others at restoring dysbiosis [148]. There are hazards associated with this procedure, though. As a matter of fact, infections that are undesirable and/or undiagnosed may also be transferred from the donor to the receiving person, occasionally even leading to death [149]. FMT has been demonstrated to be highly effective in treating recurrent Clostridium difficile infection [150], which led scientists to investigate the use of FMT in various conditions, such as allergic diseases, metabolic syndrome, irritable bowel syndrome, and inflammatory disease [151].
Among the medical conditions that may benefit from this procedure, OA treatment by FMT has also been hypothesized. An interesting attempt to explore the feasibility of FMT has been performed by Huang et al. in mice [41]. Fecal samples were collected from human donors: four were healthy controls, four had knee OA without metabolic syndrome, and four had knee OA with metabolic syndrome. These three groups of samples were transplanted into germ-free mice 2 weeks before getting meniscal ligamentous injury (MLI). After 10 weeks from the start of the experiment, the severity of histological OA in mice was assessed, along with systemic inflammation, intestinal permeability, and synovitis. The mice that had been transplanted with the samples from patients with OA and metabolic syndrome exhibited elevated cartilage damage scores, increased levels of serum inflammatory factors (IL-1b, IL-6, and macrophage inflammatory protein-1a), higher levels of serum LPS, greater intestinal permeability, and reduced diversity of the GM. The study found a significant correlation between the gut bacterial genera, cartilage histology scores, and inflammatory factors. Specifically, the presence of Fusobacterium and Faecalibacterium and the decreased abundance of Ruminococcaceae were associated with higher cartilage histology scores and increased levels of inflammatory factors [41]. This study confirmed the two-hit models of OA pathogenesis where one hit Is the emergence of gut dysbiosis and the second is the joint damage, paving the way for new studies exploring the possibility of GM modulation by FMT to treat OA. No studies have been done in humans, let alone for a single case report where FMT has been employed on a patient to treat RA [152]. Further research is urgently needed in the light of these findings.
Conclusions
The developing comprehension of the connection between this meticulous symphony director, the GM, and joints, known as the gut–joint axis, with its potential impact on OA is a promising area of investigation. Nevertheless, numerous inquiries still lack resolution. To completely understand the onset and progression of the disease, it is necessary to conduct thorough investigations into the gut bacteriome, mycobiome, and virome in individuals with OA, pushing the transition from bench to bedside. Gaining insight into these intricate relationships has the potential to unlock novel approaches for preventing and treating OA. The correlation between GM and OA signifies a captivating and dynamic area of research. The idea of the gut–joint axis emphasizes the complex interaction between GM and joint health. Currently, the main evidence points to the fact that if, on the one hand, interventions such as supplements and FMT may require further validation, on the other hand, others such as diet and exercise, may already be slowly implemented as they have been proven feasible and safe by their beneficial effect through several mechanisms on a broad spectrum of pathologies. Advancements in research in this field may reveal new and effective treatment options for addressing this widespread and incapacitating ailment. Although there is still a great deal of knowledge to acquire, the potential influence on the well-being of individuals with OA is encouraging and exciting.
Data Availability
Not applicable.
References
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. https://doi.org/10.1002/art.34453.
Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. https://doi.org/10.1002/art.23176.
Cucchiarini M, de Girolamo L, Filardo G, Oliveira JM, Orth P, Pape D, Reboul P. Basic science of osteoarthritis. J Exp Orthop. 2016;3:22. https://doi.org/10.1186/s40634-016-0060-6.
Migliorini F, Maffulli N, Pintore A, Ernst J, Eschweiler J, Hildebrand F, Betsch M. Osteoarthritis risks and sports: an evidence-based systematic review. Sports Med Arthrosc Rev. 2022. https://doi.org/10.1097/JSA.0000000000000351.
Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2008. https://doi.org/10.1016/j.rdc.2008.05.007.
van Meurs JBJ. Osteoarthritis year in review 2016: genetics, genomics and epigenetics. Osteoarthritis Cartilage. 2017;25:181–9. https://doi.org/10.1016/j.joca.2016.11.011.
Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11:2393. https://doi.org/10.3390/nu11102393.
Corriero A, Gadaleta RM, Puntillo F, Inchingolo F, Moschetta A, Brienza N. The central role of the gut in intensive care. Crit Care. 2022;26:379. https://doi.org/10.1186/s13054-022-04259-8.
Inchingolo AD, Malcangi G, Inchingolo AM, Piras F, Settanni V, Garofoli G, Palmieri G, Ceci S, Patano A, De Leonardis N, et al. Benefits and implications of resveratrol supplementation on microbiota modulations: a systematic review of the literature. Int J Mol Sci. 2022;23:4027. https://doi.org/10.3390/ijms23074027.
Inchingolo AD, Malcangi G, Semjonova A, Inchingolo AM, Patano A, Coloccia G, Ceci S, Marinelli G, Di Pede C, Ciocia AM, et al. Oralbiotica/oralbiotics: the impact of oral microbiota on dental health and demineralization: a systematic review of the literature. Child Basel Switz. 2022;9:1014. https://doi.org/10.3390/children9071014.
Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. https://doi.org/10.1111/1574-6976.12075.
Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018. https://doi.org/10.3389/fmicb.2018.01835.
Inchingolo F, Inchingolo AM, Malcangi G, De Leonardis N, Sardano R, Pezzolla C, de Ruvo E, Di Venere D, Palermo A, Inchingolo AD, et al. The benefits of probiotics on oral health: systematic review of the literature. Pharmaceuticals. 2023;16:1313. https://doi.org/10.3390/ph16091313.
Minalyan A, Gabrielyan L, Scott D, Jacobs J, Pisegna JR. The gastric and intestinal microbiome: role of proton pump inhibitors. Curr Gastroenterol Rep. 2017;19:42. https://doi.org/10.1007/s11894-017-0577-6.
Ceci S, Berate P, Candrea S, Babtan A-M, Azzollini D, Piras F, Curatoli L, Corriero A, Patano A, Valente F, et al. The oral and gut microbiota: beyond a short communication. Balneo PRM Res J. 2021. https://doi.org/10.12680/balneo.2021.471.
Olsen I, Yamazaki K. Can oral bacteria affect the microbiome of the gut? J Oral Microbiol. 2019;11:1586422. https://doi.org/10.1080/20002297.2019.1586422.
Hrncir T. Gut microbiota dysbiosis: triggers, consequences, diagnostic and therapeutic options. Microorganisms. 2022;10:578. https://doi.org/10.3390/microorganisms10030578.
Sun C, Zhou X, Guo T, Meng J. The immune role of the intestinal Microbiome in knee osteoarthritis: a review of the possible mechanisms and therapies. Front Immunol. 2023. https://doi.org/10.3389/fimmu.2023.1150572.
Liu S, Li G, Xu H, Wang Q, Wei Y, Yang Q, Xiong A, Yu F, Weng J, Zeng H. “Cross-Talk” between gut microbiome dysbiosis and osteoarthritis progression: a systematic review. Front Immunol. 2023. https://doi.org/10.3389/fimmu.2023.1150572.
Hao X, Shang X, Liu J, Chi R, Zhang J, Xu T. The gut microbiota in osteoarthritis: Where do we stand and what can we do? Arthritis Res Ther. 2021;23:42. https://doi.org/10.1186/s13075-021-02427-9.
Li J, Lv J, Cao X, Zhang H, Tan Y, Chu T, Zhao L, Liu Z, Ren Y. Gut microbiota dysbiosis as an inflammaging condition that regulates obesity-related retinopathy and nephropathy. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.1040846.
Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. https://doi.org/10.3389/fimmu.2017.01385.
Gut Microbiota and Obesity-Associated Osteoarthritis - Osteoarthritis and Cartilage Available online: https://www.oarsijournal.com/article/S1063-4584(19)31006-4/fulltext (accessed on 2 Jan 2024).
Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–95. https://doi.org/10.1146/annurev-immunol-020711-074937.
Liu Y, Wang J, Wu C. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front Nutr. 2022;8: 634897. https://doi.org/10.3389/fnut.2021.634897.
Dunand E, Burns P, Binetti A, Bergamini C, Peralta GH, Forzani L, Reinheimer J, Vinderola G. Postbiotics produced at laboratory and industrial level as potential functional food ingredients with the capacity to protect mice against salmonella infection. J Appl Microbiol. 2019;127:219–29. https://doi.org/10.1111/jam.14276.
Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via P44/42 MAPK and NF-κB. Biochem J. 2006;396:517–27. https://doi.org/10.1042/BJ20051839.
Singh S, Bhatia R, Singh A, Singh P, Kaur R, Khare P, Purama RK, Boparai RK, Rishi P, Ambalam P, et al. Probiotic attributes and prevention of LPS-induced pro-inflammatory stress in RAW264.7 macrophages and human intestinal epithelial cell line (Caco-2) by newly isolated Weissella cibaria strains. Food Funct. 2018;9:1254–64. https://doi.org/10.1039/C7FO00469A.
Boer CG, Radjabzadeh D, Uitterlinden AG, Kraaij R, van Meurs JB. The role of the gut microbiome in osteoarthritis and joint pain. Osteoarthritis Cartilage. 2017;25:S10. https://doi.org/10.1016/j.joca.2017.02.033.
Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: Is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565. https://doi.org/10.1097/BOR.0b013e32830aba34.
Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–75. https://doi.org/10.1111/j.1600-065X.2012.01146.x.
Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN. Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol. 2019;10:2441. https://doi.org/10.3389/fimmu.2019.02441.
Danger Signals and Inflammaging in Osteoarthritis Available online: https://www.clinexprheumatol.org/abstract.asp?a=14559 (accessed on 21 Jan 2024).
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. https://doi.org/10.1038/ni.1863.
Zhao Y, Chen B, Li S, Yang L, Zhu D, Wang Y, Wang H, Wang T, Shi B, Gai Z, et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci Rep. 2018;8:14305. https://doi.org/10.1038/s41598-018-32675-w.
Lambert C, Zappia J, Sanchez C, Florin A, Dubuc J-E, Henrotin Y. The Damage-Associated Molecular Patterns (DAMPs) as potential targets to treat osteoarthritis: perspectives from a review of the literature. Front Med. 2021;7:607186. https://doi.org/10.3389/fmed.2020.607186.
Pessler F, Chen LX, Dai L, Gomez-Vaquero C, Diaz-Torne C, Paessler ME, Scanzello C, Çakir N, Einhorn E, Schumacher HR. A histomorphometric analysis of synovial biopsies from individuals with Gulf War veterans’ illness and joint pain compared to normal and osteoarthritis synovium. Clin Rheumatol. 2008;27:1127–34. https://doi.org/10.1007/s10067-008-0878-0.
Both Systemic and Local Lipopolysaccharide (LPS) Burden are associated with knee OA severity and inflammation—osteoarthritis and cartilage. Available online: https://www.oarsijournal.com/article/S1063-4584(16)30086-3/fulltext (accessed on 22 Jan 2024).
Huang Z, Kraus VB. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016;12:123–9. https://doi.org/10.1038/nrrheum.2015.158.
NLRP1 and NLRP3 Inflammasomes Mediate LPS/ATP induced Pyroptosis in Knee Osteoarthritis Available online: https://doi.org/10.3892/mmr.2018.8520 (accessed on 22 Jan 2024).
Huang Z, Chen J, Li B, Zeng B, Chou C-H, Zheng X, Xie J, Li H, Hao Y, Chen G, et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann Rheum Dis. 2020;79:646–56. https://doi.org/10.1136/annrheumdis-2019-216471.
Synergistic roles of macrophages and neutrophils in osteoarthritis progression - Hsueh - 2021 - Arthritis & Rheumatology - Wiley Online Library. Available online: https://doi.org/10.1002/art.41486 (accessed on 22 Jan 2024).
Human Dendritic Cell Subsets: An Update - Collin - 2018 - Immunology - Wiley Online Library Available online: https://doi.org/10.1111/imm.12888 (accessed on 23 Jan 2024).
Figliuolo da Paz V, Jamwal DR, Gurney M, Midura-Kiela M, Harrison CA, Cox C, Wilson JM, Ghishan FK, Kiela PR. Rapid downregulation of DAB2 by Toll-like receptor activation contributes to a pro-inflammatory switch in activated dendritic cells. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.00304.
Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–8. https://doi.org/10.1074/jbc.M110.102947.
Immunomodulation of Dendritic Cells by Lactobacillus Reuteri Surface Components and Metabolites - Engevik - 2021 - Physiological Reports - Wiley Online Library Available online: https://doi.org/10.14814/phy2.14719 (accessed on 23 Jan 2024).
Kalaitzoglou E, Lopes EBP, Fu Y, Herron JC, Flaming JM, Donovan EL, Hu Y, Filiberti A, Griffin TM, Humphrey MB. TLR4 promotes and DAP12 limits obesity-induced osteoarthritis in aged female mice. JBMR Plus. 2019;3: e10079. https://doi.org/10.1002/jbm4.10079.
Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. https://doi.org/10.1016/S1471-4906(01)02060-9.
Synovial Tissue–Infiltrating Natural Killer Cells in Osteoarthritis and Periprosthetic Inflammation. https://doi.org/10.1002/art.27751.
Jaime P, García-Guerrero N, Estella R, Pardo J, García-Álvarez F, Martinez-Lostao L. CD56+/CD16−natural killer cells expressing the inflammatory protease Granzyme A are enriched in synovial fluid from patients with osteoarthritis. Osteoarthritis Cartilage. 2017;25:1708–18. https://doi.org/10.1016/j.joca.2017.06.007.
de Matos CT, Berg L, Michaëlsson J, Felländer-Tsai L, Kärre K, Söderström K. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology. 2007;122:291–301. https://doi.org/10.1111/j.1365-2567.2007.02638.x.
Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. https://doi.org/10.1038/nri2018.
Zhang H, Du M, Yang Q, Zhu M-J. Butyrate suppresses murine mast cell proliferation and cytokine production through inhibiting histone deacetylase. J Nutr Biochem. 2016;27:299–306. https://doi.org/10.1016/j.jnutbio.2015.09.020.
Folkerts J, Redegeld F, Folkerts G, Blokhuis B, van den Berg MPM, de Bruijn MJW, van IJcken WFJ, Junt T, Tam S-Y, Galli SJ, et al. Butyrate inhibits human mast cell activation via epigenetic regulation of fcεri-mediated signaling. Allergy. 2020;75:1966–78. https://doi.org/10.1111/all.14254.
Kirshenbaum AS, Swindle E, Kulka M, Wu Y, Metcalfe DD. Effect of Lipopolysaccharide (LPS) and Peptidoglycan (PGN) on human mast cell numbers, cytokine production, and protease composition. BMC Immunol. 2008;9:45. https://doi.org/10.1186/1471-2172-9-45.
IJMS | Free Full-Text | Mast cells differentiated in synovial fluid and resident in osteophytes exalt the inflammatory pathology of osteoarthritis. Available online: https://www.mdpi.com/1422-0067/23/1/541 (accessed on 24 Jan 2024).
Zhao X, Younis S, Shi H, Hu S, Zia A, Wong HH, Elliott EE, Chang T, Bloom MS, Zhang W, et al. RNA-Seq characterization of histamine-releasing mast cells as potential therapeutic target of osteoarthritis. Clin Immunol. 2022;244: 109117. https://doi.org/10.1016/j.clim.2022.109117.
de Lange-Brokaar BJE, Ioan-Facsinay A, van Osch GJVM, Zuurmond A-M, Schoones J, Toes REM, Huizinga TWJ, Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484–99. https://doi.org/10.1016/j.joca.2012.08.027.
Klein-Wieringa IR, de Lange-Brokaar BJE, Yusuf E, Andersen SN, Kwekkeboom JC, Kroon HM, van Osch GJVM, Zuurmond A-M, Stojanovic-Susulic V, Nelissen RGHH, et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J Rheumatol. 2016;43:771–8. https://doi.org/10.3899/jrheum.151068.
Frontiers | T Cells in Osteoarthritis: Alterations and Beyond. Available online: https://doi.org/10.3389/fimmu.2017.00356/full (accessed on 24 Jan 2024).
Chewning JH, Weaver CT. Development and survival of Th17 cells within the intestines: the influence of microbiome- and diet-derived signals. J Immunol. 2014;193:4769–77. https://doi.org/10.4049/jimmunol.1401835.
Hao F, Tian M, Zhang X, Jin X, Jiang Y, Sun X, Wang Y, Peng P, Liu J, Xia C, et al. Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc Natl Acad Sci. 2021;118: e2014681118. https://doi.org/10.1073/pnas.2014681118.
Rosser EC, Blair PA, Mauri C. Cellular targets of regulatory B cell-mediated suppression. Mol Immunol. 2014;62:296–304. https://doi.org/10.1016/j.molimm.2014.01.014.
Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra23-173ra23. https://doi.org/10.1126/scitranslmed.3005407.
Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, Alber DG, Krausgruber T, Catalan D, Klein N, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31:837-851.e10. https://doi.org/10.1016/j.cmet.2020.03.003.
Yao Y, Cai X, Zheng Y, Zhang M, Fei W, Sun D, Zhao M, Ye Y, Zheng C. Short-chain fatty acids regulate B cells differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br J Pharmacol. 2022;179:4315–29. https://doi.org/10.1111/bph.15852.
Zou F, Qiu Y, Huang Y, Zou H, Cheng X, Niu Q, Luo A, Sun J. Effects of short-chain fatty acids in inhibiting HDAC and activating P38 MAPK are critical for promoting B10 cell generation and function. Cell Death Dis. 2021;12:1–17. https://doi.org/10.1038/s41419-021-03880-9.
Corriero A, Giglio M, Inchingolo F, Moschetta A, Varrassi G, Puntillo F. Gut microbiota modulation and its implications on neuropathic pain: a comprehensive literature review. Pain Ther. 2024;13:33–51. https://doi.org/10.1007/s40122-023-00565-3.
Lobionda S, Sittipo P, Kwon HY, Lee YK. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms. 2019;7:271. https://doi.org/10.3390/microorganisms7080271.
Zhou Z, Sun B, Yu D, Zhu C. Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol. 2022. https://doi.org/10.3389/fcimb.2022.834485.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. https://doi.org/10.1038/s41422-020-0332-7.
Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B, Krönke G, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9:55. https://doi.org/10.1038/s41467-017-02490-4.
Chen J, Wang A, Wang Q. Dysbiosis of the gut microbiome is a risk factor for osteoarthritis in older female adults: a case control study. BMC Bioinformatics. 2021;22:299. https://doi.org/10.1186/s12859-021-04199-0.
Puntillo F, Giglio M, Paladini A, Perchiazzi G, Viswanath O, Urits I, Sabbà C, Varrassi G, Brienza N. Pathophysiology of musculoskeletal pain: a narrative review. Ther Adv Musculoskelet Dis. 2021;13:1759720X21995067. https://doi.org/10.1177/1759720X21995067.
Zolio L, Lim KY, McKenzie JE, Yan MK, Estee M, Hussain SM, Cicuttini F, Wluka A. Systematic review and meta-analysis of the prevalence of neuropathic-like pain and/or pain sensitization in people with knee and hip osteoarthritis. Osteoarthritis Cartilage. 2021;29:1096–116. https://doi.org/10.1016/j.joca.2021.03.021.
Gangadharan V, Kuner R. Pain hypersensitivity mechanisms at a glance. Dis Model Mech. 2013;6:889–95. https://doi.org/10.1242/dmm.011502.
Rai MF, Brophy RH, Sandell LJ. Osteoarthritis following meniscus and ligament injury: insights from translational studies and animal models. Curr Opin Rheumatol. 2019;31:70–9. https://doi.org/10.1097/BOR.0000000000000566.
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–75. https://doi.org/10.1038/s41584-022-00749-9.
Hameed S. Nav1.7 and Nav1.8: role in the pathophysiology of pain. Mol Pain. 2019;15:1744806919858801. https://doi.org/10.1177/1744806919858801.
Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043–56. https://doi.org/10.1016/j.joca.2015.02.163.
Volcheck MM, Graham SM, Fleming KC, Mohabbat AB, Luedtke CA. Central sensitization, chronic pain, and other symptoms: better understanding, better management. Cleve Clin J Med. 2023;90:245–54. https://doi.org/10.3949/ccjm.90a.22019.
Morreale C, Bresesti I, Bosi A, Baj A, Giaroni C, Agosti M, Salvatore S. Microbiota and pain: save your gut feeling. Cells. 2022;11:971. https://doi.org/10.3390/cells11060971.
Painful Pathways Induced by TLR Stimulation of Dorsal Root Ganglion Neurons | The Journal of Immunology | American Association of Immunologists Available online: https://journals.aai.org/jimmunol/article/186/11/6417/84213/Painful-Pathways-Induced-by-TLR-Stimulation-of (accessed on 27 Jan 2024).
Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn J-A, Fernández-Peña C, Talavera A, Kichko T, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. https://doi.org/10.1038/ncomms4125.
Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain: Neuron. Available online: https://www.cell.com/neuron/fulltext/S0896-6273(18)31000-6?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627318310006%3Fshowall%3Dtrue (accessed on 27 Jan 2024).
Gao Y-J, Ji R-R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. https://doi.org/10.1016/j.pharmthera.2010.01.002.
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. https://doi.org/10.1016/j.jpain.2009.06.012.
Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. https://doi.org/10.1038/nn.4030.
Zhang W, Li Y, Sun T, Li Z, Manyande A, Xu W, Feng M, Xiang H. Superior cervical ganglionectomy alters gut microbiota in rats. Am J Transl Res. 2022;14:2037–50.
Gallaher ZR, Ryu V, Herzog T, Ritter RC, Czaja K. Changes in microglial activation within the hindbrain, nodose ganglia, and the spinal cord following subdiaphragmatic vagotomy. Neurosci Lett. 2012;513:31–6. https://doi.org/10.1016/j.neulet.2012.01.079.
Vincent A, Whipple MO, Low PA, Joyner M, Hoskin TL. Patients with fibromyalgia have significant autonomic symptoms but modest autonomic dysfunction. PM&R. 2016;8:425–35. https://doi.org/10.1016/j.pmrj.2015.08.008.
Favazzo LJ, Hendesi H, Villani DA, Soniwala S, Dar Q-A, Schott EM, Gill SR, Zuscik MJ. The gut microbiome–joint connection: implications in osteoarthritis. Curr Opin Rheumatol. 2020;32:92–101. https://doi.org/10.1097/BOR.0000000000000681.
Coulson S, Butt H, Vecchio P, Gramotnev H, Vitetta L. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology. 2013;21:79–90. https://doi.org/10.1007/s10787-012-0146-4.
Boer CG, Radjabzadeh D, Medina-Gomez C, Garmaeva S, Schiphof D, Arp P, Koet T, Kurilshikov A, Fu J, Ikram MA, et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019;10:4881. https://doi.org/10.1038/s41467-019-12873-4.
Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage. 2016;24:1769–75. https://doi.org/10.1016/j.joca.2016.05.008.
Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis - Dunn - 2020 - Arthritis & Rheumatology - Wiley Online Library. Available online: https://doi.org/10.1002/art.41210 (accessed on 27 Jan 2024).
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. https://doi.org/10.1038/nrgastro.2017.75.
[PDF] Dietary Prebiotics: Current Status and New Definition | Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Dietary-prebiotics%3A-current-status-and-new-Gibson-Scott/84f996af4109fce9181d5c416573d9e3d999a2e5 (accessed on 29 Jan 2024).
Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. https://doi.org/10.3390/foods8030092.
Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study123. Am J Clin Nutr. 2011;93:62–72. https://doi.org/10.3945/ajcn.110.000075.
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol WJG. 2015;21:8787–803. https://doi.org/10.3748/wjg.v21.i29.8787.
Yu C, Guo C, Geng X, Yao Y, Guo J, Zhang Y, Zhang J, Mi S. Effects of fruits and vegetables on gut microbiota in a mouse model of metabolic syndrome induced by high-fat diet. Food Sci Nutr. 2022;11:794–805. https://doi.org/10.1002/fsn3.3114.
Becker B, Stoll D, Schulz P, Kulling S, Huch M. Microbial contamination of organically and conventionally produced fresh vegetable salads and herbs from retail markets in southwest Germany. Foodborne Pathog Dis. 2019;16:269–75. https://doi.org/10.1089/fpd.2018.2541.
Rios JL, Bomhof MR, Reimer RA, Hart DA, Collins KH, Herzog W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9:3893. https://doi.org/10.1038/s41598-019-40601-x.
Schott EM, Farnsworth CW, Grier A, Lillis JA, Soniwala S, Dadourian GH, Bell RD, Doolittle ML, Villani DA, Awad H, et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.95997.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. https://doi.org/10.1038/nrgastro.2014.66.
Amdekar S, Singh V, Singh R, Sharma P, Keshav P, Kumar A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines. J Clin Immunol. 2011;31:147–54. https://doi.org/10.1007/s10875-010-9457-7.
So J-S, Song M-K, Kwon H-K, Lee C-G, Chae C-S, Sahoo A, Jash A, Lee SH, Park ZY, Im S-H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011;88:358–66. https://doi.org/10.1016/j.lfs.2010.12.013.
Lei M, Guo C, Wang D, Zhang C, Hua L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benef Microbes. 2017;8:697–703. https://doi.org/10.3920/BM2016.0207.
Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, Ding C. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:703–10. https://doi.org/10.1136/annrheumdis-2013-204494.
Cells | Free Full-Text | Oral Administration of Lactobacillus Rhamnosus Ameliorates the Progression of Osteoarthritis by Inhibiting Joint Pain and Inflammation. Available online: https://www.mdpi.com/2073-4409/10/5/1057 (accessed on 30 Jan 2024).
Giaginis C, Giagini A, Theocharis S. Peroxisome proliferator-activated receptor-γ (PPAR-γ) ligands as potential therapeutic agents to treat arthritis. Pharmacol Res. 2009;60:160–9. https://doi.org/10.1016/j.phrs.2009.02.005.
Taye I, Bradbury J, Grace S, Avila C. Probiotics for pain of osteoarthritis; an N-of-1 trial of individual effects. Complement Ther Med. 2020;54: 102548. https://doi.org/10.1016/j.ctim.2020.102548.
Lee SH, Kwon JY, Jhun J, Jung K, Park S-H, Yang CW, Cho Y, Kim SJ, Cho M-L. Lactobacillus acidophilus ameliorates pain and cartilage degradation in experimental osteoarthritis. Immunol Lett. 2018;203:6–14. https://doi.org/10.1016/j.imlet.2018.07.003.
Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388:75–80. https://doi.org/10.1016/j.neulet.2005.06.044.
O-Sullivan I, Natarajan-Anbazhagan A, Singh G, Ma K, Green SJ, Singhal M, Wang J, Kumar A, Dudeja PK, Unterman TG, et al. Lactobacillus acidophilus mitigates osteoarthritis-associated pain, cartilage disintegration and gut microbiota dysbiosis in an experimental murine OA model. Biomedicines. 2022;10:1298. https://doi.org/10.3390/biomedicines10061298.
Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut Lachnospiraceae. Microorganisms. 2020;8:573. https://doi.org/10.3390/microorganisms8040573.
Lin Y-Y, Chang SL-Y, Liu S-C, Achudhan D, Tsai Y-S, Lin S-W, Chen Y-L, Chen C-C, Chang J-W, Fong Y-C, et al. Therapeutic effects of live lactobacillus plantarum GKD7 in a rat model of knee osteoarthritis. Nutrients. 2022;14:3170. https://doi.org/10.3390/nu14153170.
Lin YH, Su HL, Yu CH. Method of enhancing hyaluronic acid secretion using probiotic strain. US Patent US9289455B2. Filed 26 Mar 2015; issued 15 Oct 2015.
Lin Y-Y, Chen N-F, Yang S-N, Jean Y-H, Kuo H-M, Chen P-C, Feng C-W, Liu Y-W, Lai Y-C, Wen Z-H. Effects of Streptococcus thermophilus on anterior cruciate ligament transection-induced early osteoarthritis in rats. Exp Ther Med. 2021;21:1–1. https://doi.org/10.3892/etm.2021.9653.
Monticone M, Frizziero A, Rovere G, Vittadini F, Uliano D, LA Bruna S, Gatto R, Nava C, Leggero V, Masiero S. Hyaluronic acid intra-articular injection and exercise therapy: effects on pain and disability in subjects affected by lower limb joints osteoarthritis. A systematic review by the Italian Society of Physical and Rehabilitation Medicine (SIMFER). Eur J Phys Rehabil Med. 2016;52:389–99.
Lyu J-L, Wang T-M, Chen Y-H, Chang S-T, Wu M-S, Lin Y-H, Lin Y-H, Kuan C-M. Oral intake of Streptococcus thermophilus improves knee osteoarthritis degeneration: a randomized, double-blind, placebo-controlled clinical study. Heliyon. 2020. https://doi.org/10.1016/j.heliyon.2020.e03757.
Villafañe JH. A probiotic intervention on pain hypersensitivity and microbiota composition in patients with pain-osteoarthritis: study protocol for a randomized controlled trial. clinicaltrials.gov, 2019.
Villafañe JH. Deciphering the role of the microbiota in osteoarthritis for improving therapy. clinicaltrials.gov, 2019.
ANZCTR - Registration Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=380734&isReview=true (accessed on 31 Jan 2024).
Rahman SO, Bariguian F, Mobasheri A. The potential role of probiotics in the management of osteoarthritis pain: current status and future prospects. Curr Rheumatol Rep. 2023. https://doi.org/10.1007/s11926-023-01108-7.
Colletti A, Cicero AFG. Nutraceutical approach to chronic osteoarthritis: from molecular research to clinical evidence. Int J Mol Sci. 2021;22:12920. https://doi.org/10.3390/ijms222312920.
Shang Q, Yin Y, Zhu L, Li G, Yu G, Wang X. Degradation of chondroitin sulfate by the gut microbiota of Chinese individuals. Int J Biol Macromol. 2016;86:112–8. https://doi.org/10.1016/j.ijbiomac.2016.01.055.
Liu F, Zhang N, Li Z, Wang X, Shi H, Xue C, Li RW, Tang Q. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci Rep. 2017;7:6783. https://doi.org/10.1038/s41598-017-05860-6.
Zhang H, Qi L, Shen Q, Wang R, Guo Y, Zhang C, Richel A. Comparative analysis of the bioactive compounds in chicken cartilage: protective effects of chondroitin sulfate and type II collagen peptides against osteoarthritis involve gut microbiota. Front Nutr. 2022;9:843360. https://doi.org/10.3389/fnut.2022.843360.
Shmagel A, Demmer R, Knights D, Butler M, Langsetmo L, Lane NE, Ensrud K. The effects of glucosamine and chondroitin sulfate on gut microbial composition: a systematic review of evidence from animal and human studies. Nutrients. 2019;11:294. https://doi.org/10.3390/nu11020294.
Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, Hara H. Voluntary running exercise alters microbiota composition and increases N-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem. 2008;72:572–6. https://doi.org/10.1271/bbb.70474.
Peng L, Li Z-R, Green RS, Holzmanr IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers12. J Nutr. 2009;139:1619–25. https://doi.org/10.3945/jn.109.104638.
Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O’Reilly M, Jeffery IB, Wood-Martin R, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–20. https://doi.org/10.1136/gutjnl-2013-306541.
Bressa C, Bailén-Andrino M, Pérez-Santiago J, González-Soltero R, Pérez M, Montalvo-Lominchar MG, Maté-Muñoz JL, Domínguez R, Moreno D, Larrosa M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. 2017;12: e0171352. https://doi.org/10.1371/journal.pone.0171352.
Packer N, Hoffman-Goetz L. Exercise training reduces inflammatory mediators in the intestinal tract of healthy older adult mice. Can J Aging Rev Can Vieil. 2012;31:161–71. https://doi.org/10.1017/S0714980812000104.
Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. 2019;47:75–85. https://doi.org/10.1249/JES.0000000000000183.
Transcriptional and Translational Regulation of Heat Shock Proteins in Leukocytes of Endurance Runners. J Appl Physiol. Available online: https://doi.org/10.1152/jappl.2000.89.2.704 (accessed on 1 Feb 2024).
Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290:G204–12. https://doi.org/10.1152/ajpgi.00401.2005.
Solari E, Marcozzi C, Negrini D, Moriondo A. Interplay between gut lymphatic vessels and microbiota. Cells. 2021;10:2584. https://doi.org/10.3390/cells10102584.
Solari E, Marcozzi C, Negrini D, Moriondo A. Lymphatic vessels and their surroundings: how local physical factors affect lymph flow. Biology. 2020;9:463. https://doi.org/10.3390/biology9120463.
Gut Microbiota Regulates Lacteal Integrity by Inducing VEGF‐C in Intestinal Villus Macrophages | EMBO Reports. Available online: https://doi.org/10.15252/embr.201846927 (accessed on 27 Mar 2024).
Li K, Liu A, Zong W, Dai L, Liu Y, Luo R, Ge S, Dong G. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J Biol Sci. 2021;28:40–9. https://doi.org/10.1016/j.sjbs.2020.08.027.
Whole Body Vibration - an Overview | ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/whole-body-vibration (accessed on 1 Feb 2024).
Song N, Liu X, Feng Q, Xu M, Lan X, Li M, Liu R, Li C, Dong T, Wang D, et al. Whole body vibration triggers a change in the mutual shaping state of intestinal microbiota and body’s immunity. Front Bioeng Biotechnol. 2019. https://doi.org/10.3389/fbioe.2019.00377.
Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. https://doi.org/10.1177/1759720X12467868.
Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Ther Adv Gastroenterol. 2016;9:229–39. https://doi.org/10.1177/1756283X15607414.
Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical application and potential of Fecal microbiota transplantation. Annu Rev Med. 2019;70:335–51. https://doi.org/10.1146/annurev-med-111717-122956.
DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen Y-B, Hohmann EL. Drug-resistant E. coli Bacteremia transmitted by Fecal microbiota transplant. N Engl J Med. 2019;381:2043–50. https://doi.org/10.1056/NEJMoa1910437.
Millan B, Park H, Hotte N, Mathieu O, Burguiere P, Tompkins TA, Kao D, Madsen KL. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent clostridium difficile infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;62:1479–86. https://doi.org/10.1093/cid/ciw185.
Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. https://doi.org/10.1038/nrgastro.2011.244.
Zeng J, Peng L, Zheng W, Huang F, Zhang N, Wu D, Yang Y. Fecal microbiota transplantation for rheumatoid arthritis: a case report. Clin Case Rep. 2021;9:906–9. https://doi.org/10.1002/ccr3.3677.
Acknowledgements
To Maestro Luca Corriero and Maestro Chiara Corriero, the two great musicians to whom we dedicate our symphony.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Alberto Corriero, Mariateresa Giglio, and Rossana Soloperto wrote the first draft of the manuscript, which was critically revised by Filomena Puntillo, Francesco Inchingolo, and Giustino Varrassi; Alberto Corriero, and Filomena Puntillo prepared the figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests but Filomena Puntillo and Giustino Varrassi are Editorial Board members of Pain and Therapy. Filomena Puntillo or Giustino Varrassi were not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Corriero, A., Giglio, M., Soloperto, R. et al. Microbial Symphony: Exploring the Role of the Gut in Osteoarthritis-Related Pain. A Narrative Review. Pain Ther 13, 409–433 (2024). https://doi.org/10.1007/s40122-024-00602-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00602-9