Abstract
Neuropathic pain (NP) is a chronic pain disorder arising from somatosensory nervous system impairment. Extensive evidence supports the notion that the gut microbiota (GM) is crucial in maintaining human health by performing vital tasks. At the same time, its disruption has been linked to the emergence and advancement of an expanding range of disorders, including NP, in which GM could play a role in its pathophysiology. The crosstalk between the nervous system and GM happens through immune mediators, metabolites, and nervous structures and involves both central and peripheral nervous systems. This literature review aims to thoroughly investigate the function of modulating GM in the treatment of NP. It will achieve this by integrating existing knowledge, identifying underlying mechanisms, and evaluating the possible clinical consequences of exploiting the gut–brain axis. We will cover the main therapeutic applications of the described GM-modulators, such as probiotics, faecal microbiota transplantation, dietary supplements and emotional support, to the main kinds of NP in which any evidence, even if only pre-clinical, has been unravelled in recent years. The explored NP areas include chemotherapy-induced peripheral neuropathy, diabetic neuropathy, trauma-induced neuropathic pain, trigeminal neuralgia, postherpetic neuralgia and low back pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intricate networks of neural dysfunction, maladaptive plasticity, and immune responses characterize the pathophysiology of Neuropathic pain (NP). |
The connection between the gut microbiota (GM) and the nervous system might represent a novel treatment for NP by affecting immune mediators, metabolites and nervous structures involved in this communication. |
The gut–brain axis mediators include short-chain fatty acids, lipopolysaccharide, lipoteichoic acid, tryptophan metabolites, serotonin, gamma-aminobutyric acid, bile acids, cocaine-amphetamine regulated transcript, substance P and calcitonin gene-regulated peptide. |
This review aims to provide an overview of the potential strategies to treat NP by GM modulation, such as probiotics, faecal microbiota transplantation and diet supplements. |
The precise mechanisms by which GM influence NP are complex and multifaceted, necessitating additional research. |
Introduction
Scientists have been fascinated by the delicate interplay between the human body and its resident microbial populations for decades. The human gut hosts its residential population, which we call gut microbiota (GM). It has been estimated that the GM is composed of more than 1014 microorganisms, comprising the three main domains of life: bacteria, eukarya and archaea. Among these, the bacterial domain is the most represented [1, 2]. There are six different phyla of bacteria that are currently recognized. The most numerous are the Firmicutes and Bacteroidetes, followed by Actinobacteria and Proteobacteria [3]. The composition of the GM is unique. It varies from individual to individual, changing continuously dynamically throughout life under the influence of different factors intrinsically, such as genetic patterns and extrinsically, such as diet, medical and environmental influence in general [4].
The GM carries out numerous activities in a healthy individual, including:
-
(1)
Metabolic activities such as vitamin synthesis and production of short-chain fatty acids (SCFAs) [5].
-
(2)
Antimicrobial activities through competition for nutrients with pathogens and production of antimicrobial peptides (bacteriocins) and antibiotic substances [5].
-
(3)
Modulation activities of gene expression in intestinal epithelial cells, which allows the creation of favourable habitats for resident microorganisms [5, 6].
The GM has been recognized as a main actor shaping different physiological processes that go beyond the boundaries of the gastrointestinal tract, developing further links to the rest of the body along the so-called gut-organ axis [7]. It is common knowledge that dysbiosis of the GM, which is an alteration in the amount, composition and diversity of microbiota [8], and the host can play a role in developing various illnesses. These diseases include different conditions ranging from metabolic [9] to cardiovascular [10], neurological [11], gastrointestinal [12] and renal abnormalities [13]. The gut–brain axis is one of these axes and broadly addresses all the connections between the GM and the nervous system centrally and peripherally [14]. This very axis embracing the realm of neurobiology has sparked unprecedented interest, and the literature is thriving with data, primarily pre-clinical, that tries to study how this link between the GM and, more broadly, the gut and the nervous system works [15,16,17,18,19].
According to the latest definition by the International Association for the Study of Pain (IASP), neuropathic pain (NP) is caused by a lesion or disease of the somatosensory nervous system [11]. Its pathophysiology is characterized by intricate networks of neural dysfunction, maladaptive plasticity, and intricate immune responses [20]. NP is often described as “pricking pain” associated with electrical shock and numbness [21], and when autonomic nervous system pathways are affected, autonomic symptoms may appear [22]. NP is a considerable medical challenge affecting patient quality of life. The limited efficacy and often significant side effects of current therapeutic interventions have spurred the exploration of novel strategies, drawing inspiration from interdisciplinary perspectives. The gut microbiota modulation could represent a potential new strategy to treat NP.
This literature review embarks on a comprehensive exploration of the role of GM modulation in treating NP by synthesizing current knowledge, identifying underlying mechanistic insights, and evaluating the potential clinical implications of harnessing the gut–brain axis for NP management.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
The Interplay Between Gut Microbiota and Nervous System: Not Simply a Gut–Brain Axis
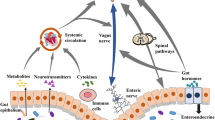
It is established that there is an intricate bidirectional communication between the gut microbiota and the nervous system through various signalling mechanisms, such as the vagus nerve [23], immune mediators [24], and metabolites [25]. This underscores the pivotal role of the gut–brain axis, where complex microbial communities in the gastrointestinal tract profoundly influence neural functions and vice versa. The connection involves the central and peripheral nervous systems, as portrayed in Fig. 1.
The interconnection between the nervous system and the gut microbiota. The neuroepithelial unit is the starting point of the cross talk where different mediators cross the epithelial barrier and through different routes as explained in the text interact with the peripheral and central nervous system. SCFA short chain fatty acids
The Gut Microbiota and the Central Nervous System
The GM can create and release active metabolites that may function as neuromodulators, interacting with the CNS [26] and influencing the neural system. The primary bacterial compounds impacting the brain include SCFAs, aromatic amino acids, and bile acids. SCFAs are mostly composed of acetate, butyrate, and propionate, which may be the byproducts of bacterial carbohydrate fermentation. These interactions with the gut may be mediated via binding to G-protein-coupled receptors [27]. Moreover, SCFAs can indirectly impact the gut–brain axis by stimulating the secretion of certain gut hormones, including glucagon-like peptide-1 (GLP-1) and leptin, via enteroendocrine cells. These enteric hormones can potentially engage with the vagus nerve and receptors in the brain [28, 29].
Neurotransmitters and their precursors generated in the gut by bacteria belonging to Lactobacillus, Bifidobacteria, Enterococcus, and Streptococcus species may also influence their levels in the brain. These include acetylcholine, serotonin and gamma-aminobutyric acid (GABA) [19].
Serotonin and serotonin receptors are essential in coordinating virtually every brain function, so it is widely used in psychiatry and neurology [30]. Its functions are numerous as serotonin is involved in a variety of biological processes, including cardiovascular function, bowel motility, ejaculatory latency, bladder control, and platelet aggregation, apart from well-known functions within the central nervous system (CNS) such as regulation of sleep, mood, and behaviour. It is then almost surprising to know that 95% of serotonin is not produced in the brain but rather in our second brain, the gut, by its microbial residents [31].
How does serotonin or its precursors as tryptophan reach the brain? Research shows that the humoral route might be one pathway by which GM products influence serotonergic neurotransmission [32]. Once it reaches the nervous system, tryptophan or serotonin serve their purpose differently. For example, serotonin binds to 5-HT receptors on microglia, leading to the release of cytokine-carrying exosomes. Tryptophan binds to an aryl hydrocarbon receptor that leads to microglial activation and influences the transcriptional program of astrocytes [32]. The tryptophan–kynurenine pathway is responsible for metabolizing about 95% of tryptophan, resulting in the synthesis of diverse bioactive metabolites, including neuroprotective antioxidants, neuroprotectants, toxic oxidants, neurotoxins, and immunomodulators [33]. A known association exists between the disruption of kynurenine metabolites and various medical conditions, including immunological disorders, malignancies, neurodegenerative illnesses, psychiatric disorders and distal NP in people with HIV [34]. The latter may be explained by the known association between NP in patients with HIV and the presence of gut dysbiosis, characterized by a decrease in microbial diversity and an increase in the ratios of Blautia and Clostridium to Lachnospira [35].
The inflammasome is another bridge that links the GM with the CNS. The inflammasome is a complex belonging to innate immunity formed when pathogens or specific signals activate them, which can happen in the gut and CNS. Inflammasome’s structure is made up of three main components, which are a receptor protein such as TLRs (toll-like receptors), an adaptor molecule called apoptosis-associated speck-like protein, and the effector molecule, which is the enzyme pro-caspase-1 [31]. The latter activates interleukin-1-β and IL-18, two proinflammatory cytokines involved in different processes in CNS, such as neuroimmunomodulation, neuroinflammation and neurodegeneration [24]. Moreover, inflammasome activation can lead to pyroptosis, a critical inflammatory mode of regulated cell death that evolved to remove intracellular pathogens. It has a distinct morphology dependent on forming plasma membrane pores, resulting in cell explosion [36].
The first link between the inflammasome and the microbiota is a receptor protein with an affinity for distinct molecular patterns, known as pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), carried by various microbes inside the gastrointestinal tract. Therefore, it is plausible that an alteration in GM could potentially induce excessive activation of the inflammasome, thereby compromising the blood–brain barrier (BBB) and allowing interleukin-1-β and IL-18 to enter the CNS and exert their proinflammatory effects. These effects have been primarily associated with neuroinflammatory conditions, which may contribute to the development of diseases such as Multiple Sclerosis [37], Alzheimer’s Disease [38], Parkinson’s Disease [39], and various neuropsychiatric disorders [40]. Notably, Kiger et al. demonstrated that pre-injury gut dysbiosis in mice with spinal cord injury exacerbated neurological impairment, slowing its recovery. Conversely, mice supported by probiotics to modulate GM showed an improved locomote recovery [41].
It is postulated that in many disorders, the variation of GM may serve as the primary driver. However, further research is necessary to determine if certain variations of GM are the underlying cause or a consequence of the pathophysiology of the disease.
The Gut Microbiota and the Peripheral Nervous System
The peripheral nervous system (PNS) encompasses the neural pathways that emanate from the CNS. These nerves constitute the neural network that facilitates communication between the CNS and the many anatomical components of the body. Overall, the PNS includes the cranial nerves, the spinal nerves with their roots, the peripheral nerves and the peripheral components of the autonomic nervous system (ANS), the sympathetic, parasympathetic and enteric divisions [42]. Indeed, the PNS can be further divided into the somatic nervous system (SNS) and the autonomic nervous system.
Recent pre-clinical evidence has proven that the different components of PNS serve as a highway connecting the CNS on one side and the gut with the other organs on the other side and are directly influenced by GM’s products [43,44,45].
Recent studies in mice have suggested that GM could be essential in regenerating damaged somatic nerves. For instance, Liu et al. investigated regeneration following corneal nerve injury caused by epithelial abrasion. They discovered that oral antibiotics inhibit regeneration of the corneal branch of the trigeminal nerve after injury. This impairment could be reverted by performing faecal microbiota transplantation (FMT) [46]. The recovery effect of GM modulation was also demonstrated in another dysbiosis-induced mice model by Rodenhouse et al., in which an eight-stain probiotic preparation could counteract impaired nerve regeneration after traumatic sciatic nerve injury [45].
Intermittent fasting (IF) is another GM modulator. A recent study showed that IF has the potential to improve nerve regeneration and support the formation of dorsal root ganglion neurites following a sciatic nerve compression injury [43].
SCFAs also directly affect the PNS as free fatty acid receptor 3, a SCFA receptor, is expressed by Schwann cells and dorsal root ganglia [44]. An in vitro study reported that when subjected to oxidative stress and treated with propionate, Schwann cells and dorsal root ganglia exhibited enhanced protection against oxidative damage. Additionally, propionate treatment was found to promote greater axon outgrowth in dorsal root ganglia, accompanied by increased expression of the growth-associated protein 43 [44].
Finally, the enteric nervous system (ENS) and the vagus nerve play a major in the interconnection between the GM and the rest of the PNS. The evidence that GM influenced the ENS was discovered in 1965 in germ-free (GF) mice by noticing an anomaly in Auerbach’s plexus development compared to control [47]. The GM communicate with the ENS through several mediators. Among these: SCFAs, lipopolysaccharide (LPS) and Lipoteichoic Acid (LTA), tryptophan metabolites, serotonin, GABA, bile acids, cocaine-amphetamine regulated transcript (CART), substance P and calcitonin gene-regulated peptide (CGRP) [48].
The vagus nerve plays a double role: on the one hand, it provides the direct connection between the GM and the brain, acting as a carrier for all the mediators previously mentioned [49]. On the other hand, vagal signalling itself is a crucial regulator of peripheral immune responses, as demonstrated in patients who underwent vagotomy [50].
Potential Therapeutic Strategies
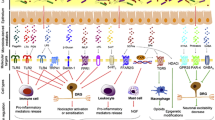
Among the many GM modulators thriving in recent years, a few could also find their target in treating or alleviating NP (Fig. 2). By GM modulators, we refer to potential tools that can change GM composition in number and diversity and, therefore, affect the GM-organ axis [51].
The main potential strategies to treat neuropathic pain by gut microbiota modulation. They consist primarily of probiotics, faecal microbiota transplantation, diet and supplements like vitamins and emotional support. Neuropathic pain may arise from peripheral, central, or both damage to the nervous system
Probiotics are living microorganisms that, when supplemented in adequate amounts, give health benefits to the host [52]. One of the main functions of probiotics is to create an environment that inhibits the growth of harmful bacteria in the gut, thus contributing to a strengthened immune system and reduced risk of gastrointestinal infections [53, 54]. Additionally, probiotics assist in breaking down complex carbohydrates and fibre that the human body cannot digest on its own, leading to improved digestion [55]. They also produce essential vitamins and SCFAs [56, 57]. Therefore, probiotics’ immunomodulation function could help treat NP, as further explained.
FMT is a medical procedure in which stool containing a mixture of beneficial bacteria and microorganisms is collected from a healthy donor and transferred into the gastrointestinal tract of a patient with suspected GM dysbiosis [58]. This transplantation is typically performed to restore or rebalance the GM, especially when it has been disrupted due to various factors like infections, antibiotic treatments, or certain medical conditions. FMT aims to introduce a diverse community of beneficial microbes into the recipient’s gut, thereby addressing gastrointestinal infections, inflammatory bowel diseases, or even certain metabolic disorders. The procedure can be administered through various methods, including colonoscopy, enema, or capsules containing freeze-dried faecal material [58].
Dietary quantity, quality, fibre content, and nutrition patterns influence the abundance and diversity of GM [59, 60]. A specific diet called low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) may decrease SCFAs production in the gut [61], which in turn might reduce abdominal hypersensitivity as it has been demonstrated in an animal model [62]. Dietary supplements like vitamin D could also alter GM, thus influencing NP [63]. For instance, pre-clinical data showed that mice with a deficiency in vitamin D had a lower microbial diversity (an increase in Firmicutes and a decrease in Verrucomicrobia and Bacteroidetes) and exhibited tactile allodynia, which was accompanied by increased neuronal excitability and changes in components of the endocannabinoid system inside the spinal cord. Alterations in endocannabinoid concentrations, specifically anandamide and 2-arachidonoylglycerol, were additionally seen in the duodenum and colon [64]. The same impairment of the endocannabinoid system was also observed when mice GM underwent antibiotic treatment [65].
Finally, psychological issues may take their toll on NP pathophysiology [61] on one hand and GM on the other hand [66]. Therefore, managing these psychological issues may affect NP also through the GM’s mediation.
In the next paragraphs, we will cover the main therapeutic applications of the described GM modulators to the main kinds of NP in which any evidence, even if only pre-clinical, has been unravelled in recent years.
Chemotherapy-Induced Peripheral Neuropathy
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect arising from treatment with many commonly used anti-cancer medications that harm patient outcomes and significantly diminish the quality of life of patients and also of cancer survivors [67]. It is distinguished by diverse symptoms, encompassing muscle twitching, discomfort, muscular weakness, numbness, burning and tingling sensations, with a typical “glove and stocking” distribution [68]. Regrettably, many patients exhibit suboptimal responses to standard chemotherapy-induced peripheral neuropathy (CIPN) treatments, leading to the need to reduce the dosages of anti-cancer drugs, ultimately impacting overall survival rates [69]. To date, there is no treatment to prevent CIPN [70].
GM’s modulation could be a novel way to address CIPN, although most data come from pre-clinical fields.
In 2017, Shen et al. explored in mice the effect of oxaliplatin [71], a chemotherapy agent used to treat colorectal cancer. They found that mechanical hyperalgesia generated by oxaliplatin was diminished in GF mice and mice pre-treated with antibiotics. The restoration of the microbiota in GF mice nullified the protection. This led the researchers to conclude that GM was a key promoter of oxaliplatin-induced hyperalgesia [72]. Moreover, the study showed that the inflammatory response of the dorsal root ganglion (DRG) to the chemotherapeutic drug was attenuated when a complete GM was not present, mostly through the lipopolysaccharide (LPS) Toll-like receptor 4 (TLR4) pathway. After oxaliplatin treatment, the DRG of mice treated with antibiotics exhibited a reduction in the presence of infiltrating macrophages and inflammatory cytokines (IL-6 and TNF-α) compared to the DRG of mice fed with water. The re-establishment of the inflammatory response following the introduction of exogenous LPS, along with the confirmation that the targeted removal of TLR4 on hematopoietic cells alone was enough to replicate the eradication of GM, provides evidence for the involvement of GM-released LPS in enhancing and amplifying the proinflammatory reaction of macrophages against oxaliplatin [72]. Another study provided additional confirmation on the role of the LPS-TLR4 pathway in the development of chemotherapy-induced pain as administration of irinotecan in mice increased the permeability of the intestinal barrier, which in turn facilitated the release of LPS from GM, leading to the development of NP associated with astrogliosis in the spinal cord. Strikingly, mice lacking the TLR-4 gene, which received the same treatment, developed less NP [73]. In another study in mice, it was proven that diosgenin, a steroid sapogenin found in fenugreek seeds and yam, could exert an analgesic effect in oxaliplatin-induced pain. The analgesic effect can be attributed to its influence on the TLR-4/NF-κB inflammatory signalling pathway [74].
Later on, Ramakrishna et al. conducted a similar pre-clinical study in mice studying the effect of paclitaxel, a chemotherapy medication used to treat, among many, ovarian cancer, oesophageal cancer and breast cancer [75]. The authors compared two mice models, wild type B6 and 129, in their response to paclitaxel, finding that B6 mice developed mechanical and cold allodynia and heat hyperalgesia. In contrast, B129 did not develop any of this [76]. Somehow 129 mice were resistant to NP. When B6 mice were treated with antibiotics that depleted their GM, the NP development was also impaired, clearly pointing to the major role of GM in mediating CIPN. Both mice models were treated with reciprocal FMT to corroborate this finding, revealing that paclitaxel-induced pain developed only in mice that hosted the GM transplanted by B6, not vice-versa. Further analysis suggested a causal relationship between spinal microgliosis and the development of pain generated by paclitaxel, as microglial expansion was reduced in 129 mice [76]. Regarding the variations in GM composition, the authors found in the B6 group, a reduction of A. muciniphila, a bacterium whose importance in promoting gut barrier integrity, has been consolidated in the last years [77]. Thus, CIPN pathophysiology might also be mediated by the decrease of this bacterium, which decreases pain levels.
In this light, a probiotic formulation DSF (DeSimoneformulation), which is a high-concentration probiotic formulation with 450 billion bacteria per sachet, has been successfully used in an vitro experiment of DRG neurons to mitigate paclitaxel-induced neurotoxicity by modulating the inflammatory response [78]. A further step in this direction was achieved by Cuozzo et al., who were able to prevent paclitaxel-induced neuropathy in mice when using another probiotic formulation called SLAB51 [79].
FMT also alleviated Paclitaxel-Induced Peripheral neuropathy in rats by interfering with the TLR4 pathway [80].
Translating these findings from bench to bedside might pose a complex challenge, but probiotics supplementation and FMT could represent a therapeutic approach to mitigate CIPN.
Diabetic Neuropathic Pain
Diabetic neuropathy (DN) is a common complication of type 2 diabetes that affects the peripheral nerves and can cause pain, numbness, and loss of sensation in the extremities [81]. It is characterized by a decline in peripheral innervations, increased neuronal inflammation, demyelination, axonal atrophy, and decreased neuronal regenerative capacity [82]. DN is present in approximately fifty per cent of patients with diabetes and affects numerous organs, leading to various complications, including cardiovascular damage with symptoms of tachycardia, orthostatic hypotension, impaired intestinal transit, profuse sweating, hormonal imbalance and gastric emptying [83]. Insulin resistance plays a major role in the pathogenesis of type 2 diabetes [84] and is intricately interconnected with DN. This connection is due to the damaging effects of prolonged high blood sugar levels on nerve cells and the surrounding microenvironment, leading to inflammation and oxidative stress, ultimately disrupting nerve conduction [85, 86].
Unfortunately, DN’s pathogenesis is not yet fully known, which equals no option to prevent or counteract this complication effectively.
The first clue that connected GM to the development of DN came from the evidence in clinical trials that the GM population from patients with DN varied significantly compared to the GM from diabetic patients without DN or healthy patients [87]. Notably, the authors found that Firmicutes and Actinobacteria were more abundant at the phylum level in the DN group, while Bacteroidetes were less abundant. At the genus level, Bacteroides and Faecalibacterium were considerably less abundant in the DPN group, whereas Escherichia-Shigella, Lachnoclostridium, Blautia, Megasphaera, and Ruminococcus were significantly more abundant [87]. Could a higher insulin resistance be a pathway harmful microorganisms use to lead to pain? Megasphera positively correlated with a higher HOMA Index, a mathematical model used to calculate insulin resistance. Conversely, there is a favourable correlation between the presence of Parabacteroidetes and the levels of both C-reactive protein and tauroursodeoxycholic acid. This correlation is connected with the improvement of metabolic illnesses [87]. Taken together, these findings indicate that insulin resistance could be related to DN.
GM modulation may be used to treat insulin resistance and DN. For instance, in 2012, Vrieze et al. demonstrated that FMT from lean donors to individuals with metabolic syndrome increased insulin sensitivity in these patients [88]. A similar result by using probiotics was achieved by Sabico et al., who found that patients with type 2 diabetes who took a multi-strain probiotic supplement with Bifidobacteria and Lactobacillus for six months as monotherapy had a substantial reduction in their HOMA index, with the probiotic treatment group demonstrating both lower inflammation and a better cardiometabolic profile [89].
Regarding DN specifically, a pre-clinical study in mice demonstrated that modulating GM by using antibiotics may improve diabetes and its correlated neuropathic pain, supporting the hypothesis that when the harmful bacteria are removed or replaced by beneficial ones, the clinical condition of DN improves [90]. Furthermore, another pre-clinical study showed that FMT from lean to obese, insulin-resistant neuropathic mice on a Western diet prevented mechanical allodynia and thermal hyperalgesia and reduced the loss of nerve fibres [91]. Most of the pain alleviation was accomplished by a process known as FMT-mediated reduction of DRG neuronal hyperexcitability, which was the direct result of a decrease in Ryanodine receptor 2-dependent Ca2 + release from the endoplasmic reticulum [91].
Unfortunately, there is a lack of human clinical studies targeting the DN by microbiota modulation, let alone from a single case report in 2018 where an obese type II diabetic woman who received FMT for 3 months achieved remission of a painful DN [92]. Overall, the modulation of the GM holds promise as a therapeutic strategy for DN. By targeting the GM, it may be possible to improve insulin resistance, reduce inflammation, and alleviate symptoms associated with diabetic neuropathy. However, further research is needed to fully understand the mechanisms underlying GM modulation’s effects on DN and optimize treatment strategies.
Trauma-Induced Neuropathic Pain
Trauma-induced neuropathic pain (TINP), referred to as traumatic neuropathy or nerve injury pain, is a chronic pain disorder that arises from nervous system impairment caused by physical trauma. This injury may occur due to various traumatic incidents, including accidents, falls, sports-related injuries, surgical procedures, or other physical trauma [93]. Numerous pathophysiological mechanisms have been proposed as potential explanations for TINP, but the first step lies in initiating spontaneous or ectopic activity within damaged sensory neurons. Most of this activity occurs within a 20-h timeframe following peripheral nerve injury, with a particular emphasis on its detection in myelinated fibres type A. Ectopic discharge has been observed to potentially start within the wounded region of the axonal segment of damaged nerves [94, 95]. However, it is more commonly observed to originate within the soma of sensory neurons in the DRG [96]. Ion channels are appealing targets in the study of neuronal excitability control. One example is sodium channels, which play a significant role in maintaining NP behaviours and abnormal nerve activity. Additionally, certain subtypes of potassium channels are strongly influenced by traumatic injury, which may account for alterations in the excitability of myelinated fibres and the development of pain-related characteristics [97].
How could the GM be linked to TINP? Pre-clinical data in mice with chronic constriction injury (CCI) points to a different GM composition compared to sham rats. CCI of the sciatic nerve has been extensively employed to induce NP in rodents after peripheral nerve injury [98]. Notably, there was a large rise in Helicobacter, Phascolarctobacterium, Christensenella, Blautia, Streptococcus, Rothia, and Lactobacillus at the genus level. Conversely, there was a significant drop in Ignatzschineria, Butyricimonas, Escherichia, AF12, and Corynebacterium. Furthermore, 72 blood metabolites and 17 spinal cord metabolites exhibited important differential expression between the rats with CCI and the sham rats [99]. Another study in mice reported that GM depletion by pretreatment with a mixture of 4 antibiotics [vancomycin (0.5g/L), ampicillin (1g/L), neomycin (1g/L) and metronidazole (1g/L)] which would therefore target different kinds of bacteria, could reduce thermal hyperalgesia and inhibit spinal glial cell activations in animals with nerve damage [90]. Akkermansia, Bacteroides, and Desulfovibrionaceae phyla were the most abundant in the feces of CCI mice, possibly indicating a key role for these specific strains in the pathophysiology of TIPN. To further verify the role of GM in the development of pain in CCI mice, FMT from control to antibiotic-treated mice restored NP with thermal hyperalgesia [90]. A similar result using the antibiotics mentioned above in the same mice model (C57BL/6) was observed by Ding et al., who reported an amelioration of CCI NP in the antibiotic-pre-treated group [100].
Strikingly, in a transition from pre-clinical to clinical, an opposite result was reported in a large case–control study in which exposure to oral fluoroquinolone or amoxicillin-clavulanate therapy correlated with an increased incidence of peripheral neuropathy [101].
This data seems contradictory, but it is not if it is considered that there is a different mixture of antibiotics implied and that antibiotics themselves might alter the GM of different animals in another way.
Future research using standardized animal models to help the transition to the clinical side could push the usage of GM modulators in the context of the multidisciplinary approach required to address and manage TINP.
Trigeminal Neuralgia
Trigeminal neuralgia (TN) is an infrequent occurrence of facial pain that manifests unilaterally, resembling electric shock sensations, and is triggered by gentle tactile stimulation. Initially, it is frequently misinterpreted as a dental issue due to its manifestation in the mandibular divisions of the trigeminal nerve [102]. Classically TN’s pathophysiology is caused by neurovascular compression in the trigeminal root entry zone, which can result in demyelination and dysregulation of voltage-gated sodium channel expression in the membrane. These alterations may be liable for TN patients’ pain attacks [reviewed in [103]].
Palmatine is an alkaloid derived from dried rhizomes, a Chinese plant. It was shown in a pre-clinical study to improve TN’s pain effectively [96]. Specifically, healthy Sprague–Dawley rats underwent surgery to expose the right infraorbital nerve and ligate it through a specific method loosely; this would constitute the TN group. Within 14 days of surgery, the TN group had a substantially lower mechanical allodynia threshold than the sham group. In contrast, the TN + palmatine group had a higher mechanical pain sensitivity threshold than the TN group. Furthermore, real-time quantitative PCR, immunohistochemistry, and immunofluorescence demonstrated that brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TRKB) expression was higher in the TN group than in the sham group. Conversely, palmatine treatment was able to rectify these changes. Therefore, the BDNF/TRKB pathway may be the target of the palmatine treatment, allowing pain mitigation in TN [104].
BDNF/TRKB pathway may not be the only cornerstone of palmatine action. Indeed, In another study in mice, experimental colitis induced by dextran sulfate sodium was ameliorated by palmatine, which improved mucosal integrity and inhibition of cell apoptosis. In addition, analysis of the gastrointestinal microbiota of mice treated with palmatine revealed an increase in the relative abundance of Bacteroidetes and Firmicutes but a decrease in Proteobacteria, preventing GM dysbiosis [105].
We hypothesize that palmatine’s modulation on GM could be another potential therapeutic pathway implied in TN worthy of being explored in future research.
Postherpetic Neuralgia
Postherpetic neuralgia (PHN) is a frequently encountered NP condition characterized by its persistence for one month or longer following the resolution of the herpes zoster rash. PHN is the most commonly observed consequence of herpes zoster infection. Clinically, this condition is distinguished by enduring pain, frequently accompanied by sensory abnormalities, sleep disturbances, and emotional comorbidities [106]. The pathogenesis comprises the impairment of peripheral and central neurons, which can potentially result from the immunological and inflammatory response associated with the reactivation and migration of the varicella-zoster virus. When damaged, both peripheral and central nerve fibres can reduce the threshold for action potentials, leading to spontaneous discharge and displaying exaggerated reactions to stimuli. This can give rise to peripheral sensitization and allodynia [107].
A recent cross-sectional study on a cohort of 27 patients diagnosed with PHN vs. 27 healthy controls revealed a preliminary connection between PNH and GM [108]. The GM analysis from stool samples showed that despite similar levels of bacterial richness and diversity in the GM of PHN and healthy individuals, there was a notable disparity in 37 genera between the two groups. Specifically, patients with PHN have higher Escherichia-Shigella, Streptococcus, Ligilactobacillus, and Clostridia than healthy controls but lower Eubacterium, Butyricicoccus, Tyzzerella, Dorea, Parasutterella, Romboutsia, Megamonas, and Agathobactergenera. Moreover, strong links between different GM and diverse clinical manifestations were reported, suggesting that GM dysbiosis could be important to PHN aetiology [108]. Despite being a preprint, currently, this study is the only paper that paves the way to further research for targeting GM as a new approach to treating PNH.
Low Back Pain
Low back pain (LBP) is defined as a pain located inferior to the costal margins and superior to the inferior gluteal folds, with or without accompanying leg pain [109]. When LBP lasts more than 12 weeks, it is considered chronic LBP [110]. Chronic LBP is a multifaceted and diverse illness characterized by the potential involvement of both nociceptive and neuropathic pain processes [111]. Neuropathic back pain refers to the sensation of pain that arises from injury or disease that directly affects the nerve roots responsible for providing sensory and motor innervation to the spine and lower limbs. It can also be caused by the abnormal growth of nerve fibres into the injured intervertebral lumbar discs (ILD) [111].
What is the link between GM and ILD in the pathogenesis of LBP? The starting point of our analysis revolves around the new concept that physiologically ILDs are not sterile but contain microbes [112]. Specifically, 355 bacterial species were identified, and 32 were exclusive to normal ILD. Moreover, when healthy ILD were compared to diseased ones, it was noted that protective microorganisms like Firmicutes and Actinobacteria (for example, Saccharopolyspora, an Actinobaterium competitor of harmful bacteria) were plentiful in normal discs. In contrast, harmful bacteria abounded in the others [112]. The interaction between GM and ILD’s microbiota, which constitutes the gut-disc-spine axis, is hypothesized through one of the following events: (1) bacterial translocation past the gut-epithelial barrier and into the ILD due to lack of immune guard at the blood-disc barrier; (2) control of the mucosal and systemic immune systems; (3) balancing nutritional absorption and metabolite production in the gut epithelium with its subsequent diffusion into the ILD [113].
This hypothesis paves the way to research the implications of GM modulation to treat LBP.
For instance, a pre-clinical study analyzed the potential benefits of a specific probiotic, Lactobacillus paracasei S16, on the symptoms of lumbar disc herniation (LDH) using a mouse model of LDH. The findings indicated that the administration of L. paracasei S16 enhanced behavioural outcomes, heightened cell proliferation, and reduced apoptosis in mice with LDH. Additionally, the administration of L. paracasei S16 mitigated the abnormal inflammatory response observed in LDH animals. This reaction is characterized by a reduction in anti-inflammatory cytokines, an increase in proinflammatory cytokines, a drop in the percentage of Th1 and Th2 cells, and a decrease in the Th17/Treg ratio [114].
Shifting to the clinical side, another study reported a slight improvement in patients with chronic LBP type 1 or mixed Modic changes by 1 year supplementation of Lactobacillus Rhamnosus GG [115], possibly hinting to a dampened inflammation as the mechanism implicated in modulation by probiotics. Inflammation was considered the cornerstone of GM modulation in LBP in a cohort study [116] where researchers investigated the connection between LBP, GM composition, and metabolic factors. The study involved 36 overweight or obese individuals, some with back pain and some without. The participants were assessed for various health markers. Interestingly, those with LBP had higher levels of specific gut bacteria, including Adlercreutzia, Roseburia, and Uncl. Christensenellaceae, thus resulting in dysbiosis. These differences persisted over different timeframes and were linked to factors like BMI, serum adipsin, and serum leptin [116].
Finally, a recent case report showed that FMT could ease pain in a patient with refractory ankylosing spondylitis, a condition associated with LBP [117].
The findings from the previous studies [112, 114,115,116] suggest that altered GM, potentially leading to increased inflammation, might play a role in LBP. Indeed, mucosal inflammation gut dysbiosis might provoke simultaneous autoimmune responses at different sites, including ILD, constituting the potential mechanism for LBP in patients with altered GM.
Conclusions
The emerging body of research on the connection between GM and NP highlights the intricate interplay between the gut and the nervous system. The bidirectional interplay happens through the vagus nerve, immune mediators such as the inflammasome, and metabolites such as SCFAs, aromatic amino acids, bile acids and even neurotransmitters. A potential way to harness GM to treat NP may be in probiotics, FMT, diet and supplements such as vitamin D and palmatine. CIPN, DN, TINP, TN, PHN and LBP are potential fields of GM modulation application in the NP context.
Although the findings are encouraging, it is essential to recognize that the field is still in its infancy and that many questions remain unanswered, mainly because most of our data come from pre-clinical models and are biased by high heterogeneity. The precise mechanisms by which GM influence neuropathic pain are complex and multifaceted, necessitating additional research.
The translation from bench to bedside has not happened yet. It will require standardized approaches to overcome the principal limit of animal models, which is the limited resemblance between various experimental models and the human organism. Future research should compare NP development and associated parameter values in long-term stable GF animals to animals with the same genetic background but harbouring a complex GM. This would enhance the reproducibility and clarify whether there is a verifiable causal relationship between GM composition and the prevalence of the development of NP due to various inducing conditions.
Overall, the evolving insights into the GM impact on NP open up exciting avenues for future research and therapeutic interventions in the realm of chronic pain management. The collaboration among pain therapists, neuroscientists, gastroenterologists, and other pertinent disciplines will play a pivotal role in comprehending the intricacies of this association and effectively applying the knowledge gained to provide practical advantages for patients afflicted by NP.
References
Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11:2393. https://doi.org/10.3390/nu11102393.
Corriero A, Gadaleta RM, Puntillo F, Inchingolo F, Moschetta A, Brienza N. The central role of the gut in intensive care. Crit Care. 2022;26:379. https://doi.org/10.1186/s13054-022-04259-8.
Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. https://doi.org/10.1111/1574-6976.12075.
Kho ZY, Lal SK. The human gut microbiome – a potential controller of wellness and disease. Front Microbiol. 1835;2018:9.
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol WJG. 2015;21:8787–803. https://doi.org/10.3748/wjg.v21.i29.8787.
Gadaleta RM, Garcia-Irigoyen O, Cariello M, Scialpi N, Peres C, Vetrano S, Fiorino G, Danese S, Ko B, Luo J, et al. Fibroblast growth factor 19 modulates intestinal microbiota and inflammation in presence of farnesoid X receptor. EBioMedicine. 2020;54: 102719. https://doi.org/10.1016/j.ebiom.2020.102719.
Ahlawat S, Asha, Sharma KK. Gut-organ axis: a microbial outreach and networking. Lett Appl Microbiol. 2021;72:636–68. https://doi.org/10.1111/lam.13333.
Hrncir T. Gut microbiota dysbiosis: triggers, consequences diagnostic and therapeutic options. Microorganisms. 2022;10:578. https://doi.org/10.3390/microorganisms10030578.
Mazidi M, Rezaie P, Kengne AP, Mobarhan MG, Ferns GA. Gut Microbiome and metabolic syndrome. Diabetes Metab Syndr Clin Res Rev. 2016;10:S150–7. https://doi.org/10.1016/j.dsx.2016.01.024.
Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018;214:153–7. https://doi.org/10.1016/j.lfs.2018.10.063.
Park J, Kim CH. Regulation of common neurological disorders by gut microbial metabolites. Exp Mol Med. 2021;53:1821–33. https://doi.org/10.1038/s12276-021-00703-x.
Antonio N, Andrea T, Claudio T, Beatrice P, Pamela C, Chiara M, Gian L, Francesco DM, Tiziana M. Digestive disorders and intestinal microbiota. Acta Bio Med Atenei Parm. 2018;89:47–51. https://doi.org/10.23750/abm.v89i9-S.7912.
Kim M-G, Yang J, Jo S-K. Intestinal microbiota and kidney diseases. Kidney Res Clin Pract. 2021;40:335–43. https://doi.org/10.23876/j.krcp.21.053.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut–brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2015;28:203–9.
Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. 2015;77:823–8. https://doi.org/10.1038/pr.2015.51.
Ferrara M, Bertozzi G, Zanza C, Longhitano Y, Piccolella F, Lauritano CE, Volonnino G, Manetti AC, Maiese A, Russa RL. Traumatic brain injury and gut brain axis: the disruption of an alliance. Rev Recent Clin Trials. 2022. https://doi.org/10.2174/1574887117666220622143423.
Giridharan VV, Generoso JS, Lence L, Candiotto G, Streck E, Petronilho F, Pillai A, Sharshar T, Dal-Pizzol F, Barichello T. A crosstalk between gut and brain in sepsis-induced cognitive decline. J Neuroinflammation. 2022;19:114. https://doi.org/10.1186/s12974-022-02472-4.
Fang H, Wang Y, Deng J, Zhang H, Wu Q, He L, Xu J, Shao X, Ouyang X, He Z;, et al. Sepsis-induced gut dysbiosis mediates the susceptibility to sepsis-associated encephalopathy in mice. mSystems. 2022;7:e01399-e1421. https://doi.org/10.1128/msystems.01399-21.
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76. https://doi.org/10.1016/j.cell.2015.02.047.
Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101:259–301. https://doi.org/10.1152/physrev.00045.2019.
Jang JY, Lee SH, Kim M, Ryu JS. Characteristics of neuropathic pain in patients with spinal cord injury. Ann Rehabil Med. 2014;38:327–34. https://doi.org/10.5535/arm.2014.38.3.327.
Jay GW, Barkin RL. Neuropathic pain: etiology, pathophysiology, mechanisms, and evaluations. Dis Mon. 2014;60:6–47. https://doi.org/10.1016/j.disamonth.2013.12.001.
Gaykema RPA, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004;18:238–45. https://doi.org/10.1016/j.bbi.2003.08.002.
Wong M-L, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, Kentish S, Xie P, Morrison M, Wesselingh SL, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. https://doi.org/10.1038/mp.2016.46.
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci Transl Med. 2014. https://doi.org/10.1126/scitranslmed.3009759.
Brodal P. The central nervous system: structure and function. USA: Oxford University Press; 2004. (ISBN 978-0-19-516560-9).
Priori D, Colombo M, Clavenzani P, Jansman AJM, Lallès J-P, Trevisi P, Bosi P. The olfactory receptor OR51E1 is present along the gastrointestinal tract of pigs, co-localizes with enteroendocrine cells and is modulated by intestinal microbiota. PLoS ONE. 2015;10: e0129501. https://doi.org/10.1371/journal.pone.0129501.
Caspani G, Swann J. Small talk: microbial metabolites involved in the signaling from microbiota to brain. Curr Opin Pharmacol. 2019;48:99–106. https://doi.org/10.1016/j.coph.2019.08.001.
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes. 2012;61:364. https://doi.org/10.2337/db11-1019.
Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66. https://doi.org/10.1146/annurev.med.60.042307.110802.
Rutsch A, Kantsjö JB, Ronchi F. The gut–brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. 2020;11: 604179.
Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut–brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–73. https://doi.org/10.1038/mp.2012.77.
Tanaka M, Török N, Tóth F, Szabó Á, Vécsei L. Co-players in chronic pain: neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines. 2021;9:897. https://doi.org/10.3390/biomedicines9080897.
Routy J-P, Mehraj V, Vyboh K, Cao W, Kema I, Jenabian M-A. Clinical relevance of kynurenine pathway in HIV/AIDS: an immune checkpoint at the crossroads of metabolism and inflammation. Aids Rev. 2015;17(2):96–106.
Ellis RJ, Heaton RK, Gianella S, Rahman G, Knight R. Reduced gut microbiome diversity in people with HIV who have distal neuropathic pain. J Pain. 2022;23:318–25. https://doi.org/10.1016/j.jpain.2021.08.006.
Pyroptosis - an Overview | ScienceDirect Topics Available online: https://www.sciencedirect.com/topics/neuroscience/pyroptosis. Accessed on 15 July 2022.
Govindarajan V, de Rivero-Vaccari JP, Keane RW. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J Neuroinflammation. 2020;17:260. https://doi.org/10.1186/s12974-020-01944-9.
Bai H, Zhang Q. Activation of NLRP3 inflammasome and onset of Alzheimer’s disease. Front Immunol. 2021;12: 701282.
Yan Y-Q, Fang Y, Zheng R, Pu J-L, Zhang B-R. NLRP3 inflammasomes in Parkinson’s disease and their regulation by parkin. Neuroscience. 2020;446:323–34. https://doi.org/10.1016/j.neuroscience.2020.08.004.
Shen Y, Qian L, Luo H, Li X, Ruan Y, Fan R, Si Z, Chen Y, Li L, Liu Y. The significance of NLRP inflammasome in neuropsychiatric disorders. Brain Sci. 2022;12:1057. https://doi.org/10.3390/brainsci12081057.
Kigerl KA, Hall JCE, Wang L, Mo X, Yu Z, Popovich PG. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213:2603–20. https://doi.org/10.1084/jem.20151345.
Birch R, Birch R, Birch R, Birch R. The peripheral nervous system: anatomy and function. In: Birch R, editor. Peripheral nerve injuries: a clinical guide. London: Springer; 2013. p. 1–67 (ISBN 978-1-4471-4613-1).
Serger E, Luengo-Gutierrez L, Chadwick JS, Kong G, Zhou L, Crawford G, Danzi MC, Myridakis A, Brandis A, Bello AT, et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature. 2022;607:585–92. https://doi.org/10.1038/s41586-022-04884-x.
Grüter T, Mohamad N, Rilke N, Blusch A, Sgodzai M, Demir S, Pedreiturria X, Lemhoefer K, Gisevius B, Haghikia A, et al. Propionate exerts neuroprotective and neuroregenerative effects in the peripheral nervous system. Proc Natl Acad Sci. 2023;120: e2216941120. https://doi.org/10.1073/pnas.2216941120.
Rodenhouse A, Talukder MAH, Lee JI, Govindappa PK, O’Brien M, Manto KM, Lloyd K, Wandling GD, Wright JR, Chen See JR, et al. Altered gut microbiota composition with antibiotic treatment impairs functional recovery after traumatic peripheral nerve crush injury in mice: effects of probiotics with butyrate producing bacteria. BMC Res Notes. 2022;15:80. https://doi.org/10.1186/s13104-022-05967-8.
Liu J, Wu M, He J, Xiao C, Xue Y, Fu T, Lin C, Dong D, Li Z. Antibiotic-induced dysbiosis of gut microbiota impairs corneal nerve regeneration by affecting CCR2-negative macrophage distribution. Am J Pathol. 2018;188:2786–99. https://doi.org/10.1016/j.ajpath.2018.08.009.
Dupont JR, Jervis HR, Sprinz H. Auerbach’s plexus of the rat cecum in relation to the germfree state. J Comp Neurol. 1965;125:11–8. https://doi.org/10.1002/cne.901250103.
Cook TM, Mansuy-Aubert V. Communication between the gut microbiota and peripheral nervous system in health and chronic disease. Gut Microbes. 2022;14:2068365. https://doi.org/10.1080/19490976.2022.2068365.
Liu Y, Forsythe P. Vagotomy and insights into the microbiota-gut–brain axis. Neurosci Res. 2021;168:20–7. https://doi.org/10.1016/j.neures.2021.04.001.
Gallaher ZR, Ryu V, Herzog T, Ritter RC, Czaja K. Changes in microglial activation within the hindbrain, nodose ganglia, and the spinal cord following subdiaphragmatic vagotomy. Neurosci Lett. 2012;513:31–6. https://doi.org/10.1016/j.neulet.2012.01.079.
Liu Y, Wang J, Wu C. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front Nutr. 2022;8: 634897. https://doi.org/10.3389/fnut.2021.634897.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. https://doi.org/10.1038/nrgastro.2014.66.
Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CGM. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci. 2007;104:7617–21. https://doi.org/10.1073/pnas.0700440104.
Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–16. https://doi.org/10.1111/j.1462-5822.2006.00836.x.
Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, Klinter S, Pudlo NA, Urs K, Koropatkin NM, et al. A discrete genetic locus confers xyloglucan metabolism in select human gut bacteroidetes. Nature. 2014;506:498–502. https://doi.org/10.1038/nature12907.
Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, Flint HJ, Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–35. https://doi.org/10.1038/ismej.2014.14.
Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 1997;6(Suppl 1):S43-45. https://doi.org/10.1097/00008469-199703001-00009.
Kim KO, Gluck M. Fecal microbiota transplantation: an update on clinical practice. Clin Endosc. 2019;52:137–43. https://doi.org/10.5946/ce.2019.009.
Kaczmarek JL, Musaad SM, Holscher HD. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr. 2017;106:1220–31. https://doi.org/10.3945/ajcn.117.156380.
Klingbeil E, de La Serre CB. Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Physiol Regul Integr Comp Physiol. 2018;315:R1254–60. https://doi.org/10.1152/ajpregu.00037.2018.
Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66:1517–27. https://doi.org/10.1136/gutjnl-2017-313750.
Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson M-P, Dechelotte P, Bommelaer G, Eschalier A, Ardid D. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology. 2005;128:1996–2008. https://doi.org/10.1053/j.gastro.2005.03.082.
Banafshe HR, Khoshnoud MJ, Abed A, Saghazadeh M, Mesdaghinia A. Vitamin D supplementation attenuates the behavioral scores of neuropathic pain in rats. Nutr Neurosci. 2019;22:700–5. https://doi.org/10.1080/1028415X.2018.1435485.
Guida F, Boccella S, Belardo C, Iannotta M, Piscitelli F, De Filippis F, Paino S, Ricciardi F, Siniscalco D, Marabese I, et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav Immun. 2020;85:128–41. https://doi.org/10.1016/j.bbi.2019.04.006.
Guida F, Turco F, Iannotta M, De Gregorio D, Palumbo I, Sarnelli G, Furiano A, Napolitano F, Boccella S, Luongo L, et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun. 2018;67:230–45. https://doi.org/10.1016/j.bbi.2017.09.001.
Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733-e575. https://doi.org/10.1111/nmo.12153.
Colvin LA. Chemotherapy-induced peripheral neuropathy (CIPN): where are we now? Pain. 2019;160:S1–10. https://doi.org/10.1097/j.pain.0000000000001540.
Park SB, Goldstein D, Krishnan AV, Lin CS-Y, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–37. https://doi.org/10.3322/caac.21204.
Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, Heijnen CJ, Walker AK. Mechanisms of chemotherapy-induced behavioral toxicities. Front Neurosci. 2015;9:131.
Managing Peripheral Neuropathy. Available online: https://www.cancer.org/cancer/managing-cancer/side-effects/nervous-system/peripheral-neuropathy/managing-peripheral-neuropathy.html Accessed on 26 Aug 2023.
Oxaliplatin - NCI Available online: https://www.cancer.gov/about-cancer/treatment/drugs/oxaliplatin. Accessed on 26 Aug 2023.
Shen S, Lim G, You Z, Ding W, Huang P, Ran C, Doheny J, Caravan P, Tate S, Hu K, et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci. 2017;20:1213–6. https://doi.org/10.1038/nn.4606.
Irinotecan-induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms | Molecular Cancer Therapeutics | American Association for Cancer Research Available online: https://aacrjournals.org/mct/article/15/6/1376/92122/Irinotecan-Induced-Gastrointestinal-Dysfunction. Accessed on 26 Aug 2023.
Man S, Xie L, Liu X, Wang G, Liu C, Gao W. Diosgenin relieves oxaliplatin-induced pain by affecting TLR4/NF-κB Inflammatory Signaling and the Gut Microbiota. Food Funct. 2023;14:516–24. https://doi.org/10.1039/D2FO02877H.
Paclitaxel Monograph for Professionals. Available online: https://www.drugs.com/monograph/paclitaxel.html. Accessed on 26 Aug 2023.
Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, Yamaki S, Adamson T, Ermel R, McKemy D, et al. Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Sci Rep. 2019;9:20324. https://doi.org/10.1038/s41598-019-56832-x.
Wade H, Pan K, Duan Q, Kaluzny S, Pandey E, Fatumoju L, Saraswathi V, Wu R, Harris EN, Su Q. Akkermansia muciniphila and its membrane protein ameliorates intestinal inflammatory stress and promotes epithelial wound healing via CREBH and miR-143/145. J Biomed Sci. 2023;30:38. https://doi.org/10.1186/s12929-023-00935-1.
Castelli V, Palumbo P, d’Angelo M, Moorthy NK, Antonosante A, Catanesi M, Lombardi F, Iannotta D, Cinque B, Benedetti E, et al. Probiotic DSF counteracts chemotherapy induced neuropathic pain. Oncotarget. 2018;9:27998–8008. https://doi.org/10.18632/oncotarget.25524.
Cuozzo M, Castelli V, Avagliano C, Cimini A, d’Angelo M, Cristiano C, Russo R. Effects of chronic oral probiotic treatment in paclitaxel-induced neuropathic pain. Biomedicines. 2021;9:346. https://doi.org/10.3390/biomedicines9040346.
Shi H, Chen M, Zheng C, Yinglin B, Zhu B. Fecal microbiota transplantation alleviated paclitaxel-induced peripheral neuropathy by interfering with astrocytes and TLR4/p38MAPK pathway in rats. J Pain Res. 2023;16:2419–32. https://doi.org/10.2147/JPR.S415642.
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primer. 2019;5:1–18. https://doi.org/10.1038/s41572-019-0092-1.
Grasset E, Burcelin R. The gut microbiota to the brain axis in the metabolic control. Rev Endocr Metab Disord. 2019;20:427–38. https://doi.org/10.1007/s11154-019-09511-1.
Vinik AI, Nevoret M-L, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42:747–87. https://doi.org/10.1016/j.ecl.2013.06.001.
Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002;90:3–10. https://doi.org/10.1016/S0002-9149(02)02553-5.
Dunnigan SK, Ebadi H, Breiner A, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care. 2013;36:3684–90. https://doi.org/10.2337/dc13-0746.
Gumy LF, Bampton ETW, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37:298–311. https://doi.org/10.1016/j.mcn.2007.10.004.
Wang Y, Ye X, Ding D, Lu Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J Int Med Res. 2020;48:0300060520936806. https://doi.org/10.1177/0300060520936806.
Vrieze A, Nood EV, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-916.e7. https://doi.org/10.1053/j.gastro.2012.06.031.
Sabico S, Al-Mashharawi A, Al-Daghri NM, Wani K, Amer OE, Hussain DS, Ahmed Ansari MG, Masoud MS, Alokail MS, McTernan PG. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Clin Nutr Edinb Scotl. 2019;38:1561–9. https://doi.org/10.1016/j.clnu.2018.08.009.
Ma P, Mo R, Liao H, Qiu C, Wu G, Yang C, Zhang Y, Zhao Y, Song X-J. Gut microbiota depletion by antibiotics ameliorates somatic neuropathic pain induced by nerve injury, chemotherapy, and diabetes in mice. J Neuroinflammation. 2022;19:169. https://doi.org/10.1186/s12974-022-02523-w.
Bonomo RR, Cook TM, Gavini CK, White CR, Jones JR, Bovo E, Zima AV, Brown IA, Dugas LR, Zakharian E, et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci. 2020;117:26482–93. https://doi.org/10.1073/pnas.2006065117.
Cai T, Ye X, Yong H, Song B, Zheng X, Cui B, Zhang F, Lu Y, Miao H, Ding D. Fecal microbiota transplantation relieve painful diabetic neuropathy. Medicine (Baltimore). 2018;97: e13543. https://doi.org/10.1097/MD.0000000000013543.
Yao C, Zhou X, Zhao B, Sun C, Poonit K, Yan H. Treatments of traumatic neuropathic pain: a systematic review. Oncotarget. 2017;8:57670–9. https://doi.org/10.18632/oncotarget.16917.
Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68:734–44. https://doi.org/10.1152/jn.1992.68.3.734.
Nassar MA, Baker MD, Levato A, Ingram R, Mallucci G, McMahon SB, Wood JN. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. Mol Pain. 2006;2:33. https://doi.org/10.1186/1744-8069-2-33.
Maratou K, Wallace VCJ, Hasnie FS, Okuse K, Hosseini R, Jina N, Blackbeard J, Pheby T, Orengo C, Dickenson AH, et al. Comparison of dorsal root ganglion gene expression in rat models of traumatic and HIV-associated neuropathic pain. Eur J Pain Lond Engl. 2009;13:387–98. https://doi.org/10.1016/j.ejpain.2008.05.011.
Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury - PMC Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3713313/. Accessed on 28 Aug 2023.
Austin PJ, Wu A, Moalem-Taylor G. Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp JoVE. 2012. https://doi.org/10.3791/3393.
Chen P, Wang C, Ren Y, Ye Z, Jiang C, Wu Z. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol Brain. 2021;14:50. https://doi.org/10.1186/s13041-021-00765-y.
Ding W, You Z, Chen Q, Yang L, Doheny J, Zhou X, Li N, Wang S, Hu K, Chen L, et al. Gut microbiota influences neuropathic pain through modulating proinflammatory and anti-inflammatory T cells. Anesth Analg. 2021;132:1146. https://doi.org/10.1213/ANE.0000000000005155.
Morales D, Pacurariu A, Slattery J, Pinheiro L, McGettigan P, Kurz X. Association between peripheral neuropathy and exposure to oral fluoroquinolone or amoxicillin-clavulanate therapy. JAMA Neurol. 2019;76:827–33. https://doi.org/10.1001/jamaneurol.2019.0887.
Zakrzewska JM, Linskey ME. Trigeminal neuralgia. BMJ. 2014;348: g474. https://doi.org/10.1136/bmj.g474.
Gambeta E, Chichorro JG, Zamponi GW. Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol Pain. 2020;16:1744806920901890. https://doi.org/10.1177/1744806920901890.
Liu L, He L, Yin C, Huang R, Shen W, Ge H, Sun M, Li S, Gao Y, Xiong W. Effects of palmatine on BDNF/TrkB-mediated trigeminal neuralgia. Sci Rep. 2020;10:4998. https://doi.org/10.1038/s41598-020-61969-1.
Zhang X-J, Yuan Z-W, Qu C, Yu X-T, Huang T, Chen PV, Su Z-R, Dou Y-X, Wu J-Z, Zeng H-F, et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol Res. 2018;137:34–46. https://doi.org/10.1016/j.phrs.2018.09.010.
Postherpetic Neuralgia | NEJM Available online: https://doi.org/10.1056/NEJMcp1403062?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed. Accessed on 29 Aug 2023.
Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. https://doi.org/10.1016/S0140-6736(99)01307-0.
Alterations of the gut microbiota in patients with postherpetic neuralgia. Available online: https://www.researchsquare.com. Accessed on 29 Aug 2023.
Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, Mannion AF, Reis S, Staal JB, Ursin H, et al. Chapter 4 European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15:s192–300. https://doi.org/10.1007/s00586-006-1072-1.
Itz CJ, Geurts JW, van Kleef M, Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain Lond Engl. 2013;17:5–15. https://doi.org/10.1002/j.1532-2149.2012.00170.x.
Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185–90. https://doi.org/10.1007/s11916-009-0032-y.
Rajasekaran S, Soundararajan DCR, Tangavel C, Muthurajan R, Sri-Vijay-Anand KS, Matchado MS, Nayagam SM, Shetty AP, Kanna RM, Dharmalingam K. Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur Spine J. 2020;29:1621–40. https://doi.org/10.1007/s00586-020-06446-z.
Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Gut-disc axis: a cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31:917–25. https://doi.org/10.1007/s00586-022-07152-8.
Wang Z, Wu H, Chen Y, Chen H, Wang X, Yuan W. Lactobacillus paracasei S16 alleviates lumbar disc herniation by modulating inflammation response and gut microbiota. Front Nutr. 2021;8: 701644. https://doi.org/10.3389/fnut.2021.701644.
Jensen OK, Andersen MH, Østgård RD, Andersen NT, Rolving N. Probiotics for chronic low back pain with type 1 modic changes: a randomized double-blind, placebo-controlled trial with 1-year follow-up using Lactobacillus rhamnosus GG. Eur Spine J. 2019;28:2478–86. https://doi.org/10.1007/s00586-019-06046-6.
Dekker Nitert M, Mousa A, Barrett H, Naderpoor N, De Courten B. Altered gut microbiota composition is associated with back pain in overweight and obese individuals. Front Endocrinol. 2020;11:605.
Wang L, Wei Z, Pan F, Song C, Peng L, Yang Y, Huang F. Case report: fecal microbiota transplantation in refractory ankylosing spondylitis. Front Immunol. 2023;14:1093233. https://doi.org/10.3389/fimmu.2023.1093233.
Acknowledgements
To professor Nicola Brienza, our brightest star in the deepest sky.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
AC and MG wrote the first draft of the manuscript which was critically revised by FP, FI and AM; AC and FP prepared the figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Corriero, A., Giglio, M., Inchingolo, F. et al. Gut Microbiota Modulation and Its Implications on Neuropathic Pain: A Comprehensive Literature Review. Pain Ther 13, 33–51 (2024). https://doi.org/10.1007/s40122-023-00565-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00565-3