Abstract
Introduction
Since 2009, a pneumococcal conjugate vaccine (PCV) covering 13 serotypes (PCV13) has been included by Germany’s Standing Committee on Vaccinations for infants, resulting in major reductions in pneumococcal disease (PD). Higher-valent vaccines may further reduce PD burden. This cost-effectiveness analysis compared 20-valent PCV (PCV20) under a 3+1 schedule with 15-valent PCV (PCV15) and PCV13, both under 2+1 schedule, in Germany’s pediatric population.
Methods
A Markov model with annual cycles over a 10-year time horizon was adapted to simulate the clinical and economic impact of pediatric vaccination with PCV20 versus lower-valent PCVs in Germany. The model used PCV13 clinical effectiveness and impact studies as well as PCV7 efficacy studies for vaccine direct and indirect effect estimates. Epidemiologic, utility, and medical cost inputs were obtained from published sources. Benefits and costs were discounted at 3% from a German societal perspective. Outcomes included PD cases, deaths, costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs).
Results
In the base case, PCV20 provided greater health benefits than PCV13, averting more cases of invasive pneumococcal disease (IPD; 15,301), hospitalized and non-hospitalized pneumonia (460,197 and 472,365, respectively), otitis media (531,634), and 59,265 deaths over 10 years. This resulted in 904,854 additional QALYs and a total cost saving of €2,393,263,611, making PCV20 a dominant strategy compared with PCV13. Compared to PCV15, PCV20 was estimated to avert an additional 11,334 IPD, 704,948 pneumonia, and 441,643 otitis media cases, as well as 41,596 deaths. PCV20 was associated with a higher QALY gain and lower cost (i.e., dominance) compared with PCV15. The robustness of the results was confirmed through scenario analyses as well as deterministic and probabilistic sensitivity analyses.
Conclusion
PCV20 3+1 dominated both PCV13 2+1 and PCV15 2+1 over 10 years. Replacing lower-valent PCVs with PCV20 would result in greater clinical and economic benefits, given PCV20’s broader serotype coverage.
Plain Language Summary
Pneumococcal diseases (e.g., ear infections, pneumonia, bloodstream infections) are among the leading causes of illness and death in children worldwide. The pneumococcal conjugate vaccine protects against pneumococcal diseases and has significantly reduced the number of newly diagnosed cases. Higher-valent vaccines (which provide coverage for a greater number of disease-causing serotypes) have recently received European Commission approval for use in adults and children. This study examined costs and health benefits associated with the 20-valent pneumococcal conjugate vaccine (PCV20) under a 3+1 (i.e., three primary doses and one booster dose) schedule in Germany’s childhood vaccination program compared with 13-valent pneumococcal conjugate vaccine (PCV13) and the 15-valent pneumococcal conjugate vaccine (PCV15), both under a 2+1 (two primary doses, one booster) schedule. PCV20 was estimated to result in greater health benefits from avoiding more cases in pneumococcal diseases and lower costs compared with both PCV13 and PCV15. PCV20, therefore, is considered the best option among the three vaccines for children in Germany.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Streptococcus pneumoniae is the leading cause of bacterial pneumonia and global mortality in children. |
Pneumococcal conjugate vaccines (PCVs) elicit robust and durable immune responses in both pediatric and adult populations. |
This study examined the cost-effectiveness of PCV20 under a 3+1 schedule in Germany’s pediatric population compared with PCV13 and a secondary comparator (PCV15), both under a 2+1 schedule. |
What was learned from the study? |
PCV20 was estimated to prevent more pneumococcal disease cases and deaths versus PCV13 and PCV15, as well as providing greater quality-adjusted life years and cost savings (i.e., dominant strategy) over 10 years. |
Implementation of PCV20 under a 3+1 schedule into the German pediatric immunization program would result in greater clinical and economic benefits versus PCV13 and PCV15, both under a 2+1 schedule. |
Introduction
Streptococcus pneumoniae is the leading cause of bacterial pneumonia and global mortality in children [2,3,4,5]. In 2016, S. pneumoniae has been estimated to account for approximately 197 million cases of pneumonia and 1.1 million deaths [6]. This encapsulated bacterium is the major cause of pneumococcal diseases ranging from otitis media (OM) and pneumonia to life-threatening invasive pneumococcal diseases (IPDs), including sepsis and meningitis.
Pneumococcal conjugate vaccines (PCV) elicit robust and durable immune responses in both pediatric and adult populations [4]. They have noticeably reduced IPD incidence across all age groups due to indirect effects (i.e., herd effects or the effect on the unvaccinated population) [7, 8]. The 7-valent PCV (PCV7) was first approved in Europe in 2001 [9], and was recommended for high-risk children in July 2001 by Germany’s Standing Committee on Vaccinations [Ständige Impfkommission (STIKO)] with a schedule of three priming doses in infancy plus one booster (3+1) [10]. The recommendation was extended to the entire infant population (< 2 years of age) in July of 2006 [11].
The 13-valent PCV (PCV13) and 10-valent PCV (PCV10) replaced PCV7 and were introduced in 2009, and administered based on physician’s choice [12]. In 2015, STIKO changed its recommendations for full-term infants from a 3+1 schedule with the priming series administered at 2, 3, and 4 months and a booster at 11–14 months, to a 2+1 vaccination schedule with a priming series administered at 2 months and 4 months plus a booster at 11 months [3, 13]. The 3+1 schedule remains in place for preterm infants [14].
Next-generation PCVs with increased serotype coverage [15-valent PCV (PCV15) and 20-valent PCV (PCV20)] were approved for adults aged 18 years and older by the European Commission in October 2021 and February 2022, respectively [15, 16]. Since September 2023, PCV20 is recommended in Germany for all individuals aged 60 years and older and for all individuals aged 18‒59 years with underlying diseases. A cost-effectiveness analysis (CEA) in the German adult population concluded that a single dose of PCV20 for adults aged ≥ 60 years and adults aged 18‒59 years with moderate- and high-risk conditions would prevent pneumococcal disease cases, save lives, and would be cost-saving compared to the pneumococcal polysaccharide vaccine (PPSV23) alone, PCV13 followed by PPSV23, or PCV15 followed by PPSV23 [17].
Prior to the licensure of PCV15 in October of 2022, PCV13 was considered as the standard of care. With the inclusion of PCV15 in the STIKO recommendation for infants in Germany, the current clinical practice includes a market basket of PCV13 and PCV15. PCV20 covers all PCV15 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F) and five additional serotypes (8, 10A, 11A, 12F, and 15B). On January 25, 2024, the Committee for Medicinal Products for Human Use adopted a positive opinion for the higher-valent option of PCV20 for the use in children [18] PCV20 was approved by the European Commission in a 3+1 schedule on March 12, 2024 [16]. The purpose of this CEA was to examine the health benefits and costs of implementing a PCV20 vaccination program under a 3+1 schedule in Germany’s pediatric population compared with PCV13 and PCV15, both administered in a 2+1 schedule.
Methods
Conceptual Framework and Model Structure
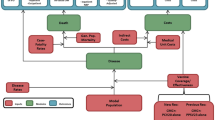
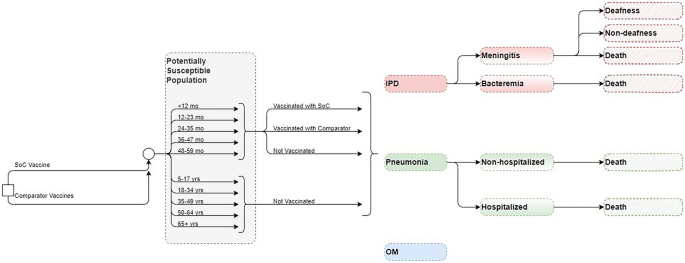
The CEA was structured in Microsoft Excel® (Redmond, WA, US) using a decision-analytic Markov (state-transition) cohort model. The Markov model estimated pneumococcal disease-related events in both unvaccinated and vaccinated individuals (Fig. 1). The model captured an individual’s possible transition to several clinical events, including IPD (developing into either meningitis or sepsis/bacteremia), all-cause pneumonia (non-hospitalized or hospitalized), all-cause OM, no pneumococcal disease state, and death. Death captured both general mortality and case fatality, which could occur in any disease state and non-disease state. The transition occurred on an annual cycle and was age- and vaccination-specific. The non-mutually exclusive nature of pneumococcal disease was reflected through each 12-month interval during which persons could transition to one or more disease states or remain in a non-disease state. In the case of more than one pneumococcal disease, costs and quality-adjusted life year (QALY) decrements associated with all events were considered. At the beginning of each annual cycle, a new cohort of children (i.e., incoming birth cohort) entered the model and was eligible for vaccination.
The full health benefit of vaccination was applied to the entire German population, of which the vaccinated cohort experienced the direct effects of vaccination immediately, while the rest of the unvaccinated population gradually received indirect effects over the model time horizon.
Target Population and Subgroups
The target population was composed of infants aged < 2 years (i.e., ,a vaccination cohort), while the model assumed that the groups aged 2–4 years, 5–17 years, and 18–49 years were not vaccinated with the higher-valent PCVs. In clinical practice, a proportion of the group ≥ 60 years is vaccinated under the adult immunization program [19, 20]; hence, within this pediatric model, that proportion of adults was excluded from receiving indirect effects, while the remainder of the population ≥ 60 years remained unvaccinated and benefitted from indirect effects of pediatric vaccination.
Intervention and Comparator Strategies
STIKO currently recommends PCV10, PCV13, and PCV15 for infants and children in Germany [21]. However, PCV13 was shown to avoid more cases than PCV10 [22] and accounts for the majority of vaccination rate, at more than 90%, in children, remaining the most used PCV in the past decade in Germany [13, 23]. Therefore, PCV10, although included in the STIKO recommendation, was not considered among comparators in this analysis. The analysis evaluated the clinical and economic outcomes of PCV20 in a 3+1 schedule as a potential vaccination strategy compared with PCV13 (i.e., standard of care) and PCV15, both in a 2+1 schedule.
Perspective, Time Horizon, Cycle Length, and Discount Rate
The base-case analysis was conducted from a German societal perspective using a 3% annual discount rate for both costs and benefits, according to the recommendations of the Institute for Quality and Efficiency in Health Care and STIKO [24, 25]. The model used an annual cycle length over a 10-year time horizon to capture relevant costs and outcomes. The 10-year time horizon sufficiently captures the health benefits of the PCV vaccination program, based on the observation of the accrual and stabilization of indirect effects over a 5- to 10-year period following the introduction of PCV7 and PCV13 [26, 27]. The life years and QALY loss is accumulated over the time loss between death occurrence and life expectancy (with the QALYs being age-dependent and discounted from the year the death occurs). Lifetime long-term costs related to a clinical event, such as sequalae following meningitis, were incorporated in the model as a one-time discounted cost in the cycle in which the event happens.
This study was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors; as such, ethical approval was not required.
Inputs
Population and Epidemiology Data
Population data were obtained from the German Federal Statistical Office to determine the size of the stratified population age groups [28] (Table S1; Supplementary Material) and the incoming birth cohort each year [29] (Table S2; Supplementary Material).
Age-specific disease incidence rates per 100,000 individuals were informed by German-specific published literature [30,31,32,33], adjusted for relevant age groups using population size from the German Federal Statistical Office [28] (Table 1).
Mortality in the analysis was considered as a combination of general mortality [34] and case fatality, which were applied to meningitis, sepsis/bacteremia, and all-cause hospitalized and non-hospitalized pneumonia (Table 1), while no mortality was assumed for OM [22, 31].
The model considered sequela following meningitis (i.e., deafness and non-deafness) in the base case as sequela following meningitis are quite common among patients with IPD [22]. The data for the proportion of patients developing complications with IPD were sourced from published literature [22] (Table S3; Supplementary Material).
IPD serotype-specific distribution by each PCV stratified by age groups was obtained from [35, 36] (Table 2). The serotype coverage for PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) was incorporated as one input, while the coverage for each additional serotype included in higher valent vaccines was input separately for each age group. The analysis did not consider cross-reactive serotypes. Non-invasive serotype distributions (i.e., for pneumonia and OM) were assumed to be the same as IPD serotype distribution.
Vaccine Effectiveness and Efficacy
The direct effect of PCVs against IPD for a complete vaccine schedule was assumed to be equivalent to the adjusted PCV13 effectiveness against PCV13-type IPD, of which 78.2% [95% confidence interval (CI) 56.0, 89.0] was applied for vaccines in a 2+1 schedule while 89.7% (95% CI 82.0, 94.0) was used for PCV20 in 3+1 schedule (Table 3) [37]. To estimate the direct effects of the higher-valent PCVs against all-cause pneumonia (non-hospitalized and hospitalized) and OM, the model adopted an approach commonly used in CEAs [38,39,40,41,42], in which the effectiveness of higher-valent PCVs against non-invasive disease was assumed to be the same as the reported trial-based efficacy data of PCV7, which was then adjusted based on study design, period, and country-specific factors. These results demonstrated an efficacy of 25.5% (95% CI 4.4, 34.0) [43], 6.0% (95% CI − 1.5, 11.0) [44], and 7.8% (95% CI 5.2, 10.5) [45] against radiographically confirmed non-invasive hospitalized pneumonia, non-hospitalized pneumonia, and OM, respectively (Table 3).
In addition, a < 12-month effect modifier was used to account for potential reduced effectiveness in the first year of life during which children have only received the priming series of the full vaccination schedule [i.e., at two-thirds (~ 67%) of the full effect for vaccination with a 2+1 schedule for PCV13 and PCV15, and at 75.6% for PCV20 3+1 based on the Advisory Committee on Immunization Practices’ assumption [46]]. Vaccine coverage was set at 89.9% for the priming series and at 76.8% for the booster dose [47].
Evidence has shown that direct effects of PCVs remain stable for a few years after the final dose. For example, the efficacy of PCV13 was steady for up to 4 years in infants after vaccination was completed [37] and for more than 5 years in people aged ≥ 65 years after a single dose [48]. Therefore, in the base case, a full direct effect for the first 5 years after the booster dose was assumed for all vaccines, followed by an annual waning of 10% from year 6 through year 10.
The analysis considered indirect effects in unvaccinated individuals since they are an important benefit from pediatric PCV national immunization programs. The indirect effect against serotypes covered represents the maximum protection the unvaccinated population could receive from a vaccine regimen. This was modeled as a percent reduction in the expected age-specific disease incidence. Indirect effect was not realized immediately and was only applied to newly covered serotypes in PCV15 and PCV20, as the indirect effect for PCV13 serotypes was assumed to have already reached a steady state. These benefits accrued gradually until a new steady state was reached for additional serotypes. Indirect effect for PCV15 and PCV20 was assumed to have no added effects on PCV13 steady-state serotypes. The model assumed that incidence trends for all newly covered serotypes would decrease consistently across ages. For IPD and non-hospitalized and hospitalized pneumonia, indirect effect was assumed for all age groups, while for OM, indirect effect was assumed only for the < 5 years age group. To estimate indirect effects, the model incorporated the reduction in incidence of the newly covered serotypes and the accrual of the indirect effects of higher-valent PCVs (see Supplementary Material, Appendix A).
Resource Use and Costs
Vaccine costs for PCV13, PCV20, and PCV15 were derived from retail pharmacy price per dose [49], and the administration cost were from [22], inflated to 2022 Euros (€). Additional vaccine cost and administration cost, which accounted for an additional visit, were applied to the extra dose under 3+1 schedule for PCV20. Medical costs per episode related to each disease state sourced from [22] were included for all relevant age groups in the model [22]. Lifetime medical costs per episode of sequela were assumed to be the same across all age groups for deafness and non-deafness [22]. Societal costs considered productivity loss per episode of meningitis, sepsis/bacteremia, inpatient and outpatient pneumonia and OM, as well copayment for adult patients. All costs were in Euros (€) and obtained from German published sources and the literature, then inflated to 2022 prices using the healthcare component of the consumer price index [50] where relevant. The summary of cost inputs is listed in Table 3.
Utility
The model used baseline utility for the general population [51] minus disutilities related to disease states and acute events to assess quality of life related to each vaccination strategy (Table 3) [42, 52,53,54,55,56,57].
Assessment of Uncertainty
Uncertainty around the analyses was evaluated using deterministic sensitivity analyses (DSA), probabilistic sensitivity analyses (PSA), and scenario analyses. DSA assessed uncertainty around the following variables: disease incidence, breakdown of IPD cases, case fatality rate (CFR), serotype distribution by age, vaccine effectiveness and utilities.
In the PSA, all parameters subject to any degree of uncertainty were assessed The incremental results for costs, QALYs, and incremental cost-effectiveness ratios (ICER) were recorded for each simulation of a total of 1000 simulations to examine the stability of the model findings.
Several scenarios were conducted, the description and results of which are reported in Table 6. In addition to the scenario assessments, threshold analyses were conducted to assess the price per dose of PCV20 under a 3+1 schedule. These analyses aim to determine the price range at which PCV20 stays a cost-saving strategy compared to PCV13 2+1 and PCV15 2+1, assuming consistent input parameters for other variables (including the prices of PCV13 and PCV15).
Results
Base-case Results
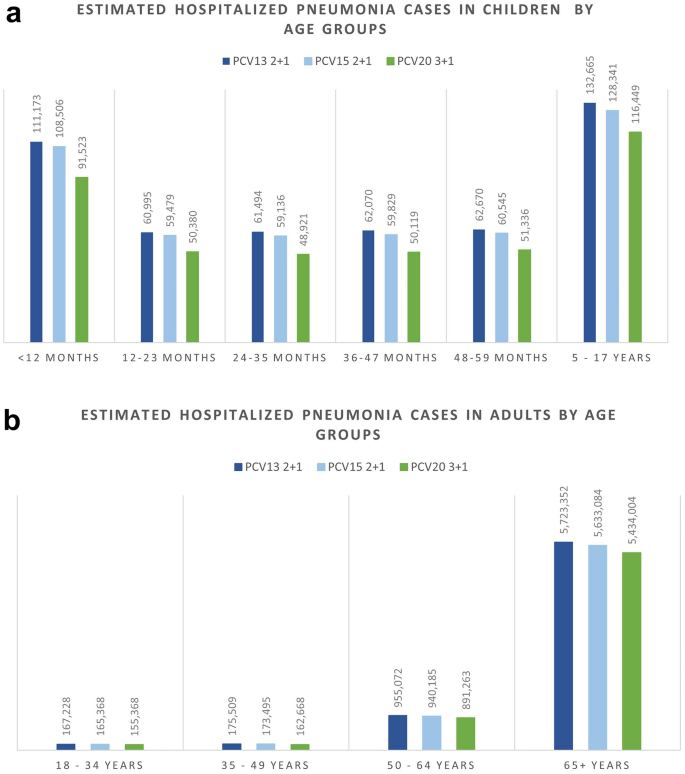
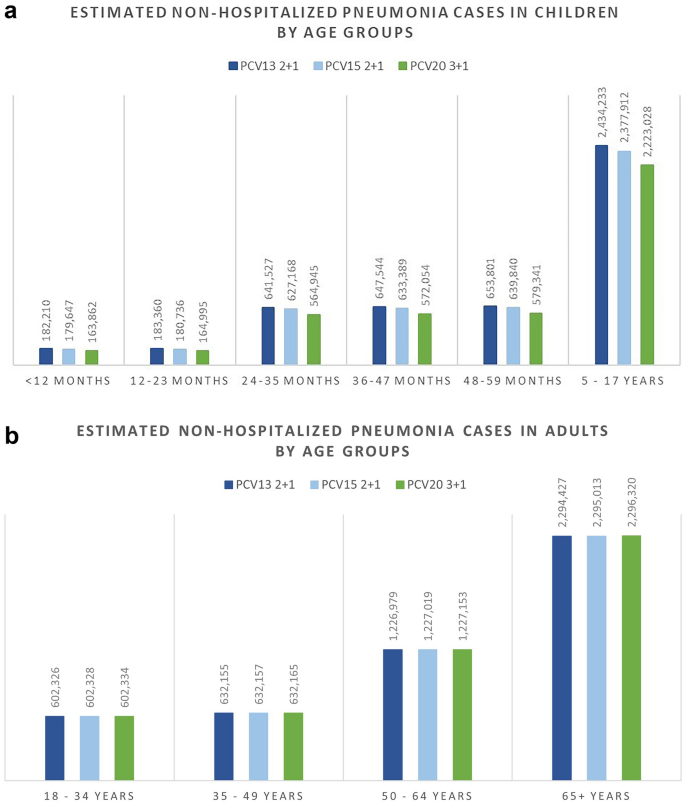
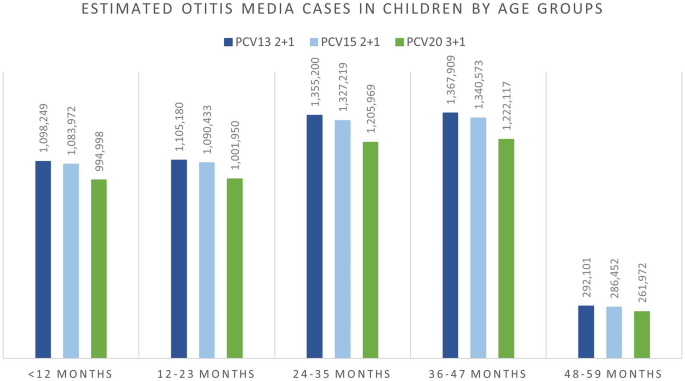
Over the 10-year time horizon, compared to both PCV13 2+1 and PCV15 2+1, PCV20 3+1 provided substantially greater health benefits and broader protection (Table 4). Compared to PCV13, PCV20 is estimated to result in greater health benefits due to a greater number of cases averted and total QALYs gained (Table 4). Compared with PCV13, PCV20 averted an additional 15,301 cases of IPD; 460,197 and 472,365 cases of hospitalized and non-hospitalized pneumonia, respectively; 531,634 cases of OM; and 59,265 deaths due to disease across all ages. Consequently, PCV20 was estimated with a higher QALY gain of 904,854. In comparison with PCV15, the number of additional cases averted by PCV20 were 11,334, 335,937, 369,012, and 441,643 in IPD, hospitalized pneumonia, non-hospitalized pneumonia, and OM, respectively. Additionally, an estimation of a reduction of 41,596 deaths due to disease was estimated for PCV20 versus PCV15, and PCV20 was associated with an incremental QALY of 646,235.
PCV20 was associated with higher direct vaccine costs, at €525,362,283 and €522,747,819 more than that of PCV13 and PCV15, respectively. This is the result of the higher price per dose considered for PCV20 and the additional dose included in PCV20 under the 3+1 schedule. However, it resulted in significant cost savings from lower direct costs of disease and lifetime costs of sequela compared to both comparators (Table 4) due to broader serotype coverage compared to PCV13 and PCV15. PCV20 was the dominant strategy in both comparisons, with a total cost saving of €2,393,263,611 versus PCV13 and of €1,628,000,506 versus PCV15.
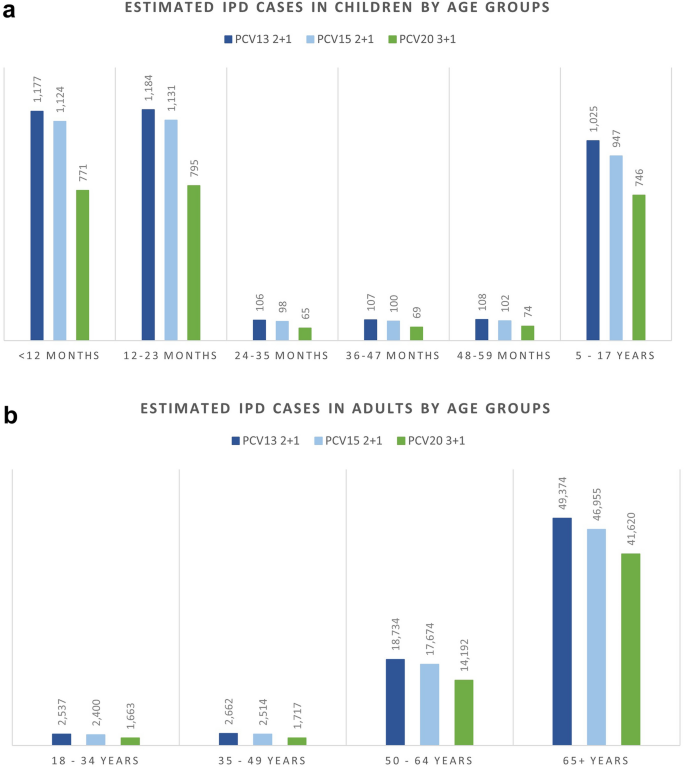
The breakdown results by age groups were mostly consistent with the overall results where more cases were avoided related to PCV20 in all included age groups for IPD, hospitalized pneumonia, and OM than to PCV13, especially in the oldest group of 65+ year olds. Similarly, PCV20 is estimated to prevent more cases of non-hospitalized pneumonia than PCV13 in children less than 5 years old. The higher valent vaccines showed an increasing number of prevented non-invasive pneumonia. One could observe a shift from severe cases (i.e., hospitalized cases) in the lower valent vaccine strategies to less severe cases (i.e., non-hospitalized pneumonia) in the higher valent vaccine strategies. Furthermore, better health outcomes from PCV20 were shown by a reduction in deaths due to disease in all age groups, with the greatest reduction observed in those 65 years old and above (50,064 deaths averted) (Table S4; Supplementary Material). Similar results broken down by age groups were also observed when comparing PCV20 with PCV15 (Figs. 1, 2, 3, 4, 5; Table S4; Supplementary Material).
Sensitivity Analyses
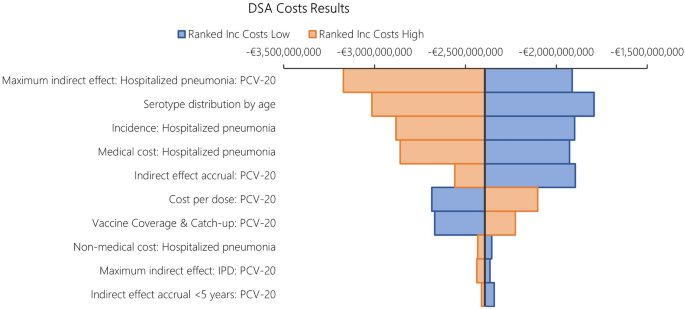
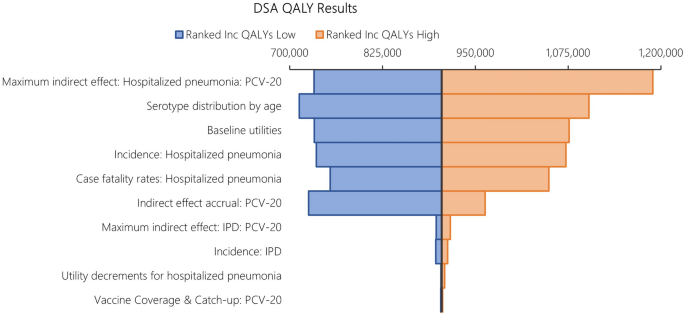
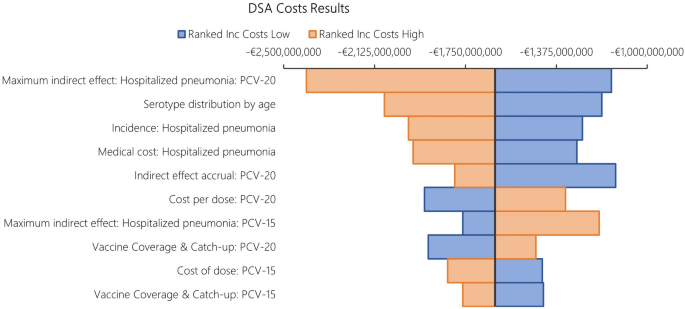
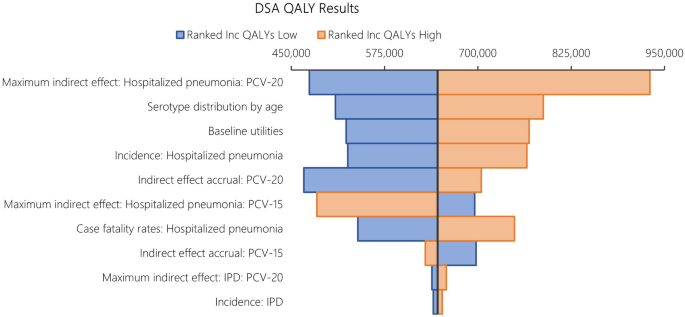
The results from DSA, where one parameter was varied in one direction while all other inputs were held constant, are reported in Fig. 6 for costs and Fig. 7 for QALYs. When compared to PCV13, the key drivers for costs included maximum indirect effect against hospitalized pneumonia (PCV20), serotype distribution by age, incidence of hospitalized pneumonia and medical costs of per episode of hospitalized pneumonia.
The DSA of PCV20 versus PCV13 also illustrated that the top five most impactful parameters on QALYs were maximum indirect effects on hospitalized pneumonia (PCV20) and serotype distribution by age, baseline utilities, followed by hospitalized pneumonia incidence and CFR for hospitalized pneumonia. When comparing PCV20 and PCV15, the results were largely similar (Figs. 8 and 9).
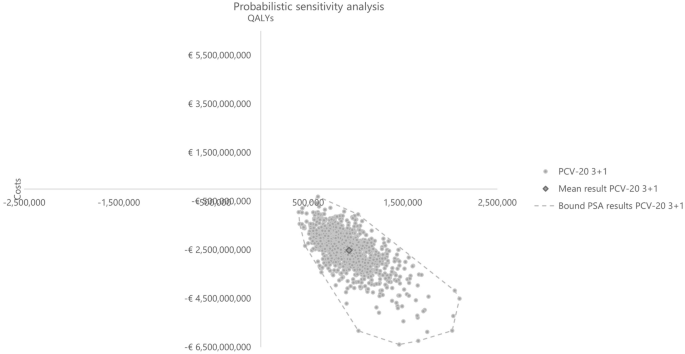
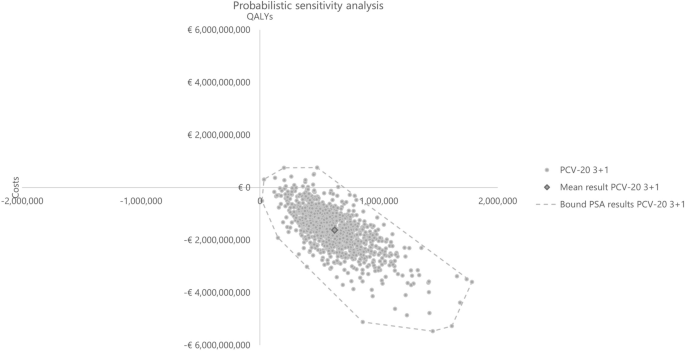
Probabilistic results from the PSA based on 1,000 iterations aligned with the base case results, confirming robust findings with PCV20 being dominant in all simulations. Compared with PCV13, PCV20 was the dominant strategy in all iterations, while PCV20 dominated PCV15 in 98.40% of the total 1000 iterations (Table 5). The cost-effectiveness plane plots are reported in Figs. 10 and 11.
Scenario Analyses
Scenario analysis results are summarized in Table 6. In the three scenarios where different discount rates were applied for costs and benefits, the qualitative conclusion of PCV20 being the dominant strategy compared to both comparators remained robust. When reducing indirect effects (i.e., maximum reduction in disease incidence) by half, PCV20 was still estimated to have better health benefits and lower costs compared to both PCV13 and PCV15. Similarly, extending time to realize indirect effects (i.e., accrual data in the first 2 years at 0%) and increased vaccination uptake in adults 65+ years old aligned with the base case results of PCV20 being the dominant strategy versus both comparators. Using a payer perspective led to a decrease by 15% and 17% in ICER in the comparison between PCV20 versus PCV13 and PCV20 versus PCV15, respectively. However, the qualitative conclusion remained the same. Other scenarios testing a different waning assumption (i.e., reducing duration of full protection to 3 years) and serotype replacement were in line with the base case with minimal change in ICER. Overall, the results and conclusion were relatively robust. When assuming a high vaccine uptake in infant (90%), ICERs increased slightly, at less than 1%, for both PCV20 versus PCV13 and PCV20 versus PCV15. Considering disutility related to adverse events related to the administration of all vaccines (e.g., local reaction and systematic reaction or fever) resulted in the same conclusion of PCV20 3+1 being the dominant strategy among the three PCVs. In the threshold analyses, threshold prices per dose for PCV20 3+1 were €170 versus PCV13 2+1 and €135 versus PCV15 2+1. Given that all price ranges exceed double the current list price per dose of PCV20 for cost-saving analyses, it is reasonable to conclude that changes in the price per dose are unlikely to impact the study's conclusions.
This article is based on previously conducted studies or collected published data and does not contain any new studies with human participants or animals performed by any of the authors.
Discussion
The introduction of next-generation PCVs with increased serotype coverage in Europe has provided options to be considered in national childhood immunization programs. This study examined the cost and health outcomes related to PCV20 under a 3+1 schedule compared with PCV13 and PCV15, both under a 2+1 schedule, in the pediatric population in Germany.
The base-case results demonstrated PCV20 as the dominant strategy over both lower-valent alternative vaccines. PCV20 was estimated to have greater health benefits than both PCV13 and PCV15 by averting more cases of pneumococcal diseases, including IPD, pneumonia, and OM, over a 10-year time horizon. This resulted in higher QALY gained and lower total costs related to PCV20, implying dominance of PCV20 compared with PCV13 and PCV15. Several scenarios assessed additional uncertainty, such as effects of different discount rates for costs and outcomes, several assumptions on vaccine effects (i.e., reduction in indirect effects and extension in accrual time of indirect effects), and waning duration. In addition, serotype replacement assumptions were examined to test how sensitive the results were to reduction in vaccine-type serotype coverage over time. Finally, payer perspective and an assumption of higher vaccine uptake in infant were explored. The results were robust across all sensitivity analyses including PSA and DSA.
Our findings are consistent with published studies comparing PCV20 to PCV13 and PCV15 in other settings. In Canada [58] and Greece [59], PCV20 was estimated to be cost-saving compared with PCV15 in a 2+1 schedule. In the United Kingdom, PCV20 2+1 was estimated to be cost-saving compared to PCV13 1+1 and cost-effective compared with PCV20 1+1 [60]. Moreover, a public health impact analysis in the Netherlands estimated that PCV20 could avert 45,127 pneumococcal cases compared to PCV10 over 5 years [61]. Our findings are also consistent with a historic economic evaluation in Germany comparing higher-valent versus lower-valent PCVs, in which PCV13 dominated both PCV10 and PCV7 [56]. The PCV13 infant immunization program in Germany was expected to have a substantial public health impact because of its broader serotype coverage compared with both PCV7 and PCV10, similar to our findings for PCV20 compared with both PCV13 and PCV15. Differences in disease incidence have been noted; for example, Strutton et al. 2012 [56] used an IPD incidence among those aged < 2 years of 43.35 per 100,000 individuals, whereas our analysis uses 15.8 per 100,000. The lower incidence values in our economic evaluation reflect the historic impact of PCV13 on disease incidence; however, substantial disease associated with PCV20-unique serotypes remains in Germany.
Our analyses have several limitations. Firstly, a Markov cohort model was considered appropriate for the decision problem based on previous cost-effectiveness assessments of PCVs [62]. Static models are commonly used for economic evaluations of PCVs in Germany [56, 63] and globally [42, 64,65,66,67,68]. While dynamic models are known to capture indirect effects, the decision-analytic Markov cohort model in this study utilizes the simplistic static Markov framework and incorporates components such as indirect effects. This notable improvement in the modeling approach helps quantify the far-reaching effects of vaccination at the population level while maintaining the clarity and transparency of the model.
The methodological framework adopted in this study was based on the examination of overall vaccine-type serotypes rather than individual serotypes, owing to the limited data suitable for modeling clinical outcomes at the individual serotype level, in particular non-invasive disease. While the analysis encompassed diverse distributions in serotype coverage pertaining to additional serotypes facilitated by higher-valent PCVs in contrast to PCV7, the capacity to scrutinize outcomes specific to distinct serotypes within this paradigm remained constrained. Future modeling endeavors could attempt to explore the nuanced dynamics inherent to pneumococcal serotypes, especially for IPD for which data are more readily available.
German data were prioritized to parameterize the model. When German data were not available, data were sourced from other high-income European countries with similar health care systems. Direct effects were estimated from different sources using PCV13 and PCV7 studies, given no studies have measured the effectiveness of PCV15 or PCV20 against pneumococcal disease outcomes. Differential herd effects were not modeled based on a PCV schedule but have been observed in countries that have implemented PCV13 in infant national immunization programs (i.e., increase in disease reduction under a 3+1 vs. 2+1) [26, 69]. There are potential confounding factors, such as the rate of reduction and time to stabilization of IPD incidence across age groups and countries may be associated with multiple factors, including vaccine uptake, implementation of a catch-up program, duration of PCV use, availability of an adult pneumococcal vaccination program, serotypes in circulation, and general epidemiologic variability. To assess the uncertainty around the indirect effect estimations for IPD and non-invasive disease, extensive sensitivity analyses were conducted, such as PSA, DSA, and several scenarios.
The base-case analysis did not include serotype replacement. It is difficult to predict how the characteristics or composition of non-vaccine serotypes will change following higher-valent PCV introduction. Model simulations suggest that replacement may be less for high-valency PCVs [70, 71]. To address uncertainty, we tested the impact of increasing non-vaccine serotypes over time, which all led to similar directional results as the base-case (i.e., PCV20 remained dominant).
We did not consider adverse events originating from vaccination by PCV20, PCV15, or PCV13 in our base-case analysis. Although adverse events after pneumococcal vaccination resulting in healthcare seeking are rare and event rates are similar for the different pneumococcal vaccines, PCV20, licensed in a 3+1 schedule, is likely to result in more adverse events than PCV15 and PCV13, both under a 2+1 schedule. We tested this scenario and found that, although PCV20 3+1 was estimated with slightly higher disutility related to the extra dose, the strategy still provides higher total QALY gain compared to lower-valent alternatives and remained dominant.
Despite a numerically higher immunological response reported against serotype 3 for PCV15, we did not model higher vaccine effectiveness against this serotype for PCV15, as data on clinical effectiveness of PCV15 against serotype 3 are unknown. In contrast, a meta-analysis of observational studies supports direct PCV13 protection against serotype 3 IPD in children. Without any real-world effectiveness data for PCV15, there is no way to assess the actual impact of PCV15 on serotype 3. For that reason, PCV15 and PCV20 are assumed to provide comparable protection to PCV13 against serotype 3 disease.
Recently, STIKO recommended PCV20 for adults aged 60 years and older and for adult patients with underlying diseases [19]. Vaccination rates among adults may increase in the future as PCV20 is only administered once in adults. However, given the recent recommendation and implementation (since January 2024), we were not able to draw conclusions regarding the full impact of adult PCV20 vaccination on epidemiology at this time. To account for the direct impact of adult vaccination, we assumed a proportion of adults received a PCV and therefore received no additional benefit of indirect effects from the pediatric program. We also tested the impact of less pronounced herd effects associated with higher-valent PCVs. Changes to the assumptions resulted in fewer cases avoided and smaller life expectancy gains under a PCV20 pediatric program. However, PCV20 under a 3+1 program remained the dominant strategy avoiding more cases, increasing life expectancy while costing less than PCV13 or PCV15 under a 2+1 program.
Indirect cost estimates were not based on a rigorous German database cost assessment. We applied estimates based on published assumptions. To avoid assumptions on the indirect costs, we carried out a scenario from the payer perspective, not accounting for any indirect costs. This is a very conservative approach given that parents stay at home or have another caregiver for their child. Even under conservative assumptions, PCV20 vaccination strategy was clearly dominating PCV15 and PCV13 strategies resulting in fewer cases and fewer costs.
Conclusion
The results of this CEA estimated that the implementation of PCV20 under a 3+1 schedule into the German immunization recommendation would be less costly and more effective than PCV13 and PCV15, both under a 2+1 schedule. PCV20 has the potential to substantially decrease the clinical and economic burden of pneumococcal diseases in Germany by providing substantially broader protection compared with lower-valent vaccines.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
Ta A, et al. Cost-effectiveness of PCV20 to prevent pneumococcal disease in the pediatric population—a German societal perspective analysis. medRxiv. 2024.03.14.24304296; https://doi.org/10.1101/2024.03.14.24304296.
Dadonaite, B. and M. Roser, Pneumonia. 2019. OurWorldInData.org.
European Centre for Disease Prevention and Control, Factsheet about pneumococcal disease. 2023.
Ganaie F, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio. 2020. https://doi.org/10.1128/mbio.00937-20.
World Health Organization. Pneumococcal Disease. [cited 2023 June]; Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/pneumococcal-disease.
Troeger C, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
Ladhani SN, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51.
Wahl B, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–57.
Tin Tin Htar M, Christopoulou D, Schmitt H-J. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15(1):419.
Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut. Begründung der STIKO-Empfehlungen zur Impfung gegen Pneumokokken und Meningokokken vom Juli 2006. Epidemiologisches Bulletin 2006;4(31).
Robert-Koch-Institut. Impfempfehlungen der Ständigen, I., Begründungen zur allgemeinen Empfehlung der Impfungen gegen Pneumokokken und Meningokokken im Säuglings- und Kindesalter, in Epidemiologisches Bulletin. Robert Koch-Institut. 2006.
van der Linden M, et al. Four years of universal pneumococcal conjugate infant vaccination in Germany: Impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine. 2012;30(40):5880–5.
Perniciaro S, et al. Regional variations in serotype distribution and vaccination status in children. PLoS One. 2019;14(1932-6203(electronic)):e0210278.
Robert Koch-Instituts (RKI). Epidemiologisches Bulletin. Robert Koch-Institut. 2015.
European Medicines Agency. European public assessment report. Vaxneuvance. 2021 [cited 2024 March]; Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxneuvance.
European Medicines Agency. European public assessment report. Prevenar 20 (previously Apexxnar). [cited 2024 March]; Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/prevenar-20-previously-apexxnar.
Kühne Felicitas, et al. Cost-effectiveness of use of 20-valent pneumococcal conjugate vaccine among adults in Germany. Expert Rev Vacc. 2023;22(1):921–32. https://doi.org/10.1080/14760584.2023.2262575.
European Medicines Agency. Apexxnar—opinion on variation to marketing authorisation. [cited 2024 February]; Available from: https://www.ema.europa.eu/en/medicines/human/variation/apexxnar.
Robert-Koch-Institut. STIKO: Aktualisierung der Empfehlungen zur Pneumokokken-Impfung in Epidemiologisches Bulletin. Robert-Koch-Institut. 2023.
Robert-Koch-Institut. Impfquoten bei Erwachsenen in Deutschland in Epidemiologisches Bulletin. Robert-Koch-Institut. 2022.
Robert-Koch-Institut. Impfempfehlungen der Ständigen, I., Stellungnahme der STIKO zum Einsatz von Pneumokokken-Konjugatimpfstoffen im Säuglings-, Kindes- und Jugendalter, in Epidemiologisches Bulletin. Robert Koch-Institut. 2023.
Kuhlmann A, von der Schulenburg JMG. Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany. Eur J Health Econ. 2017;18(3):273–92.
Weiss S, et al. Impact of 10-and 13-valent pneumococcal conjugate vaccines on incidence of invasive pneumococcal disease in children aged under 16 years in Germany, 2009 to 2012. Eurosurveillance. 2015. https://doi.org/10.2807/1560-7917.ES2015.20.10.21057.
Institut für Qualität und Wirtschaftlichkeit im, G., Allgemeine Methoden. 2022.
Ständige Impfkommission am Robert-Koch-Institut, Standardvorgehensweise (SOP) der Ständigen Impfkommission (STIKO) für die systematische Entwicklung von Impfempfehlungen Version 3.1 (Stand: 14.11.2018). Berlin. 2018.
Shiri T, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(1):e51–9.
Centers for Disease Control and Prevention. 2019. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2019. [cited 2024 March]; Available from: https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf.
Statistisches Bundesamt (Destatis), Population: Germany Reporting date, age years. 2022.
Destatis Statistiches B. Current population of Germany. 2023 13 June 2023]; Available from: https://www.destatis.de/EN/Themes/Society-Environment/Population/Current-Population/_node.html.
Weinberger R, et al. Invasive pneumococcal disease in children under 16 years of age: Incomplete rebound in incidence after the maximum effect of PCV13 in 2012/13 in Germany. Vaccine. 2018;36(4):572–7.
Deb A, et al. Clinical and economic burden of pneumococcal disease among individuals aged 16 years and older in Germany. Epidemiol Infect. 2022;150: e204.
Kuhlmann A, von der Schulenburg JMG. Authors’ reply to Gandjour: “Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany.” Eur J Health Econ. 2018;19(3):473–81.
Hu T, et al. Incidence of acute otitis media in children < 16 years old in Germany during 2014–2019. BMC Pediatr. 2022;22(1):204.
van der Linden M, Itzek A. Invasive pneumococcal disease among adults in Germany. Reporting period: 1 July 1992–30 June 2022, in Report for Pfizer Pharma GmbH. 2022, data on file.
van der Linden M, Itzek A. Invasive pneumococcal disease among adults in Germany. Reporting period: 1 July 1992–30 June 2022, in Report for Pfizer Pharma GmbH. 2022.
van der Linden M, Itzek A. Effects of the National Immunization Program for Pneumococcal Conjugate Vaccination (PCV7, PCV10, PCV13) on invasive pneumococcal disease among children in Germany. Reporting period: 1 July 1997–30 June 2022. Pfizer data on file. 2022.
Savulescu C, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine. 2022;40(29):3963–74.
Ansaldi F, et al. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to the 10-valent pneumococcal conjugate vaccine (PCV10) in Italy. Pathogens. 2020. https://doi.org/10.3390/pathogens9020076.
Kim H-Y, et al. Cost-effectiveness of a national immunization program with the 13-valent pneumococcal conjugate vaccine compared with the 10-valent pneumococcal conjugate vaccine in South Korea. Hum Vaccin Immunother. 2021;17(3):909–18.
Klok RM, et al. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin Ther. 2013;35(2):119–34.
Kulpeng W, et al. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31(26):2839–47.
Rozenbaum MH, et al. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ. 2010;340: c2509.
Hansen J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using world health organization standardized interpretation of chest radiographs. Pediat Infect Dis J. 2006;25(9):779–81.
Black SB, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5.
Black S, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19(3):187–95.
Stoecker C. Economic assessment of PCV15 &PCV20. In: Advisory Committee on Immunization Practices. Atlanta, GA. 2021.
Schley K, et al. Did the change of the vaccination schedule effect pneumococcal conjugate vaccination compliance and adherence of premature and mature born infants in Germany? Answers from a claims database analysis. Vaccine. 2023;41(28):4081–91. https://doi.org/10.1016/j.vaccine.2023.05.045.
Patterson S, et al. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia immunization Trial in Adults). Trials Vaccinol. 2016;5:92–6.
Lauer-Fischer GmbH. LAUER-TAXE® Kompetenz online. 2023.
Statistisches Bundesamt (Destatis), Consumer Price Index: Germany Reporting date, application [Table 61111]. 2022. [cited 2024 March]. Available from: https://www.destatis.de/EN/Home/_node.html.
Grochtdreis T, et al. Health-related quality of life measured with the EQ-5D-5L: estimation of normative index values based on a representative German population sample and value set. Eur J Health Econ. 2019;20(6):933–44.
Mangen M-JJ, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31):4203–14.
Rozenbaum MH, et al. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ Br Med J. 2012;345: e6879.
Stoecker C, et al. Cost-Effectiveness of Using 2 vs 3 Primary Doses of 13-Valent Pneumococcal Conjugate Vaccine. Pediatrics. 2013;132(2):e324–32.
Strutton DR, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: Germany, Greece, and The Netherlands. J Infect. 2012;64(1):54–67.
Mangen M-JJ, Bonten MJM, de Wit GA. Rationale and design of the costs, health status and outcomes in community-acquired pneumonia (CHO-CAP) study in elderly persons hospitalized with CAP. BMC Infect Dis. 2013;13(1):597.
Lytle D, et al. Cost-effectiveness analysis of PCV20 to prevent pneumococcal disease in the Canadian pediatric population. Hum Vaccin Immunother. 2023;19(2):2257426.
Warren S, et al. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to higher-valent options in Greek infants. Vaccines. 2023;11(8):1369.
Wilson M, et al. Estimating the cost-effectiveness of switching to higher-valency pediatric pneumococcal conjugate vaccines in the United Kingdom. Vaccines. 2023;11(7):1168.
Wilson M, et al. Assessing public health impact of four pediatric pneumococcal conjugate vaccination strategies in the Netherlands. Infect Dis Ther. 2023;12(7):1809–21. https://doi.org/10.1007/s40121-023-00828-8.
Løchen A, Anderson RM. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: a quality appraisal and limitations. Clin Microbiol Infect. 2020;26(1):60–70.
Talbird SE, et al. Outcomes and costs associated with PHiD-CV, a new protein D conjugate pneumococcal vaccine, in four countries. Vaccine. 2010;28:G23–9.
Wilson M, et al. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71.
Chuck AW, et al. Pharmacoeconomic evaluation of 10- and 13-valent pneumococcal conjugate vaccines. Vaccine. 2010;28(33):5485–90.
Earnshaw SR, et al. Cost-effectiveness of 2 + 1 dosing of 13-valent and 10-valent pneumococcal conjugate vaccines in Canada. BMC Infect Dis. 2012;12(1):101.
Wals P, et al. Simulation model for comparing the costs and effectiveness of different pneumococcal conjugate vaccines. Proc Vaccinol. 2009;1:67–72.
Syeed MS, et al. Pneumococcal vaccination in children: a systematic review and meta-analysis of cost-effectiveness studies. Value Health. 2023;26(4):598–611.
Perdrizet J, et al. Historical population-level impact of infant 13-valent pneumococcal conjugate vaccine (PCV13) National immunization programs on invasive pneumococcal disease in Australia, Canada, England and Wales, Israel, and the United States. Infect Dis Ther. 2023;12(5):1351–64.
Croucher NJ, Løchen A, Bentley SD. Pneumococcal vaccines: host interactions, population dynamics, and design principles. Annu Rev Microbiol. 2018;72:521–49.
Masala GL, et al. Exploring the role of competition induced by non-vaccine serotypes for herd protection following pneumococcal vaccination. J R Soc Interface. 2017;14(136):20170620.
Robert Koch Institut; 2016. available from: RKI - Abgeschlossene Projekte - Evaluation unterschiedlicher Impfstrategien zur Prävention von Pneumokokken-Infektionen bei älteren Erwachsenen: Ergebnisse eines dynamischen Transmissionsmodells.
Levy C, et al. Impact of PCV13 on community-acquired pneumonia by C-reactive protein and procalcitonin levels in children. Vaccine. 2017;35(37):5058–64.
Rodrigo C, et al. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur Respir J. 2015;45(6):1632.
Lau WCY, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–9.
Claes C, Reinert RR, von der Schulenburg J-MG. Cost effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur J Health Econ. 2009;10:25–38.
Statistisches Bundesamt (destatis.de). 40% der Mütter von Kindern unter drei Jahren sind erwerbstätig. 2023: destatis.de.
Mangen M-JJ, et al. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis. 2017;17(1):208.
Erickson LJ, et al. Complications of meningococcal disease in college students. Clin Infect Dis. 2001;33(5):737–9.
Oostenbrink R, Henriëtte AM, Essink-Bot ML. The EQ-5D and the health utilities index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol. 2002;55(8):791–9.
Janoir C, et al. Insight into resistance phenotypes of emergent non 13-valent pneumococcal conjugate vaccine type pneumococci isolated from invasive disease after 13-valent pneumococcal conjugate vaccine implementation in France. In: Open forum infectious diseases. Oxford: Oxford University Press; 2016.
Medical Writing/Editorial Assistance
The original model was developed by Des Dillon-Murphy, PhD, and Ruth Chapman, PhD, of Evidera. Writing support for this manuscript was provided by Colleen Dumont and Sally Neath of Cytel Inc, Waltham, MA, US and by Allison Brackley, PhD, and Elizabeth Hubscher, PhD, who were employed by Cytel Inc, Waltham, MA, US at the time of the manuscript development. This support was funded by Pfizer. We thank Mark van der Linden for providing IPD data. An Ta is an employee of Cytel, which received funding from Pfizer in connection with the development of this manuscript.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Felicitas Kühne, Maren Laurenz, Christof von Eiff, Johnna Perdrizet and Sophie Warren contributed to the study conceptualization, investigation, and methodology. An Ta conducted the formal analysis and was responsible for project administration. Johnna Perdrizet was responsible for funding acquisition. Johnna Perdrizet and Felicitas Kühne provided supervision and validation. All authors contributed to writing the original draft, as well as reviewing and editing subsequent drafts, and approving the final version.
Corresponding author
Ethics declarations
Conflict of Interest
Felicitas Kühne, Maren Laurenz, Christof von Eiff, and Johnna Perdrizet are employees of Pfizer. Sophie Warren was employed by Pfizer at the time of the manuscript development (current affiliation: Adelphi Values, UK). An Ta is an employee of Cytel, which received consulting fees from Pfizer for the study and manuscript development.
Ethical Approval
This article was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors; as such, ethical approval was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: DGGÖ 2024 presentation and preprint (medRxiv) [1].
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ta, A., Kühne, F., Laurenz, M. et al. Cost-effectiveness of PCV20 to Prevent Pneumococcal Disease in the Pediatric Population: A German Societal Perspective Analysis. Infect Dis Ther 13, 1333–1358 (2024). https://doi.org/10.1007/s40121-024-00977-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00977-4