Abstract
Introduction
In Argentina, vaccination with 13-valent pneumococcal conjugate vaccine (PCV13) followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23; PCV13 → PPSV23) has been recommended for all adults aged ≥ 65 years and younger adults with chronic medical (“moderate-risk”) or immunocompromising (“high-risk”) conditions since 2017. With the approval of a 20-valent PCV (PCV20), we evaluated the cost-effectiveness of PCV20 versus current recommendations for moderate-/high-risk adults aged 18–64 years and all adults 65–99 years.
Methods
A probabilistic cohort model was used to project lifetime outcomes and costs associated with invasive pneumococcal disease (IPD) and all-cause non-bacteremic pneumonia (NBP), and the expected impact of vaccination. Clinical outcomes were projected annually based on Argentinean data. Economic costs were estimated based on cases and corresponding medical costs (adjusted to 2023 USD) and costs of vaccine and administration. Cost-effectiveness of PCV20 was evaluated versus the current strategy, PCV13 → PPSV23, and alternatively, versus sequentially administered 15-valent PCV and PPSV23 (PCV15 → PPSV23), and presented as cost per quality-adjusted life year gained; a healthcare system perspective was used. Costs and benefits were discounted at 3%/year.
Results
PCV20 in lieu of PCV13 → PPSV23 among moderate-/high-risk adults aged 18–64 years and all adults 65–99 years (N = 13.4M) prevented 3838 IPD, 4377 inpatient NBP, and 6003 outpatient NBP cases, and 1865 disease-related deaths; relative to PCV15 → PPSV23 the corresponding reductions were 2775, 3285, 4518, and 1348. PCV20 was projected to be the dominant strategy versus PCV13 → PPSV23 and PCV15 → PPSV23 as overall costs were lower by $87.6M and $80.8M, respectively. In probabilistic sensitivity analyses, PCV20 was dominant (i.e., more effective, less costly) in 100% of 1000 simulations.
Conclusions
Analyses suggest implementing a PCV20 vaccination program in moderate-/high-risk adults aged 18–64 years and all adults ≥ 65 years—in lieu of PCV13 → PPSV23—would yield substantial reductions in pneumococcal disease and would be cost saving to the Argentinean healthcare system.
Plain Language Summary
Pneumococcal pneumonia has a high disease burden in both children and adults. Older adults and those with certain underlying conditions are more susceptible to severe pneumococcal disease resulting in considerable economic burden on the healthcare system. In Argentina, vaccination with 13-valent pneumococcal conjugate vaccine (PCV13) followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23) a year later is recommended for all adults aged ≥ 65 years and adults aged 18–64 years with underlying risk conditions. Despite vaccination efforts, prevalence of pneumococcal disease remains high. Two higher-valent PCVs—15-valent PCV (PCV15) and 20-valent PCV (PCV20)—are available for use in adults with PCV20 offering additional serotype coverage. This study assessed the cost-effectiveness of replacing current (PCV13 → PPSV23) and alternative (PCV15 → PPSV23) vaccination strategies with PCV20 alone. The use of PCV20 was evaluated among Argentinean adults aged 18–64 years with underlying risk conditions and all adults aged 65–99 years (N = 13 million). Over a lifetime time horizon, compared to PCV13 → PPSV23, PCV20 use would avert 14,218 cases and 1865 deaths, and increase quality-adjusted life years by 8655. Compared to PCV15 → PPSV23, PCV20 reduced cases and deaths by 10,578 and 1348, respectively, and increased quality-adjusted life years by 6341. In both comparisons, PCV20 use was cost saving with $87.6 million and $80.8 million lower costs compared to PCV13 → PPSV23 and PCV15 → PPSV23, respectively. Results of the cost-effectiveness analyses suggest that the use of PCV20 is a cost-saving strategy, reducing overall costs to the healthcare system and improving public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Pneumococcal disease continues to cause significant morbidity and mortality in Argentinean adults despite existing vaccination programs. |
The objective of this study was to assess the clinical and economic impact of replacing existing vaccination strategies with a new 20-valent pneumococcal conjugate vaccine (PCV20), which has the potential to reduce disease burden and associated costs. |
What was learned from the study? |
PCV20 was estimated to reduce invasive pneumococcal disease (IPD) cases by 3838, non-bacteremic pneumonia (NBP) cases by 10,380, and deaths by 1865 compared to PCV13 → PPSV23; corresponding reductions for PCV20 vs. PCV15 → PPSV23 were 2775, 7803, and 1348. |
The use of PCV20 is projected to be cost-saving among Argentinean adults, lowering total costs by $87.6 million and $80.8 million compared to PCV13 → PPSV23 and PCV15 → PPSV23, respectively. |
This economic evaluation of PCV20 will help inform decision-makers in Argentina and in other Latin American countries who will soon consider the use of novel pneumococcal conjugate vaccines. |
Introduction
Infections due to Streptococcus pneumoniae manifest as invasive pneumococcal disease (IPD) and non-bacteremic pneumonia (NBP) causing significant morbidity and mortality in both children and adults [1]. Between 2008 and 2018, 65,497 deaths occurred due to IPD (43.3%) and NBP (56.7%) in Argentina [2]. To reduce the risk of pneumococcal infection, the Ministry of Health of Argentina recommended the use of 13-valent pneumococcal conjugate vaccine (PCV13) among children < 2 years of age beginning in 2012 [3, 4]. Among adults, a 23-valent pneumococcal polysaccharide vaccine (PPSV23) has been available for routine vaccination among all adults aged ≥ 65 years and those at increased risk of disease due to underlying risk conditions (i.e., immunocompromising conditions and other conditions such as chronic heart disease, chronic pulmonary disease, diabetes, alcoholism, chronic liver disease, smoking) since 2001 [3, 4]. Due to the added benefits of pneumococcal conjugate vaccines (PCVs), PPSV23 was replaced with a sequential strategy involving administration of PCV13 followed by PPSV23 (PCV13 → PPSV23) among adults aged ≥ 65 years and adults aged 18–64 years with underlying risk conditions in 2017. PCV13 has been shown to protect against vaccine-type (VT) IPD and VT-NBP [5] whereas evidence suggests PPSV23 protects against VT-IPD only [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21].
Two new, higher valent PCVs—15-valent PCV (PCV15) and 20-valent PCV (PCV20)—are expected to be available soon in Argentina. While PCV15 includes the serotypes in PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F) plus two additional serotypes (22F, 33F), PCV20 includes the serotypes in PCV15 plus five additional serotypes (8, 10A, 11A, 12F, 15B) [22,23,24]. Moreover, PCV20 also includes 19 of the 20 serotypes present in PPSV23 and is expected to provide more robust protection against common serotypes due to its immune response mechanism [25]. We therefore evaluated the cost-effectiveness of replacing the current NIP recommendation of PCV13 → PPSV23 with PCV20 alone among all adults aged ≥ 65 years and younger adults at elevated risk of pneumococcal disease. Additionally, given the broader serotype coverage of PCV20 compared to PCV15, we also evaluated the cost-effectiveness of PCV20 versus sequential administration of PCV15 and PPSV23 (PCV15 → PPSV23) in the same population.
Methods
Model Description
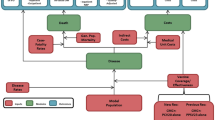
Lifetime clinical outcomes and economic costs of IPD and all-cause NBP were projected using a probabilistic cohort model with a Markov-type process in a hypothetical population of Argentinean adults aged 18–99 years. Figure 1 depicts the model structure, which has previously been used in evaluation of PCV20 vaccine [26]. The model population is initially characterized based on age in 1-year increments and risk profile (i.e., low, moderate, and high risk). Persons are allowed to transition to from low-risk to moderate-risk or from moderate-risk to high-risk group, but not to a lower risk group, during the modeling horizon.
Persons in the model population may receive PCV20 alone, PCV13 → PPSV23, PCV15 → PPSV23, or no vaccine at model entry, depending on the vaccination strategy and coverage. Clinical outcomes and economic costs are projected annually for the model population based on age, risk profile, disease/fatality rates, vaccination status, vaccine type, and time since vaccination. IPD includes bacteremia and meningitis, and NBP is stratified by care setting (inpatient vs. outpatient). Persons vaccinated at model entry may be at lower risk of future IPD and NBP; the magnitude of vaccine-associated risk reduction depends on clinical presentation (i.e., IPD or NBP), as well as the vaccine(s) received, proportion of disease that is vaccine-preventable, age, time since vaccination, and risk profile. Risk of death from IPD, NBP requiring inpatient care, and other causes (i.e., other than IPD and NBP) depends upon age and risk profile. Expected costs of medical treatment for IPD and NBP are generated based on event rates and unit costs in relation to the setting of care (i.e., inpatient vs. outpatient), age, and risk profile. Costs of vaccination—including the vaccine and its administration—are tallied in the year(s) in which the vaccination occurs (i.e., year 1 for single vaccine strategies, years 1 and 2 for sequential vaccination strategies). Clinical outcomes and economic costs are projected over the modeling horizon for the alternative vaccination strategies considered and include expected numbers of cases of IPD and NBP (inpatient and outpatient), number of deaths due to IPD and inpatient NBP, number of life-years (unadjusted and quality-adjusted), costs of medical treatment for IPD and NBP, and costs of vaccination.
Model Estimation
A summary of methods used in estimation of key model parameters is presented below; base case model inputs are included in Table 1.
Population
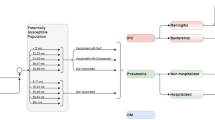
The size of the Argentinean population was based on the 2019 Argentina Encuesta Permanente de Hogares (EPH) Survey [27]. Persons in each age group (i.e., 18–49, 50–64, 65–74, 85–99 years) were allocated into low-, moderate-, and high-risk subgroups based on risk distribution presented in two Spanish database studies [28, 29]. The low-risk group included immunocompetent persons without underlying medical conditions, the moderate-risk group included immunocompetent persons with ≥ 1 underlying medical conditions (e.g., chronic heart disease, chronic pulmonary disease, diabetes, alcoholism, chronic liver disease, smoking, obesity), and the high-risk group included immunocompromised persons (e.g., due to immunosuppressive therapy, HIV, cancer) [28, 29].
Rates of Disease
IPD incidence was estimated using incidence of hospitalized pneumonia from Lopardo et al. (which was based on prospective active surveillance) [30], and the proportion of hospitalized pneumonia attributable to pneumococcus and the proportion of pneumococcal pneumonia that is invasive from Said et al. [31]. Because Lopardo et al. reported results for all persons aged 65–99 years in a single age group, estimates of pneumonia incidence were allocated across ages 65–74, 75–84, and 85–99 years based on relative rates of incidence by age group from Buzzo et al. [32] and the Argentinean population distribution [27]. As Lopardo et al. captured all cases of pneumonia (i.e., including all-cause NBP, treated in the inpatient and outpatient settings), incidence of IPD was derived by multiplying incidence of all cases of pneumonia by the proportion of pneumonia requiring hospitalization (68%) [30], proportion of hospitalized pneumonia attributable to pneumococcus (27.3%) and proportion of pneumococcal pneumonia that is invasive (24.8%) based on Said et al. [31]. These estimates were further apportioned across bacteremia (95%) and meningitis (5%) based on Drijkoningen et al. [33]. Age-specific rates of bacteremia and meningitis were then allocated across risk groups based on US CDC 2017/2018 data provided to Pfizer Inc. [34] and underlying population risk distributions.
Age-specific rates of inpatient and outpatient NBP were based on values reported by Buzzo et al. [32]. Because Buzzo et al. reported rates only for persons aged ≥ 50 years, incidence of inpatient NBP for persons aged 18–49 years was estimated using relative rates for 18–49 years (vs. 50–64 years) from Pelton et al. [35]. Similarly, incidence of outpatient NBP for persons aged 18–49 years was estimated using relative rates for 18–49 years (vs. 50–64 years) from Nelson et al. [36]. These estimates were further apportioned across risk profile using assumed relative risk-based published sources [35, 37] and underlying population risk distributions.
Case-Fatality and Mortality Rates
Age-specific case-fatality rates (CFRs) for bacteremia were based on data from a longitudinal study conducted in a single center in Argentina [38]. To derive CFRs across age groups of interest, point estimates were anchored to selected ages within the available age groups and methods of linear interpolation/extrapolation were employed. CFRs were apportioned across risk groups based on relative risk of mortality for moderate and high risk [34] and the age- and risk-specific distributions of bacteremia and meningitis cases. The CFR for meningitis was assumed to be the same as that for bacteremia. CFR for inpatient NBP was based on Buzzo et al. [32]. Because Buzzo et al. reported results only among persons aged ≥ 50 years, relative risk of death for persons aged 18–49 years (vs. 50–64 years) based on Averin et al. [39] was used to estimate inpatient NBP CFR for those aged 18–49 years. Age-specific CFRs were then apportioned across risk groups based on relative risk of mortality for moderate and high risk (vs. low risk) [39] and the age-specific risk distributions of inpatient NBP cases. Persons affected by outpatient NBP were assumed to not have an elevated risk of mortality.
Age-specific risk of death due to other causes (i.e., not related to IPD or inpatient NBP) was based on 2019 general population mortality rates in Argentina reported by INDEC [40] and apportioned across risk groups based on assumed RRs for mortality and age-specific risk distributions. Age- and risk-specific mortality rates were downwardly adjusted to account for death due to IPD and inpatient NBP.
Vaccine Effectiveness
For PCV20, vaccine effectiveness (VE) against vaccine type (VT) disease was assumed to be the same as that for PCV13 based on published results of an evaluation of PCV20 safety, tolerability, and immunogenicity [41]. Lacking alternative data, VE-PCV15 against VT disease was also assumed to be the same as VE-PCV13. For low-/moderate-risk persons aged ≥ 65 years, initial (i.e., year 1 of modeling horizon) VE of PCVs against vaccine type (VT) IPD and VT-NBP was assumed to be the same for all persons based on data from the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA) per-protocol population; for persons aged 50–64 years, initial VE was derived from post hoc analyses of CAPiTA; and for persons aged 18–49 years, initial VE was assumed to be the same as that for persons aged 50 years [5, 42]. For high-risk persons aged 18–99 years, initial VE against VT-IPD and VT-NBP was assumed to be equal to 80% of corresponding values for low-/moderate-risk persons [43, 44]. Initial VE-PCV was assumed to persist for the first 5 years of the modeling horizon [5, 45]. Beyond year 5 of the modeling horizon, VE-PCV was assumed to wane, as follows: 5% annual decline during years 6–10, 10% annual decline during years 11–15, and no efficacy from year 16 through the end of the modeling horizon [42].
Initial VE of PPSV23 against VT-IPD for low-, moderate-, and high-risk persons aged ≥ 18 years was estimated from published literature [46, 47]. VE-PPSV23 against VT-CAP was assumed to be zero based on various published sources and consistent with base case assumptions employed in several economic studies [6,7,8,9,10,11,12,13,14,15,16,17,18,19, 48,49,50,51,52]. Beyond year 1 of the modeling horizon, VE-PPSV23 against VT-IPD was assumed to wane, as follows: linear decline to 76.2% of initial VE by year 5, and linear decline to no efficacy by year 10 and through the end of the modeling horizon [46].
Serotype Coverage
The percentage of IPD and NBP due to vaccine serotypes in year 1 of the modeling horizon was based on age-specific data from the Surveillance Network System of Agents Responsible for Bacterial Pneumonia and Meningitis (SIREVA II) (Supplemental Tables S1 and S2) [53]. Serotype coverage for IPD and NBP was assumed to be invariant across risk groups.
As childhood vaccination programs for PCV15 and PCV20 are expected to be implemented in model years 2 and 3, respectively, herd effects for serotypes unique to PCV15 and PCV20 were assumed to begin in model years 3 and 4, respectively. We assumed no serotype replacement in the model and consequently re-based the proportion of disease due to vaccine serotypes annually (model years 3–10) to account for the reduction in overall disease.
Vaccine Coverage
Vaccine coverage estimates were based on internal Pfizer data and varied by age (18–64 years, 17%; ≥ 65 years, 65%) [54]. For persons receiving sequential strategies, all persons who received a PCV in year 1 were assumed to receive PPSV23 in year 2 (if alive).
Costs
Costs of medical care for persons aged 18–64 years and for those aged ≥ 65 years were based on a micro-costing study conducted by Soul Consulting (data on file) [54]. For both IPD and outpatient NBP, age-specific costs were apportioned across risk groups using relative costs of disease (i.e., for moderate- and high-risk vs. low risk)—for IPD and outpatient NBP, respectively—from Weycker et al. [37] and risk distribution among cases. Medical care costs for inpatient NBP among those in low- and high-risk groups were based on results from the study by Soul Consulting [54]. The average of medical care costs for low- and high-risk groups was used as the estimated medical care costs for moderate-risk persons with inpatient NBP.
Since PPSV23 is procured through PAHO’s revolving fund, the list price was used as the vaccination price [55]. The price of PCVs were markedly higher than the price of PPSV23 and were based on confidential Pfizer pricing. Vaccine administration cost varied across 18–64- and 65–99-year-old age groups and was assumed to be $6.45 and $7.58, respectively, based on an internal analysis conducted by Soul Consulting [54].
Utility and Disutilities
Age-specific health state utilities for the general population in Argentina were based on Szende et al. [56] where a self-report of population health was converted to utility values using a visual analogue scale value set specific to Argentina. Estimates were apportioned across risk groups using relative utilities (moderate and high risk vs. low risk) [57] and underlying population risk distribution [28, 29].
Disease-related disutilities were based on published literature. Disutility due to IPD (bacteremia and meningitis) and inpatient NBP was assumed to be 0.13 in the year of occurrence based on Mangen et al. [58]. For NBP requiring outpatient care only, disutility was assumed to be 0.004 based on were based on Melegaro et al. [59]. Disutility was assumed to be invariant by age or risk.
Analyses
Clinical outcomes and economic costs over the remaining years of life for moderate-risk and high-risk adults aged 18–64 years and all adults aged 65–99 years at model entry were evaluated for PCV20 versus PCV13 → PPSV23 and, alternatively, considering the use of PCV20 versus PCV15 → PPSV23. Analyses were conducted from the healthcare system perspective; future costs, life-years (LYs), and quality-adjusted LYs (QALYs) were discounted at 3% annually [60]. Incremental cost-effectiveness ratios (ICERs) were calculated based on differences in costs and QALYs for each comparison. All costs are expressed in January 2023 US dollars (1 USD = 183.25 ARS $).
Probabilistic sensitivity analyses (PSAs) were conducted (1000 replications) to account for uncertainty surrounding key model parameters. For each input parameter, the appropriate distribution was determined based on information available in the source material (Supplemental Table S3). Certain parameters such as population risk distribution, vaccine coverage and vaccine price were not varied in the PSA either because their impact on results was believed to be minimal, or due to limitations of the model structure that prevented varying these estimates. Lastly, price sensitivity analysis was conducted where PCV20 vaccine price was varied across a range of values and the corresponding ICERs were estimated.
Ethics Compliance
This study was conducted using publicly available data and previously conducted studies. It does not contain any new studies with human participants or animals performed by any of the authors, and hence was not subject to institutional review.
Results
The use of PCV20 in lieu of PCV13 → PPSV23 among moderate- and high-risk adults aged 18–64 years and all adults aged 65–99 years (N = 13,403,444) at model entry would reduce expected lifetime cases of IPD by 3838, all-cause hospitalized NBP by 4377, and all-cause outpatient NBP by 6003, and total disease-related deaths by 1865 (Table 2). Subsequently, lifetime total medical care costs would be reduced by $25.5 million. Compared to the sequential strategy, the use of PCV20 alone would reduce vaccination costs by $62.1 million. With total costs lower by $87.6 million, and discounted life years higher by 10,788 (unadjusted) and 8655 (quality-adjusted), PCV20 was dominant (i.e., more effective, less costly) versus PCV13 → PPSV23.
Similarly, the use of PCV20 remained dominant when compared with PCV15 → PPSV23 (Table 3). The use of PCV20 in lieu of PCV15 → PPSV23 reduced expected lifetime cases by 2775 for IPD, 3285 for hospitalized NBP, and 4518 for outpatient NBP, and disease-related deaths by 1348. Lifetime total medical care costs and vaccination costs would be reduced by $18.8 million and $62.1 million, respectively, and discounted life years would be higher by 7912 (unadjusted) and 6341 (quality-adjusted), yielding a dominant ICER. PCV20 was also found to be dominant versus PCV13 → PPSV23 and PCV13 → PPSV23 among both subgroups of the population (Supplemental Tables S4–S7).
In probabilistic sensitivity analyses, 100% of 1000 of simulations generated ICERs located in the southeast quadrant of the scatterplot, and thus projected lower costs and higher QALYs with the use of PCV20 vs. PCV13 → PPSV23 (Fig. 2). Results were comparable for PCV20 vs. PCV15 → PPSV23 (Fig. 3). PCV20 (vs. PCV13 → PPSV23 and PCV15 → PPSV23) remained dominant for both population subgroups in 100% of replications in probabilistic sensitivity analyses (Supplemental Figures S1 and S2). Considering a willingness-to-pay threshold of 1x to 3x gross domestic product per capita, the value-based price of PCV20 ranged from $58.8–$96.8 (vs. PCV13 → PPSV23) and $52.4–$80.2 (vs. PCV15 → PPSV23).
Discussion
We evaluated the public health and economic impact of replacing current (PCV13 → PPSV23) and alternative (PCV15 → PPSV23) vaccination strategies with PCV20 for prevention of pneumococcal disease among Argentinean adults. Results suggest that the use of PCV20 would substantially reduce the clinical and economic burden of pneumococcal disease among adults aged 18–64 years at moderate and high risk and all adults aged 65–99 years compared to PCV13 → PPSV23 and PCV15 → PPSV23, resulting in $87.6 million and $80.8 million in net savings, respectively. Results from probabilistic sensitivity analyses were consistent with the base case results. Our findings therefore suggest that the use of PCV20 is more efficient than the currently recommended PCV13 → PPSV23 strategy and the alternative sequential strategy including PCV15. To the best of our knowledge, this evaluation is the first cost-effectiveness analysis of PCV20 for the prevention of pneumococcal disease among Argentinean adults and the findings are largely consistent with those from previous evaluations of PCV13 among adults and children in Argentina and elsewhere, which suggested that higher-valency vaccines are either cost-effective or cost-saving versus lower-valency vaccines or no vaccination [26, 61, 62].
A higher valency conjugate vaccine—PCV20—was recently approved for use in Argentina [63] and offers several benefits compared to existing vaccinations against pneumococcal disease. First, PCV20 protects against additional serotypes of S. pneumoniae not covered by PCV13 and PCV15 and shares 19 of the 23 serotypes covered by PPSV23. Second, compared to PPSV23, which induces immune response by releasing immunoglobulin from B cells, PCV20 offers durable protection because of T cell-dependent response with memory B cell formation [64]. Lastly, PCV20, a single-dose vaccination strategy may improve vaccine coverage rates compared to the current sequential strategy.
Implementation of a PCV20 adult vaccination program is especially relevant given the current serotype-epidemiology of pneumococcal disease in Argentina [65]. Serotypes 3, 8, 12F, 7F and 1, which are covered by PCV20, were the most prevalent disease-causing serotypes among Argentinean adults between 2013 and 2017 [65]. Additionally, individuals who had regular contact with children had a higher prevalence of carriage [66]. Analyses in Argentinean children < 5 years have shown that pneumococcal disease burden attributable to PCV20 serotypes is substantially higher than that attributable to lower valent PCVs [67]. The data on serotype distribution that was employed in these analyses suggest that much of the adult burden of disease is also attributable to PCV20 serotypes. Therefore, direct immunization with PCV20 is expected to further reduce the disease burden in adults.
The major limitation of our study is uncertainty regarding estimation of certain parameter values. The risk distribution in the population was estimated based on two studies conducted in Spain because they provide detailed data on risk profiles by age, and comparable Argentinean data were not available. Although estimated using data specific to Argentina, the current disease rates are another source of uncertainty. IPD estimates were based on an active surveillance study by Lopardo et al. [30] conducted in a single city (General Roca) between 2012 and 2015 and may not be generalizable to the entire population. CFR for meningitis was assumed to be the same as for bacteremia due to lack of data specific to meningitis. Lastly, only pre-COVID-19 pandemic data on vaccine coverage rates of sequential strategies among Argentinean adults are available, and hence the estimates used in the analysis were based on assumptions accounting for an increase in vaccine uptake in the post-pandemic period. However, as the uptake of the second dose of the sequential strategy (i.e., PPSV23) is likely to be lower than uptake of the first dose, it may be somewhat overestimated. Our model also does not account for PPSV23 booster doses at age 65 years among persons who received PCV13 → PPSV23 between the ages of 18–64 years, which may bias outcomes against PCV20, however, uptake of these booster doses is believed to be very low.
Another area of parameter uncertainty is the assumed effectiveness of vaccines. The recently published study by Djennad et al. [46], which showed that PPSV23 provides some protection against VT-IPD for 10 years, is the best-available real-word effectiveness study conducted to date. Although recent evidence suggests that PPSV23 may provide some, albeit limited, protection against VT-NBP, our study did not account for effectiveness of PPSV23 against VT-NBP [68, 69]. VE-PCV20 against VT-IPD and VT-CAP was based principally on data from CAPiTA and post hoc analyses as PCV20 was developed on the foundation of PCV13 [5, 42]. We acknowledge, however, that the effectiveness of PCV20 may vary somewhat from PCV13 [70]. Finally, our model, like all such health economic models, simplifies reality to some extent. Our model also did not include other potential downstream adverse outcomes and costs associated with pneumococcal disease, which confers a conservative bias against the use of PCV20.
Conclusions
The use of PCV20 among adults aged 18–64 years with risk conditions and all adults aged 65–99 years would substantially reduce the number of cases and deaths due to pneumococcal disease in Argentina. Moreover, the incremental costs of PCV20 acquisition would be fully offset by the savings from averted disease. Therefore, PCV20 alone among adults for whom PCV13 → PPSV23 is currently recommended is a more efficient use of public healthcare budget than sequential (i.e., PCV → PPSV23) vaccination.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chalmers J, et al. Community-acquired pneumonia in the United Kingdom: a call to action. Pneumonia. 2017;9(1):15.
Giglio N, et al. 1311. Population-based mortality rates of clinical syndromes potentially associated with pneumococcal disease in Argentina from 2008–2018. Open Forum Infect Dis. 2021;8(Supplement_1):S744.
Minesterio de Salud Argentina, Introducción de la Vacuna Conjugada Contra el Neumococo al Calendario Nacional de Inmunizaciones de la República Argentina; 2011.
Ministerio de Salud Argentina, Vacunación Contra Neumococo. Estrategia Argentina 2017–2018. Lineamientos técnicos manual del vacunador; 2017.
Bonten MJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25.
Boccalini S, et al. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother. 2013;9(3):699–706.
Cho B-H, et al. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21.
Evers SMAA, et al. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur J Clin Microbiol Infect Dis. 2007;26(8):531–40.
Fry AM, et al. Comparing potential benefits of new pneumococcal vaccines with the current polysaccharide vaccine in the elderly. Vaccine. 2002;21(3):303–11.
Heo JY, et al. Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea. PLoS ONE. 2017;12(5): e0177342.
Hoshi S-L, Kondo M, Okubo I. Economic evaluation of immunisation programme of 23-valent pneumococcal polysaccharide vaccine and the inclusion of 13-valent pneumococcal conjugate vaccine in the list for single-dose subsidy to the elderly in Japan. PLoS ONE. 2015;10(10): e0139140.
Johnstone J, et al. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167(18):1938–43.
Kuhlmann A, et al. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Heal Econ Rev. 2012;2(1):4.
Moberley S, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD000422.pub3.
Örtqvist Å, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351(9100):399–403.
Simberkoff MS, et al. Efficacy of pneumococcal vaccine in high-risk patients. N Engl J Med. 1986;315(21):1318–27.
Smith KJ, et al. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in U.S. older adults. Am J Prevent Med. 2013;44(4):373–81.
Stoecker C, et al. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016;31(8):901–8.
Stoecker C, et al. Cost-effectiveness of continuing pneumococcal conjugate vaccination at age 65 in the context of indirect effects from the childhood immunization program. Vaccine. 2020;38(7):1770–7.
Latifi-Navid H, et al. Pneumococcal disease and the effectiveness of the PPV23 vaccine in adults: a two-stage Bayesian meta-analysis of observational and RCT reports. Sci Rep. 2018;8(1):11051.
Tin TinHtar M, et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PLoS ONE. 2017;12(5): e0177985.
PCV13 Package Insert, F.a.D. Administration, Editor. 2017.
PCV15 Package Insert, F.a.D. Administration, Editor. 2021.
PCV20 Package Insert, F.a.D. Administration, Editor. 2021.
Clutterbuck EA, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205(9):1408–16.
Mendes D, et al. Cost-effectiveness of using a 20-valent pneumococcal conjugate vaccine to directly protect adults in England at elevated risk of pneumococcal disease. Expert Rev Pharmacoecon Outcomes Res. 2022;22(8):1285–95.
INDEC. Encuesta Permanente de Hogares (EPH). 2019. Available from https://www.indec.gob.ar/indec/web/Institucional-Indec-BasesDeDatos. Accessed Dec 2023.
Rejas J, et al. All-cause community acquired pneumonia cost by age and risk in real-world conditions of care in Spain. Expert Rev Pharmacoecon Outcomes Res. 2022;22(5):853–67.
Ochoa-Gondar O, et al. Prevalence of high, medium and low-risk medical conditions for pneumococcal vaccination in Catalonian middle-aged and older adults: a population-based study. BMC Public Health. 2017;17(1):610.
Lopardo GD, et al. Incidence rate of community-acquired pneumonia in adults: a population-based prospective active surveillance study in three cities in South America. BMJ Open. 2018;8(4): e019439.
Said MA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8(4): e60273.
Buzzo AR, et al. Morbidity and mortality of pneumonia in adults in six Latin American countries. Int J Infect Dis. 2013;17(9):e673–7.
Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51.
Pfizer Inc., Active bacterial core surveillance (ABCs) 2017/2018 (data on file).
Pelton SI, et al. Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood PCV13 immunization. Clin Infect Dis. 2019;68(11):1831–8.
Nelson JC, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26(38):4947–54.
Weycker D, et al. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16:182.
Gentile JH, et al. Bacteremic pneumococcal pneumonia: a longitudinal study in 279 adult patients from a single center. Univ Louisville J Respir Infect. 2018. https://doi.org/10.18297/jri/vol2/iss1/10/.
Averin A, et al. Mortality and readmission in the year following hospitalization for pneumonia among US adults. Respir Med. 2021;185:106476.
INDEC. Tablas de mortalidad. 2019. Available from https://www.indec.gob.ar/indec/web/Nivel3-Tema-2-42. Accessed Dec 2023.
Essink B, et al. Pivotal phase 3 randomized clinical trial of the safety, tolerability, and immunogenicity of 20-valent pneumococcal conjugate vaccine in adults Aged ≥18 years. Clin Infect Dis. 2022;75(3):390–8.
Mangen MJ, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–16.
Klugman KP, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–8.
French N, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362(9):812–22.
Patterson S, et al. A post hoc assessment of duration of protection in CAPiTA (community acquired pneumonia immunization trial in adults). Trials Vaccinol. 2016;5:92–6.
Djennad A, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6:42–50.
van Hoek AJ, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65(1):17–24.
Sisk JE, et al. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med. 2003;138(12):960–8.
Smith KJ, et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine. 2008;26(11):1420–31.
Part 4—Active vaccines: pneumococcal vaccine, in Canadian immunization guide, P.H.A.o. Canada, Editor; 2021.
Kraicer-Melamed H, et al. Update on the use of pneumococcal vaccines in adults 65 years of age and older—a public health perspective: an advisory committee statement (ACS) National Advisory Committee on Immunization (NACI). Canada: Public Health Agency of Canada; 2018.
Kuhne F, et al. Cost-effectiveness of use of 20-valent pneumococcal conjugate vaccine among adults in Germany. Expert Rev Vaccines. 2023;22(1):921–32.
National Institute of Infectious Diseases—Argentina’s National Administration of Laboratories and Institutes of Health (INEI-ANLIS), SIREVA II; 2021.
Pfizer Inc., Data on File.
Pan American Health Organization. PAHO Revolving Fund Vaccine Prices for 2021. Available from https://www.paho.org/en/documents/paho-revolving-fund-vaccine-prices-2021. Accessed Dec 2023.
Szende A, Janssen B. Cross-country analysis of EQ-5D data. In: Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht, NL: Springer; 2014. p. 31–6.
Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539–45.
Mangen M-JJ, et al. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis. 2017;17(1):208.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14.
Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020;35(1):107–14.
Uruena A, et al. Cost-effectiveness analysis of the 10- and 13-valent pneumococcal conjugate vaccines in Argentina. Vaccine. 2011;29(31):4963–72.
Giglio ND, et al. Cost-effectiveness of pneumococcal vaccines for adults aged 65 years and older in Argentina. Value Health Reg Issues. 2022;28:76–81.
Administración Nacional De Medicamentos, A.y.T.M.A., DI-2023-4988-APN-ANMAT#MS. 2023, ANMAT.
Shah AA. Simplifying pneumococcal immunizations for adults. Am Fam Physician. 2022;105(6):580–1.
Zintgraff J, et al. Distribution of PCV13 and PPSV23 Streptococcus pneumoniae serotypes in Argentinean adults with invasive disease, 2013–2017. Rev Argent Microbiol. 2020;52(3):189–94.
Wyllie AL, et al. Persistence of pneumococcal carriage among older adults in the community despite COVID-19 mitigation measures. Microbiol Spectr. 2023;11(3): e0487922.
Mac Mullen M, et al. POSA198. Clinical and economic burden attributable to serotypes included in current and future pneumooccal conjugate vaccines in Argentinean children under five years of age, in ISPOR Europe 2021. Value Health. 2021;25(1):S125.
Lawrence H, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case–control test-negative design study. PLoS Med. 2020;17(10): e1003326.
Suzuki M, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313–21.
Essink B, et al. 3 phase 3 pivotal evaluation of 20-valent pneumococcal conjugate vaccine (PCV20) safety, tolerability, and immunologic noninferiority in participants 18 years and older. Open Forum Infect Dis. 2020;7(Suppment_1):S2.
Medical Writing/Editorial Assistance
Editorial assistance in the preparation of this article was provided by Vanya Nikolova of Avalere Health, which was funded by Pfizer.
Funding
The research described herein was supported by Pfizer Inc. The journal’s Rapid Service Fee was funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Authorship was designated based on guidelines promulgated by the International Committee of Medical Journal Editors (2004). All persons who met criteria for authorship are listed as authors on the title page. All authors contributed to the study conception and design. Lucila Rey-Ares, Mercedes Mac Mullen, and Ahuva Averin were responsible for data acquisition. Liping Huang was responsible for funding acquisition. Formal analyses were conducted by Mark Atwood, Ahuva Averin, and Dhwani Hariharan, and all authors contributed to the interpretation of data. The first draft of the manuscript was written by Dhwani Hariharan, and all authors critically reviewed the manuscript and commented on all subsequent versions. All authors have read and approved the final version of the manuscript. The study sponsor, Pfizer Inc., reviewed the study research plan and study manuscript; data management, processing, and analyses were conducted by Avalere Health. All final analytic decisions and the decision to submit for publication were made solely by study investigators.
Corresponding author
Ethics declarations
Conflict of Interest
Lucila Rey-Ares, Mercedes Mac Mullen, Carolina Carballo and Liping Huang are employed by Pfizer Inc. Ahuva Averin, Dhwani Hariharan, and Mark Atwood are employees of Avalere Health, which received financial support from Pfizer Inc. for this study (including manuscript preparation).
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rey-Ares, L., Averin, A., Mac Mullen, M. et al. Cost-Effectiveness of 20-Valent Pneumococcal Conjugate Vaccine in Argentinean Adults. Infect Dis Ther 13, 1235–1251 (2024). https://doi.org/10.1007/s40121-024-00972-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00972-9