Abstract
Introduction
A 20-valent pneumococcal conjugate vaccine (PCV20) was recently recommended for use among US children. We evaluated the cost-effectiveness of PCV20 among children aged 6 years with chronic medical conditions (CMC+) and children aged 6 years with immunocompromising conditions (IC) versus one and two doses of 23-valent pneumococcal polysaccharide vaccine (PPSV23), respectively.
Methods
A probabilistic model was employed to depict 10-year risk of clinical outcomes and economic costs of pneumococcal disease, reduction in life years from premature death, and expected impact of vaccination among one cohort of children with CMC+ and IC aged 6 years. Vaccine uptake was assumed to be 20% for both PCV20 and PPSV23. Cost per quality-adjusted life year (QALY) gained was evaluated from the US societal and healthcare system perspectives; deterministic and probabilistic sensitivity analyses (DSA/PSA) were also conducted.
Results
Among the 226,817 children with CMC+ aged 6 years in the US, use of PCV20 (in lieu of PPSV23) was projected to reduce the number cases of pneumococcal disease by 5203 cases, medical costs by US$8.7 million, and nonmedical costs by US$6.2 million. PCV20 was the dominant strategy versus PPSV23 from both the healthcare and societal perspectives. In the PSA, 99.9% of the 1000 simulations yielded a finding of dominance for PCV20. Findings in analyses of children with IC aged 6 years in the USA were comparable (i.e., PCV20 was the dominant vaccination strategy). Scenario analyses showed that increasing PCV20 uptake to 100% could potentially prevent > 22,000 additional cases of pneumococcal disease and further reduce medical and nonmedical costs by US$70.0 million among children with CMC+ and IC.
Conclusions
Use of PCV20 among young children with CMC+ and IC in the USA would reduce the clinical burden of pneumococcal disease and yield overall cost savings from both the US healthcare system and societal perspectives. Higher PCV20 uptake could further reduce the number of pneumococcal disease cases in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Until recently, the US Center for Disease Control (CDC) recommended the 23-valent pneumococcal polysaccharide vaccine (PPSV23) for children aged 2–18 years with underlying medical conditions. |
PCV20 was recently approved by the Food and Drug Administration (FDA) for use among children and has been recommended by the ACIP for use among US infants as well as for children with underlying medical conditions. |
This study evaluated whether PCV20 use would be cost effective compared with PPSV23 among young children with underlying medical conditions. |
What was learned from the study? |
Findings suggest that use of PCV20 (versus PPSV23) would markedly reduce the burden of pneumococcal disease among young children at elevated risk of pneumococcal disease and, thus, make an important contribution to pediatric public health. |
Use of PCV20 among children with underlying medical conditions would reduce the clinical burden of pneumococcal disease and yield overall cost savings from both the US healthcare system and societal perspectives. |
Introduction
Streptococcus pneumoniae causes significant morbidity and mortality in children of all ages, especially infants and those with underlying medical conditions [1]. Immunization is the principal means of preventing pneumococcal disease and is currently recommended for infants as well as toddlers, and older children aged ≤ 18 years with chronic medical conditions in the USA. The Advisory Committee on Immunization Practices (ACIP) has recommended pneumococcal vaccination for subgroups of US children since 1997 [2].
Infant vaccination with pneumococcal conjugate vaccines (PCVs) has proven extremely effective in reducing the risk of pneumococcal disease among young children [3]. Rates of IPD among children aged < 5 years in the US decreased by 93% between 1998 and 2019, which is largely credited to infant vaccination with 7-valent PCV (PCV7, introduced in 2000) and 13-valent PCV (PCV13, introduced in 2010) [3]. Despite considerable reductions in overall pediatric pneumococcus rates, children with underlying medical conditions remain at an elevated risk of pneumococcal disease [4]. Unpublished US CDC data presented at the February 2023 meeting of the ACIP show that risk of IPD among children aged < 5 years is 231× higher for those with hematologic malignancy (versus no condition) and 29× higher for those with sickle cell disease (versus no condition) [4].
Until recently, the US CDC recommended a single dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23) for children aged 2–18 years with underlying medical conditions who have completed the infant PCV 3 + 1 series, as well as a second dose of PPSV23 5 years after the first dose for children with immunocompromising conditions [5]. To provide additional protection against pneumococcal disease, a novel 20-valent PCV (PCV20) was recently developed and approved for use in US adults aged 18 years and over, and more recently, children under the age of 18 years [6]. The additional seven vaccine serotypes in PCV20 (versus PCV13) were targeted based on several factors, including associations with heightened disease severity, invasive potential, antibiotic resistance, and prevalence as a cause of pediatric and adult disease across widespread geographic areas in the post-PCV13 era [7,8,9,10,11,12,13].
As of June 2023, PCV20 is recommended for US infants under a 3 + 1 schedule, as well as for children with increased risk of pneumococcal disease [14]. This study evaluates the cost effectiveness of PCV20 versus previous vaccine recommendations (i.e., use of PPSV23) among US children aged 6 years with underlying medical conditions.
Methods
Model Description
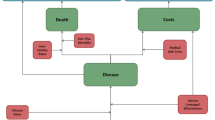
The model employs a probabilistic Markov framework to depict the risk of clinical outcomes and economic costs of pneumococcal disease over a 10-year time horizon (Fig. 1). The population at model entry comprises a single cohort of US children aged 6 years with underlying medical conditions at model entry and is stratified into those who are immunocompetent with chronic medical conditions (CMC+) and those who have immunocompromising conditions (IC); children are followed through age 15 years. Because the pediatric population aged 2–18 years with underlying conditions is heterogeneous with respect to age and condition, we selected 6-year-old children with one or more of the chronic medical or immunocompromising conditions included in the pneumococcal vaccine recommendations as the exemplar age for the population.
At model entry, 6-year-old children in the model population receive either PCV20 (intervention) or PPSV23 (standard of care). All children also are assumed to have received the 3 + 1 dose regimen of PCV13, as infants and are therefore (partially) protected (see below). For children with IC vaccinated with PPSV23 at model entry, a second dose of PPSV23 was provided 5 years after the first dose.
Expected clinical outcomes and economic costs are projected for the model population on an annual basis, based on age, risk profile (i.e., CMC+ versus IC), disease/fatality rates, PCV13 vaccination history, vaccination status/type (PCV20, PPSV23), and time since vaccination. Clinical outcomes include cases of invasive pneumococcal disease (IPD), all-cause nonbacteremic pneumonia (NBP), and all-cause otitis media (OM), as well as disease-associated death. IPD includes bacteremia and meningitis, all-cause NBP is stratified by setting of care (inpatient versus outpatient), and all-cause OM includes cases treated in all care settings. Children vaccinated at model entry, and those children who received PCV13 prior to model entry (assumed to be 100%), may be at lower risk of IPD, all-cause NBP, and all-cause OM. The magnitude of vaccine-associated risk reduction depends on clinical presentation, as well as the vaccine (effectiveness and serotype coverage), time since vaccination, and risk profile. The model assumes age-specific mortality due to IPD and all-cause NBP requiring inpatient care and other causes (i.e., other than IPD and all-cause NBP). All-cause NBP requiring outpatient care only and all-cause OM are assumed not to elevate risk of death.
Expected costs of medical treatment for IPD, all-cause NBP, and all-cause OM are generated based on event rates and unit costs in relation to the setting of care (i.e., inpatient versus outpatient) and age. Costs of vaccination—including the vaccine and its administration—are tallied at the time of administration. The value of morbidity- and mortality-related work loss is also tallied in the model.
Future life-years, QALYs, and costs are discounted in the base-case analysis, and both societal and healthcare system perspectives are employed.
Model Estimation
Model parameter values are summarized in Table 1. A detailed description of methods employed to estimate parameter values, along with tables reporting values employed in base-case analyses, scenario analyses, and probabilistic sensitivity analyses (PSA), are set forth in the supplemental material.
Model population: The model population included children with CMC+ (N = 226,817) and children with IC (N = 15,189) aged 6 years at model entry, estimated based on data from the US Census Bureau (number of US children aged 6 years) and a study by Pelton et al. (distribution of children by risk profile) [15, 16].
Disease rates: Age- and risk-specific rates of IPD, all-cause NBP, and all-cause OM were derived using age-specific rates and risk-specific relative rates/population weights from published studies [17,18,19,20,21,22]. From sources providing age-specific values, rates by single year of age from 6 to 15 years were calculated using techniques of linear interpolation between reported point estimates. Age-specific rates were then apportioned across risk groups based on relative rates and corresponding population weights [23].
Case-fatality and mortality rates: For IPD, age-specific values were calculated based on 2017–2019 Active Bacterial Core surveillance (ABCs) data [17]; for all-cause inpatient NBP, age-specific values were based on the CDC 15-valent PCV (PCV15) cost-effectiveness assessment and a published study [24,25,26,27]. The background mortality rate was calculated based on age-specific mortality rates among the general population and was downwardly adjusted to account for death due to IPD and inpatient NBP [28]. Case-fatality and mortality rates were assumed to be the same for children with CMC+ and IC.
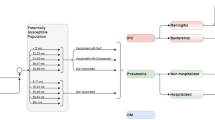
Vaccine effectiveness: Vaccine effectiveness (VE) for PCV13 and PCV20 against vaccine-type IPD among children with CMC+ was based on the Moore et al. case–control study of PCV13 (≥ one dose) among US children aged 2–59 months, which demonstrated effectiveness of 86.0% [95% confidence interval (CI) 75.5–92.3%] (Fig. 2) [29]. VE-PPSV23 against VT-IPD among children with CMC+ was assumed to be 63.0% based on Fiore et al. [30]. VE-PCV20 and VE-PCV13 versus all-cause noninvasive disease among children with CMC+ was estimated by multiplying the effectiveness estimates of inpatient NBP (25.5%), all-cause ambulatory NBP (6.0%), and all-cause OM (7.8%) from the PCV7 pivotal trial with the ratio of current serotype coverage level for each vaccine to serotype coverage level for PCV7 (80.6%) in the 2000 calendar year (i.e., the year PCV7 was introduced in the USA) [31,32,33]. VE-PPSV23 against NBP and OM was assumed to be 0% based on various published sources and consistent with base-case assumptions employed in a number of economic studies [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. For children with IC, VE-PCV20 and VE-PPSV23 were reduced by 15.6% relative to corresponding values for children with CMC+, based on the ratio of VE-PCV7 against VT-IPD for children with comorbidities (81%) versus healthy children (96%) from Whitney et al. [50].
Age-specific proportions of invasive disease attributable to vaccine serotypes were sourced from 2019 ABCs data, reduced due to herd effects from projected use of PCV20 among infants, and assumed to be the same for children with CMC+ and IC. Age-specific proportions for noninvasive disease were assumed to be the same as those for invasive disease; additional details are provided in Supplemental Material [51]. It was assumed that VE-PCV would persist at the initial level for 4 years and, thereafter, was subject to 10% exponential decay annually. For PPSV23, VE was assumed to wane based on data reported by Djennad et al. [52]. Serotype replacement was assumed not to occur.
Medical and nonmedical costs: Costs of medical treatment for IPD, all-cause inpatient NBP, and all-cause outpatient NBP were based on retrospective healthcare claims studies by Weycker et al. [53]. Cost of OM (all care settings) was based on reported values in a study by Tong et al. [22]. Unit costs were assumed to be the same in the CMC+ and IC populations. Costs of medical treatment were inflated to 2022 US dollars (USD) using the Consumer Price Index for medical care [54]. Prices of vaccines (per dose) are set forth in Table 1. Nonmedical costs per episode of disease were estimated based on productivity loss incurred by caregivers of infected children [17, 55,56,57]. A human capital approach was employed to estimate indirect costs, which were based on work force participation, work loss days, and average daily salary [58,59,60].
Utilities and disutilities: General population utility for children aged 6–15 years was assumed to be 0.93, based on EQ-5D-5L population norms for persons aged 5–19 years [61]. Baseline utility values were assumed to be the same across risk groups. Disease-related utility decrements were taken from published cost-effectiveness analyses of PCVs and were assumed to be invariant by age [62,63,64]. Because disutility varies for meningitis (0.0232) and bacteremia (0.0079), a single estimate for all IPD was derived based on the weighted average of disutilities (i.e., assuming 7% meningitis and 93% bacteremia) [17].
Analyses
Cost-effectiveness of PCV20 [versus PPSV23 (CMC+, one dose and IC, two dose)] was calculated in terms of the cost per QALY gained, based on the ratio of the difference in total costs to the difference in total QALYs. Costs and QALYs were discounted at a rate of 3% per annum [65]. Vaccine uptake at model entry was assumed to be 20% among each population subgroup, irrespective of vaccination strategy [66]. In addition, all children in the model were assumed to have received PCV13 prior to model entry [67]. One-way deterministic sensitivity analyses (± 25% of the base-case values), scenario analyses, and PSAs (1000 replications) were also conducted; additional details on scenario analyses and the PSAs are set forth in the supplemental material. A US healthcare system perspective [including direct (i.e., medical and vaccination) costs] and societal perspective [including direct and indirect (i.e., productivity loss-related) costs] were employed.
Ethics Approval
Human subjects did not participate in this study; thus, ethical approval was not required and informed consent was not applicable.
Results
Immunocompetent Children with Chronic Medical Conditions
Base-case analysis: Use of PCV20 (in lieu of PPSV23) among children with CMC+ aged 6 years (N = 226,817) was projected—during the 10-year modeling horizon—to reduce IPD cases by 4, all-cause inpatient NBP by 392, all-cause outpatient NBP by 516, all-cause OM cases by 4291, disease-related deaths by 4, medical costs by US$8.7 million, and nonmedical costs by US$6.2 million (Table 2). Although vaccination costs were higher with PCV20 (by US$5.5 million), they were offset by reductions in medical and nonmedical costs, yielding cost savings of US$3.2 million from the healthcare system perspective and US$9.4 million from the societal perspective. Use of PCV20 was therefore projected to dominate PPSV23 (i.e., to yield higher QALYs and lower costs) from the US societal and healthcare system perspectives.
Deterministic sensitivity, scenario, and probabilistic sensitivity analyses
In all one-way deterministic sensitivity analyses PCV20 remained dominant versus PPSV23 (Supplementary Table 19). Additionally, in scenario analyses employing alternative values for disease-related disutilities, reduced direct effectiveness of PCV against serotype 3, no history of PCV13 use, and higher (100%) PCV20 uptake, PCV20 dominated PPSV23 (Supplementary Tables 20–23). In the PSA, 99.9% of the 1000 simulations yielded estimates of cost per QALY gained that were in the southeast quadrant of the scatterplot, indicating lower costs and higher QALYs—and thus dominance—with PCV20 (Fig. 3).
Immunocompromised Children
Base-case analysis: Use of PCV20 (in lieu of two doses of PPSV23) among children with IC aged 6 years (N = 15,189) was projected—during the 10-year modeling horizon—to reduce IPD cases by 2, all-cause inpatient NBP by 54, all-cause outpatient NBP by 70, all-cause OM cases by 242, disease-related deaths by 1, medical costs by US$1.2 million, and nonmedical costs by $0.7 million (Table 2). Although vaccination costs were higher with PCV20 (by US$0.1 million), they were offset by reductions in medical and nonmedical costs, yielding cost savings of US$1.1 million from the healthcare system perspective and US$1.8 million from the societal perspective. Use of PCV20 was, therefore, projected to dominate PPSV23 from the US societal and healthcare system perspectives.
Deterministic sensitivity, scenario, and probabilistic sensitivity analyses: In all one-way deterministic sensitivity analyses, PCV20 remained dominant versus PPSV23 (Supplementary Table 19). Additionally, in scenario analyses employing alternative values for disease-related disutilities, reduced direct effectiveness of PCV against serotype 3, no history of PCV13 use, and higher (100%) PCV20 uptake, PCV20 dominated PPSV23 (Supplementary Tables 20–23). In the PSA, 97.3% of the 1000 simulations yielded estimates of cost per QALY gained that were in the southeast quadrant of the scatterplot, indicating lower costs and higher QALYs—and thus dominance—with PCV20 (Fig. 3).
Discussion
This is the first cost-effectiveness study evaluating the impact of PCV20 use among US children with underlying comorbidities. In this study, we employed a probabilistic model with a Markov-type process to evaluate the public health and economic impact of PCV20 (versus PPSV23) among US children aged 6 years with underlying medical conditions. Study findings indicated that—under reasonable assumptions, and variations thereof, concerning disease burden, vaccine effectiveness, and vaccine cost—use of PCV20 among young children with CMC+ and IC would reduce the clinical burden and overall economic costs [direct (including vaccination) and indirect] of pneumococcal disease and, thus, would be a dominant vaccination strategy versus previous vaccination recommendations. Compared with PPSV23, PCV20 would prevent almost 400 hospitalizations for pneumococcal disease and about 4800 ambulatory encounters for pneumococcal disease among children with CMC+ aged 6 years in the US. Moreover, incremental acquisition costs of PCV20 were fully offset by savings from averted disease, yielding US$9.4 million in net savings from the societal perspective. The same trend was observed when comparing PCV20 versus PPSV23 among children with IC aged 6 years. It is important to interpret the findings of our analysis with caution, as the cohort under analysis omits 2–5- and 7–18-year-olds; vaccine use across the pediatric risk groups would result in much broader public health impact. Study findings therefore support the adoption of recent CDC recommendations for use of PCV20 among children with CMC+ and IC aged 2–18 years who have a history of use of PCV15 or PCV13 [14].
While the economic benefits of PCV20 were somewhat lower from a healthcare system perspective, PCV20 remained dominant versus PPSV23 for both children CMC+ and IC. Deterministic sensitivity, scenario, and probabilistic sensitivity analyses yielded the same findings as base-case analyses (i.e., lower costs and higher QALYs with PCV20). Importantly, if recommendation of the simpler, single dose PCV20 strategy led to higher vaccine uptake among children with CMC+ and IC, disease burden and medical and nonmedical costs could be further reduced (cases by 22,314 and costs by US$70.0 million).
While this study is, to the best of our knowledge, the first peer-reviewed economic evaluation of PCV20 in young children with underlying medical conditions, the findings described herein are consistent with other recent studies presented during the June 22, 2023 ACIP meeting [68, 69]. Both models presented during the meeting (i.e., Stoecker et al. and Huang et al.) showed that vaccination of children with CMC+ or children with IC with PCV20 was cost saving compared with vaccination with the PCV13 + PPSV23 series. PCV20 was also found to be cost saving in both models when compared with the PCV15 + PPSV23 series.
Some limitations of the study should be noted. While the CDC recommendations considered herein are applicable to children with CMC+ or IC aged ≥ 2 years who previously received PCV13, our model population was limited to children aged 6 years (which was assumed to be a “representative” age for the CMC+ and IC population aged 2–18 years). Nevertheless, when we ran the analyses for 12-year-olds, the conclusion remained unchanged (results not shown). Although age-specific disease rates and costs were employed in the model, we note that the US pediatric population and healthcare delivery system are highly heterogeneous, and thus, the generalizability of results may be limited. We also note that recent infant recommendations include optional use of PCV15 in the 3 + 1 schedule (i.e., in lieu of PCV13), which means that in the future, some children aged 2–18 years may be protected against serotypes 22F and 33F through the infant vaccination series. Nonetheless, it is our expectation that the incremental benefit of PCV20 administered during early childhood would remain substantial.
Perhaps the greatest area of uncertainty concerns our estimates of vaccine effectiveness. Consistent with findings from PPSV23 effectiveness studies published over the past 30 years, as well as a number of previously published cost-effectiveness analyses of pneumococcal vaccination, we assumed PPSV23 provides protection against IPD only [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49, 70,71,72,73]. Although recently published data suggest that PPSV23 may provide some level of protection against NBP, the quality of evidence generated via these retrospective evaluations is not yet sufficient to warrant a different assumption [74, 75].
Estimates of the effectiveness of PCVs for children aged 6–15 years are not currently available in published literature. However, based on the study by Whitney et al. [50] in which there was little variation in VE by age [i.e., VE with one dose of PCV7 at age ≥ 24 months was 94% (95% CI 49–99%) and VE among children aged 12–23 months receiving 1 dose of PCV7 was 93% (95% CI 68–98%)], we assumed initial effectiveness of PCVs among children aged 6 years to be the same as that for children aged 2–59 months reported by Moore et al. [29]. Moreover, because clinical trials have not been conducted for PCV20, VE-PCV20 was derived using data from a variety of sources, with the process of interpretation and synthesis potentially subject to bias. However, the approach employed to estimate vaccine effectiveness for PCV20 was robust, using the best available evidence, and was consistent with previous research. Furthermore, it was assumed that vaccine serotype coverage for IPD was generalizable to noninvasive diseases, an appropriate assumption given the lack of data to suggest otherwise.
In the absence of evidence on the duration of protection for PCV20, we assumed that the duration of protection would be the same as that for PCV13, which is aligned with the assumption that initial VE-PCV20 versus vaccine-type disease is the same as that for PCV13. We assumed a stable duration of protection for 4 years based on a study by Savulescu et al. [76]. After 4 years, we conservatively assumed an annual waning rate of 10% relative to the prior year (i.e., exponential decay). For this reason, the full benefits of PCV20 vaccination (i.e., those beyond 10 years) may be underestimated.
Our model, like all such health economic models, simplifies reality to some extent. Our model does not, for example, consider possible indirect effects of vaccination within (or outside) the population of interest or broader societal benefits that may be conferred by vaccines (e.g., reducing antimicrobial resistance or reducing use of antibiotics). Finally, our model did not include other potential downstream adverse outcomes and costs associated with pneumonia, which confers a conservative bias against use of PCV20.
Conclusions
The results of this evaluation suggest that use of PCV20—in lieu of PPSV23—would yield important reductions in the clinical burden of pneumococcal disease among children with CMC+ and IC conditions as well as net savings from both the US societal and healthcare system perspectives. Accordingly, use of PCV20 in this population would have a favorable impact on individual patients and public health, as well as is a good use of resources in the US. Moreover, higher PCV20 uptake in this population could substantially increase the number of pneumococcal disease cases averted through vaccination.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Centers for Disease Control and Prevention. Pneumococcal disease: prevention 2022. [Accessed August 14, 2023]. Available from: https://www.cdc.gov/pneumococcal/surveillance.html.
Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46(Rr-8):1–24.
Centers for Disease Control and Prevention. Pneumococcal disease: surveillance and reporting 2020. Available from: https://www.cdc.gov/pneumococcal/surveillance.html. Accessed Oct 5, 2022.
Gierke R, editor. Current epidemiology of pediatric pneumococcal disease, United States. Meeting of the Advisory Committee on Immunization Practices; 2023; Atlanta: Centers for Disease Control and Prevention.
Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(Rr-11):1–18.
Pfizer. Prevnar 20 (Pneumococcal 20-valent Conjugate Vaccine), suspension for intramuscular injection, prescribing information. 2021.
Cohen R, Levy C, Ouldali N, Goldrey M, Bechet S, Bonacorsi S, et al. Invasive disease potential of pneumococcal serotypes in children after PCV13 implementation. Clin Infect Dis. 2021;72(8):1453–6.
Tomczyk S, Lynfield R, Schaffner W, Reingold A, Miller L, Petit S, et al. Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2016;62(9):1119–25.
Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6(5): e1000081.
Oligbu G, Collins S, Sheppard CL, Fry NK, Slack M, Borrow R, et al. Childhood deaths attributable to invasive pneumococcal disease in England and Wales, 2006–2014. Clin Infect Dis. 2017;65(2):308–14.
Cui YA, Patel H, O’Neil WM, Li S, Saddier P. Pneumococcal serotype distribution: a snapshot of recent data in pediatric and adult populations around the world. Hum Vaccin Immunother. 2017;13(6):1–13.
Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS ONE. 2017;12(5): e0177113.
Hausdorff WP, Hanage WP. Interim results of an ecological experiment—conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–74.
Kobayashi M, editor. Evidence to recommendations framework and policy options: use of 20-valent pneumococcal conjugate vaccine in US children. Meeting of the Advisory Committee on Immunization Practices; 2023; Atlanta, GA.
US Census Bureau. Annual estimates of the resident population by single year of age and sex for the United States: April 1, 2020 to July 1, 2021 (NC-EST2021-SYASEXN): U.S. Census Bureau, Population Division; 2022. Accessed Mar 23, 2023.
Pelton SI, Weycker D, Farkouh RA, Strutton DR, Shea KM, Edelsberg J. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clin Infect Dis. 2014;59(5):615–23.
Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, Streptococcus pneumoniae, 2019 2019. Available from: www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf. Accessed Nov 17, 2022.
Stoecker C, editor. Economic assessment of PCV15 & PCV20. Advisory Committee on Immunization Practices Meeting; 2021; Atlanta, GA.
Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415–27.
Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806–12.
Tong S, Amand C, Kieffer A, Kyaw MH. Trends in healthcare utilization and costs associated with pneumonia in the United States during 2008–2014. BMC Health Serv Res. 2018;18(1):715.
Tong S, Amand C, Kieffer A, Kyaw MH. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008–2014. BMC Health Serv Res. 2018;18(1):318.
Lapidot R, Averin A, Weycker D, Huang L, Vietri J, Mohs AGA, et al. Disparities by age, comorbidity profile, and insurance type in the burden of invasive pneumococcal disease and respiratory syndromes among children in the United States. Unpublished.
Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report emerging infections program network Streptococcus pneumoniae, 2018 2018. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/spneu18.pdf. Accessed Nov 17, 2022.
Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report emerging infections program network Streptococcus pneumoniae, 2017 2017. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/spneu17.pdf. Accessed Nov 17, 2022.
Kobayashi M, Farrar JL, Gierke R, Leidner AJ, Campos-Outcalt D, Morgan RL, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the advisory committee on immunization practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(37):1174–81.
Averin A, Shaff M, Weycker D, Lonshteyn A, Sato R, Pelton SI. Mortality and readmission in the year following hospitalization for pneumonia among US adults. Respir Med. 2021;185: 106476.
US Census Bureau. National demographic analysis tables: 2020. 2021. Available from: https://www.census.gov/data/tables/2020/demo/popest/2020-demographic-analysis-tables.html. Accessed Nov 17, 2022.
Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case–control study. Lancet Respir Med. 2016;4(5):399–406.
Fiore AE, Levine OS, Elliott JA, Facklam RR, Butler JC. Effectiveness of pneumococcal polysaccharide vaccine for preschool-age children with chronic disease. Emerg Infect Dis. 1999;5(6):828–31.
Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95.
Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5.
Hansen J, Black S, Shinefield H, Cherian T, Benson J, Fireman B, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25(9):779–81.
Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother. 2013;9(3):699–706.
Cho B-H, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21.
Fry AM, Zell ER, Schuchat A, Butler JC, Whitney CG. Comparing potential benefits of new pneumococcal vaccines with the current polysaccharide vaccine in the elderly. Vaccine. 2002;21(3):303–11.
Heo JY, Seo YB, Choi WS, Lee J, Noh JY, Jeong HW, et al. Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea. PLoS ONE. 2017;12(5): e0177342.
Hoshi S-L, Kondo M, Okubo I. Economic evaluation of immunisation programme of 23-valent pneumococcal polysaccharide vaccine and the inclusion of 13-valent pneumococcal conjugate vaccine in the list for single-dose subsidy to the elderly in Japan. PLoS ONE. 2015;10(10): e0139140.
Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167(18):1938–43.
Örtqvist Å, Hedlund J, Burman L-Å, Elbel E, Höfer M, Leinonen M, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351(9100):399–403.
Sisk JE, Whang W, Butler JC, Sneller V-P, Whitney CG. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med. 2003;138(12):960–8.
Smith KJ, Wateska AR, Nowalk MP, Raymund M, Lee BY, Zimmerman RK. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in US older adults. Am J Prev Med. 2013;44(4):373–81.
Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016;31(8):901–8.
Stoecker C, Kobayashi M, Matanock A, Cho B-H, Pilishvili T. Cost-effectiveness of continuing pneumococcal conjugate vaccination at age 65 in the context of indirect effects from the childhood immunization program. Vaccine. 2020;38(7):1770–7.
Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database of Syst Rev. 2013;(1).
Evers SMAA, Ament AJHA, Colombo GL, Konradsen HB, Reinert RR, Sauerland D, et al. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur J Clin Microbiol Infect Dis. 2007;26(8):531–40.
Kuhlmann A, Theidel U, Pletz MW, von der Schulenburg JMG. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Heal Econ Rev. 2012;2(1):4.
Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, et al. Efficacy of pneumococcal vaccine in high-risk patients. N Engl J Med. 1986;315(21):1318–27.
Smith KJ, Zimmerman RK, Lin CJ, Nowalk MP, Ko F-S, McEllistrem MC, et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine. 2008;26(11):1420–31.
Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case–control study. Lancet. 2006;368(9546):1495–502.
Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) data provided by M Kobayashi 2022 under the DUA. 2022.
Djennad A, Ramsay ME, Pebody R, Fry NK, Sheppard C, Ladhani SN, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6:42–50.
Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16:182.
US Bureau of Labor Statistics. Consumer price index for all urban consumers. 2020.
Wilson MR, Wasserman MD, Breton MC, Peloquin F, Earnshaw SR, McDade C, et al. Health and economic impact of routine pediatric pneumococcal immunization programs in Canada: a retrospective analysis. Infect Dis Ther. 2020;9(2):341–53.
Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–412.
Barber C, Ille S, Vergison A, Coates H. Acute otitis media in young children—what do parents say? Int J Pediatr Otorhinolaryngol. 2014;78(2):300–6.
US Bureau of Labor Statistics. Average hours employed people spent working on days worked by day of week. Available from: https://www.bls.gov/charts/american-time-use/emp-by-ftpt-job-edu-h.htm. Accessed Nov 29, 2022.
US Bureau of Labor Statistics. Civilian labor force participation rate. Available from: https://www.bls.gov/charts/employment-situation/civilian-labor-force-participation-rate.htm. Accessed Nov 29, 2022.
Organisation for Economic Co-operation and Development. Average wages. Available from: https://data.oecd.org/earnwage/average-wages.htm. Accessed Nov 29, 2022.
Jiang R, Janssen MFB, Pickard AS. US population norms for the EQ-5D-5L and comparison of norms from face-to-face and online samples. Qual Life Res. 2021;30(3):803–16.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14.
Rozenbaum MH, van Hoek AJ, Fleming D, Trotter CL, Miller E, Edmunds WJ. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ. 2012;345: e6879.
Stoecker C, Hampton LM, Link-Gelles R, Messonnier ML, Zhou F, Moore MR. Cost-effectiveness of using 2 vs 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics. 2013;132(2):e324–32.
Centers for Disease Control and Prevention. Guidance for health economics studies presented to the Advisory Committee on Immunization Practices (ACIP), 2019 update. 2019. Available from: https://www.cdc.gov/vaccines/acip/committee/downloads/Economics-Guidance-for-ACIP-2019.pdf. Accessed Nov 18, 2022.
Harris JG, Harris LA, Olarte L, Elson EC, Moran R, Blowey DL, et al. Improving pneumococcal vaccination rates in high-risk children in specialty clinics. Pediatrics. 2022;149(4).
Centers for Disease Control and Prevention. Vaccination coverage among young children (0–35 months). 2020. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/interactive-reports/index.html. Accessed Dec 16, 2022.
Stoecker C, editor. Economic assessment of routine PCV20 for children. Meeting of the Advisory Committee on Immunization Practices; 2023; Atlanta, GA.
Ayabina DV, editor Summary of three economic analyses of the use of 20-valent pneumococcal conjugate vaccine (PCV20) in children in the United States. Meeting of the Advisory Committee on Immunization Practices; 2023; Atlanta, GA.
Marra LP, Sartori AL, Martinez-Silveira MS, Toscano CM, Andrade AL. Effectiveness of pneumococcal vaccines on otitis media in children: a systematic review. Value Health. 2022;25(6):1042–56.
Klein JO, Teele DW, Sloyer JL, et al. Use of pneumococcal vaccine for prevention of recurrent episodes of otitis media. In: Weinstein L, Fields BN, editors., et al., Seminars in infectious disease. New York: Thieme-Stratton Inc; 1982. p. 305–10.
Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174(6):1271–8.
Straetemans M, Sanders EA, Veenhoven RH, Schilder AG, Damoiseaux RA, Zielhuis GA. Pneumococcal vaccines for preventing otitis media. Cochrane Database Syst Rev. 2004;1:C001480.
Lawrence H, Pick H, Baskaran V, Daniel P, Rodrigo C, Ashton D, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case–control test-negative design study. PLoS Med. 2020;17(10): e1003326.
Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313–21.
Savulescu C, Krizova P, Valentiner-Branth P, Ladhani S, Rinta-Kokko H, Levy C, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine. 2022;40(29):3963–74.
Funding
The research described herein, including the journal’s Rapid Service fee, was supported by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Authorship was designated based on guidelines promulgated by the International Committee of Medical Journal Editors (2004). All persons who met criteria for authorship were listed as authors on the title page. The contribution of each of these persons to this study is as follows: (1) conception and design (all authors), acquisition of data (Rozenbaum, Chilson, Averin, Weycker, Hariharan), analysis or interpretation of data (all authors); and (2) preparation of manuscript (Averin, Rozenbaum, Weycker), critical review of manuscript (all authors). All authors have read and approved the final version of the manuscript. The study sponsor, Pfizer Inc., reviewed the study research plan and study manuscript; data management, processing, and analyses were conducted by PAI. All final analytic decisions and the decision to submit for publication were made solely by study investigators.
Corresponding author
Ethics declarations
Conflict of Interest
Ahuva Averin, Mark Atwood, Dhwani Hariharan, and Derek Weycker are employees of Policy Analysis Inc. (PAI), which received financial support from Pfizer Inc. for this study (including manuscript preparation). Mark H. Rozenbaum, Erica Chilson, Raymond Farkouh, Liping Huang, Alejandro Cane, Adriano Arguedas, Maria J. Tort, and Vincenza Snow are employed by Pfizer Inc.
Ethical Approval
Human subjects did not participate in this study; thus, ethical approval was not required and informed consent was not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: findings from this study have been submitted in the form of an abstract for presentation at the 13th Meeting of the International Society of Pneumonia and Pneumococcal Disease (ISPPD) 17–20 March 2024, Cape Town, South Africa.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rozenbaum, M.H., Chilson, E., Farkouh, R. et al. Cost-Effectiveness of 20-Valent Pneumococcal Conjugate Vaccine Among US Children with Underlying Medical Conditions. Infect Dis Ther 13, 745–760 (2024). https://doi.org/10.1007/s40121-024-00944-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00944-z