Abstract
Introduction
The 10-valent pneumococcal conjugate vaccine (PCV10, Synflorix) was introduced into the Dutch pediatric national immunization program (NIP) starting in 2011. However, there is substantial pneumococcal disease burden due to increases in non-PCV10 covered serotypes. Higher-valent vaccines for pediatrics (PCV13, PCV15, and PCV20) may alleviate much of the remaining disease burden upon implementation through broader serotype coverage. This article assesses the public health impact of different pediatric vaccination strategies (switching to PCV13, PCV15 or PCV20) versus maintaining PCV10 at different time intervals in the Netherlands.
Methods
A population-based, decision-analytic model was developed using historical pneumococcal disease surveillance data to forecast future invasive pneumococcal disease (IPD), pneumonia, and otitis media (OM) cases over a 7-year period (2023–2029) under the following strategies: continued use of PCV10, switching to PCV13 in 2023, switching to PCV15 in 2023, and switching to PCV20 in 2024. Scenario analyses were performed to account for uncertainties in future serotype distributions, disease incidence reductions, and epidemiologic parameters.
Results
Switching to PCV13 in 2023 was found to avert 26,666 cases of pneumococcal disease compared to continuing PCV10 over a 7-year period (2023–2029). Switching to PCV15 in 2023 was found to avert 30,645 pneumococcal cases over the same period. Switching to PCV20 once available in 2024 was estimated to avert 45,127 pneumococcal cases from 2024–2029. Overall conclusions were maintained after testing uncertainties.
Conclusions
For the Dutch pediatric NIP, switching to PCV13 in 2023 would be an effective strategy compared with continued use of PCV10 for averting pneumococcal disease cases. Switching to PCV20 in 2024 was estimated to avert the most pneumococcal disease cases and provide the highest protection. However, in the face of budget constraints and the undervaluation of prevention strategies, it remains challenging to implement higher valent vaccines. Further research is needed to understand the cost-effectiveness and feasibility of a sequential approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What carry out the study? |
There is substantial pneumococcal disease burden due to increases in non-PCV10 covered serotypes in the Netherlands. |
This paper assesses the public health impact of different pediatric vaccination strategies (switching to PCV13, PCV15, or PCV20) versus maintaining PCV10 at different time intervals in the Netherlands. |
Why was learned from the study? |
Switching to PCV13 in 2022, and subsequently PCV20 in 2024, was estimated to avert the most pneumococcal disease cases and provide the highest protection in the Netherlands. |
Further research is needed to understand the cost-effectiveness and feasibility of a sequential approach. |
Introduction
Before pneumococcal conjugate vaccines (PCVs) were introduced, 70% of IPD cases globally in children under 5 years of age were caused by 6–11 serotypes [19]. In 2000, the heptavalent vaccine (PCV7) was introduced into pediatric national immunization programs (NIPs) for protection against seven of the most prevalent disease-causing serotypes worldwide. Implementing PCV7 dramatically decreased invasive pneumococcal disease (IPD) incidence in both vaccinated children and unvaccinated individuals across all ages in many countries [9, 18, 32]. However, this benefit was countered by an increase in non-vaccine serotype disease, of which serotype 19A became the predominant cause of childhood IPD globally accounting for 22% of cases [2]. Given these emerging non-vaccine serotypes, 10-valent (PCV10, Synflorix) and 13-valent (PCV13) PCVs were introduced globally in 2009 and 2010. Following their introduction, significant reductions in vaccine-type IPD were observed [11]. However, non-vaccine serotypes still cause a considerable proportion (72%) of childhood IPD cases in European PCV13 settings [2]. Although serotype replacement occurs in most countries after PCV implementation, its impact may be of particular concern in PCV10 countries, where there is an emergence of dominating serotypes covered by PCV13 (e.g., 19A) [19, 33].
In 2006, the Netherlands introduced PCV7 into the pediatric NIP, which was replaced by PCV10 in 2011 [26, 34]. After PCV7 introduction, IPD incidence rates significantly declined by 69% in children < 5 years, 31% in those 18–49 years, and 19% in adults ≥ 65 years [4]. After PCV10's introduction, IPD incidence was reduced by an additional 30% in individuals < 50 years, while no further reduction was observed in adults ≥ 50 years [4]. An increase in non-PCV10 type IPD following PCV10 introduction, countering the decline in PCV10 type disease, may explain the lack of IPD incidence reductions in older adults. Thus, the potential for PCV10 to reduce pneumococcal disease incidence is likely maximized, given only 11% of IPD cases in 2016–2018 were caused by PCV10 serotypes [4].
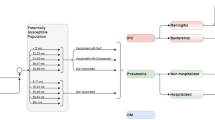
There are three higher-valent pediatric PCVs which have the potential to reduce substantial disease burden in the Netherlands (Fig. 1). The 15-valent (PCV15) and the 20-valent (PCV20) vaccines are now licensed for adults [14, 15] and are expected to be available for children soon, although neither is currently recommended as part of the NIP. Given that non-vaccine type disease burden remains substantial in the Netherlands, decision makers should carefully consider and assess the potential impact of implementing higher-valent vaccines. Therefore, the objective of this article is to evaluate the potential public health impact of broader serotype coverage in Dutch children by comparing different higher-valent vaccination strategies versus maintaining PCV10. We estimate the public health impact of switching to PCV13 or PCV15 in the near-term (2023) or switching to PCV20 in 2024 compared with maintaining PCV10 until 2028. With one higher-valent infant PCV available and two new PCVs on the horizon, comparing the public health impact of PCV13, PCV15, and PCV20 implementation can inform decision makers regarding which higher-valent vaccine should be incorporated into future pediatric NIPs.
Methods
Model Structure
A previously published decision-analytic forecasting model was adapted to include pediatric higher-valent PCVs in development. The model was used to compare the potential public health impact of different vaccination strategies in the Netherlands: continued use of the current standard of care (PCV10) or switching to one of the higher-valent vaccines (PCV13, PCV15, or PCV20) [29, 33, 42,43,44]. This population-based model utilizes historical real-world invasive pneumococcal disease (IPD) surveillance data stratified by age group and serotype to forecast disease incidence and serotype distribution changes under PCV10, PCV13, PCV15, and PCV20 pediatric NIPs for the overall population. The model predicts age-specific prospective incidence trends and various serotype behaviors based on observed retrospective incidence and serotype dynamics with and without having a specific PCV coverage. This methodology not only captures vaccine pressure on covered serotypes based on real-world evidence but also captures the replacement of non-vaccine serotypes observed from surveillance data. The projected incidence in pneumococcal disease is used to calculate total cases and deaths associated with each PCV program. Instead of using clinical trial data, this model better reflects real-world effectiveness of PCVs by using historical IPD trends to project the future number of IPD, inpatient pneumonia, outpatient pneumonia, and acute otitis media (AOM) cases and deaths over a 7-year time horizon (i.e., 2023 through 2029). The 7-year time horizon is used to allow for multiple years of vaccine effect following the anticipated introduction of higher-valent vaccines to be available in 2023 or 2024. This study is based on previously conducted studies and does not contain any new studies with human participants or animals.
Population
The total population of the Netherlands (17,282,163) is included in this analysis and stratified into seven age groups: 0–< 2, 2–4, 5–17, 18–34, 35–49, 50–64, and 65+ [8]. We assumed 95% of children < 2 years old in the Netherlands (339,259) are eligible for vaccination [25, 33].
Comparison of Vaccination Strategies
As outlined in Fig. 2, our model estimates the public health impact of four vaccination strategies: maintaining PCV10 (Strategy 1); switching to PCV13 in 2023 (Strategy 2); switching to PCV15 in 2023 (Strategy 3); and switching to PCV20 once available in 2024 (Strategy 4). To address the objectives of this paper, we compare Strategies 2–4 with the status quo (Strategy 1).
Base Case Analysis
For each vaccination strategy, a base case is calculated in which the numbers of disease cases and deaths are estimated over a 7-year time horizon (to allow for a 5-year time horizon after the introduction of either PCV15 or PCV20). By comparing the number of disease cases and deaths prevented by switching to these higher-valent vaccines, we can determine which vaccine strategies provide the greatest public health impact.
Invasive Pneumococcal Disease Incidence
Age and serotype-specific IPD incidence rates were obtained from 2011 to 2018 surveillance data reported by the Dutch National Institute of Public Health and Environment (RIVM) [23, 24]. IPD incidence was reported for the following serotype groups: PCV7 covered (i.e., serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F), incremental PCV10 (i.e., serotypes 1, 5, 7F), incremental PCV13 (i.e., serotypes 3, 6A, 19A), and non-PCV13 serotypes. Serotype-specific incidence rates for newly covered PCV15-unique (i.e., 22F, 33F) and PCV20-unique (i.e., 8, 10A, 11A, 15B, 12F) are needed to understand the impact of PCV15 and PCV20 vaccine programs on IPD incidence. However, PCV15- and PCV20-unique serotype-specific incidence data were not available between 2011 and 2018. Thus, based on data provided by Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM), we applied the serotype distribution from 2019 to 2011–2018 historical IPD incidence data to calculate serotype-specific incidence for PCV15-unique and PCV20-unique serotypes [31]. Estimated age-specific IPD incidence is reported in Table 1. The estimated IPD incidence by age and serotype group is presented in supplemental material Tables 1–3. Based on recent data from multiple European countries [4, 6, 30], IPD incidence in 2021–2022 has rebounded to pre-COVID19 pandemic levels after social distancing measures were lifted, and serotype distributions have not significantly changed. As such, we assume that 2021 incidence was similar to 2019 incidence and modeled prospectively beginning with 2022.
Future IPD incidence, assuming continued use of PCV10 (Strategy 1), was forecasted based on trend lines that were fit to Dutch historical surveillance data according to methodologies published in previous studies [29, 33, 42,43,44]. Trend lines were independently fit to historical data of covered (i.e., after PCV10 introduction) and non-covered (i.e., before PCV10 introduction) periods. Based on R-squared values reflecting best fit, linear or logarithmic functions are used to forecast serotypes during non-covered periods to minimize unrealistic increases in disease incidence, while linear, logarithmic, exponential, or power functions are used to forecast serotypes during covered periods. Future IPD incidence for newly covered serotypes, assuming a switch to PCV13 (Strategy 2), PCV15 (Strategy 3) or PCV20 (Strategy 4), is forecasted by estimating annual percent reductions in PCV13-10 type IPD by linearizing data from a systematic review [38].
Scenario analyses were included on the estimated impact of vaccination based on observed incidence trends (percentage reduction in incidence over time) following the introduction of PCV13 as observed in Australia, Canada, Israel, and the United Kingdom (UK), as well as the average percentage incidence reduction across the four countries [1, 3, 16, 18, 27, 28, 45]. For each country, the annual relative change in IPD incidence was calculated compared to the incidence in the year prior to introduction of a PCV13 program. Specifically, the relative change in incidence was estimated for seven age groups (< 2 years; 2–4 years; 5–17 years; 18–34 years; 35–49 years; 50–64 years; ≥ 65 years) by serotype group (PCV13 minus PCV7 serotypes). Each of the country annual relative incidence changes was used for each year and age group in the scenario analysis [27]. Finally, a scenario analysis was considered only including children (0–18 years) and excluding adult age groups.
Non-invasive Pneumococcal Disease Incidence
Non-invasive pneumococcal disease is comprised of pneumococcal AOM and pneumonia. Because the etiology of AOM is largely unknown, serotype-specific historical data for both pneumococcal AOM and pneumococcal pneumonia are unavailable. Global estimates of the proportion of AOM attributable to Streptococcus pneumoniae are highly variable, ranging from 20 to 50% [10, 12]. Thus, based on prior studies, we conservatively assume that 20% of AOM and pneumonia are due to S. pneumoniae [33, 37, 42, 43]. We test this assumption with a scenario analysis assuming 10% of non-invasive disease is pneumococcal. Given that the serotype distribution is unknown for non-invasive disease, we forecast future AOM, hospitalized pneumonia, and non-hospitalized pneumonia cases using the same approach outlined by previous models, which relies on the underlying assumption that future non-invasive disease cases will vary proportionately to future IPD cases [40]. This assumption posits that vaccination programs will impact the serotype distribution of both IPD and non-invasive disease similarly.
Incidence of all-cause AOM for children < 5 years old is obtained from the published literature [17]. Age-specific incidence of non-hospitalized all-cause pneumonia is obtained from national surveillance data from the Netherlands [34], while hospitalized pneumonia incidence was derived from earlier published study and assuming a similar proportion of longitudinal decline in incidence as observed for non-hospitalized pneumonia [13, 35, 36]. Age-specific AOM and pneumonia are reported in Table 1.
Mortality
All-cause mortality rates per 100,000 individuals by age group were calculated based on population estimates and number of deaths reported by Statistics Netherlands (CBS), the Dutch Statistics Authority (Table 2) [7]. Case fatality rates from previous epidemiology and cost-effective analysis (CEA) studies range from 8.1 to 17.8% and 1.4 to 15.2% for IPD and hospitalized pneumonia, respectively [4, 20, 21]. To be consistent with other CEAs, the assumption was made that non-hospitalized pneumonia and AOM are not associated with an increased risk of death [33, 37, 42, 43].
Results
Results for each scenario can be seen in Table 3. With the status quo scenario (Strategy 1), we estimated the Netherlands would incur 23,856 cases of IPD over the 7-year period from 2023 to 2029. Compared with Strategy 1, switching to any higher-valent vaccine would result in a greater reduction in cases. Switching to PCV13 in 2023 (Strategy 2) would avert 2983 cases of IPD over 7 years, while switching to PCV15 in 2023 (Strategy 3) would avert 3926 IPD cases. Switching to PCV20 in 2024 (Strategy 4) would result in the greatest amount (5717) of cases averted.
Results were similar for pneumonia and AOM. Switching to Strategy 2 would result in 7233 fewer hospitalized pneumonia cases, 2981 fewer outpatient pneumonia cases, and 13,468 fewer cases of AOM compared with PCV10. Strategy 4 would result in the fewest cases, averting 21,291 AOM cases, 13,400 inpatient pneumonia cases and 4719 outpatient pneumonia cases compared with PCV10 over the 7-year period. Pneumococcal-related deaths would be substantially reduced with Strategies 2–4 compared with Strategy 1: Strategy 2 would avert 1235 deaths, whereas Strategy 4 would avert 2127 deaths.
The results from scenario analyses illustrate the range of effects that switching from PCV10 a higher-valent vaccine would have when assuming disease reductions based on various countries’ historical data (Table 4). In the base case analyses, continued PCV10 use would result in 739,042 total cases of pneumococcal disease, whereas switching to PCV13, PCV15, and PCV20 would avert 26,666, 30,645, and 45,127 cases of disease, respectively. In scenario analyses, the impact of switching to PCV13 varied from 14,830 cases averted (assuming just a 10% of non-invasive disease is pneumococcal) to 31,518 cases averted (assuming a similar vaccination impact as seen in Israel). Other vaccines saw a similar variation: PCV15 impact varied from 17,293 to 35,497 cases averted; PCV20 averted 25,431–49,478 cases.
Discussion
We developed a decision-analytic model to forecast and compare the public health impact of sustained use of PCV10 with a switch to a higher-valent PCV (i.e., PCV13, PCV15, PCV20) in the Dutch pediatric NIP. The analysis estimated that switching to higher-valent vaccines would substantially reduce disease incidence across all ages. Switching to PCV20 in 2024 was expected to generate the largest decrease in pneumococcal disease cases and deaths because of having the broadest serotype coverage.
These results are consistent with findings from previous studies. Pugh et al. [33] estimated that introduction of PCV13 in the Netherlands would reduce morbidity by 450 IPD cases, 50,651 AOM cases, 818 hospitalized pneumonia cases, and 2072 outpatient pneumonia cases over 5 years, leading to 167 fewer deaths. By comparison, we estimate a reduction of 2983 IPD cases, 7233 AOM cases, 2981 inpatient pneumonia cases, and 1235 deaths over 7 years. The differences in results are driven largely by differences in data availability. Whereas Pugh et al. [30] used data from 2004 to 2011 and projected forward, we used published Dutch data through 2019. Another study by Thorrington et al. [39] followed a similar approach utilizing real-world surveillance data to assess the cost-effectiveness of PCV13 and a 23-valent polysaccharide vaccine (PPV23) recommended for older individuals aged ≥ 65 years from a healthcare provider’s perspective. Although this study focuses on adult vaccination strategies, it concluded that the combined use of PCV13 in infants and PPV23 in older individuals has the greatest impact on pneumococcal disease burden. Thus, our study builds upon this review by using a population-based model to assess the impact of an infant-based strategy on both vaccinated and unvaccinated populations.
Following the implementation of PCV10, serotype replacement has been a significant concern in the Netherlands, and, as of 2019, IPD incidence had rebounded to pre-PCV10 levels [4]. Despite largely eliminating PCV10 incidence in children, increases in current, noncovered serotypes (most notably serotypes 19A and 8 in children and serotypes 3, 19A, and 8 in adults) have outpaced the reduction in PCV10 disease. This has implications regarding the near-term value of a future PCV program. The estimated incremental benefit of PCV15 over PCV13 was smaller than that of PCV20 over PCV15, as serotype 19A (covered by PCV13, PCV15, and PCV20) and serotype 8 (covered only by PCV20) cause most of the disease in The Netherlands.
Sensitivity analyses illustrated the potential variability of impact of the higher-valent vaccination strategies. When varying the effect of the higher-valent vaccines based on incidence reductions seen in other countries, we find results relatively similar to the base case. When running a scenario restricting the analysis to those < 18 years of age, higher-valent vaccines averted 75–80% as many cases as in the base case. This is primarily due to the assumption that infant PCV programs have no effect on outpatient pneumonia cases in those > 5 years of age. Thus, even when excluding a large proportion of the population who would benefit from pediatric vaccination, we still observed a substantial impact on disease incidence. As expected, the scenario assuming only 10% pneumonia and AOM are pneumococcal had the largest impact on results. However, even in this extremely conservative scenario, the higher-valent vaccines (PCV13, PCV15, and PCV20) were still expected to avert approximately 15,000–25,000 disease cases compared with PCV10 over 7 years.
Since there are no modeling studies which have considered PCV15 or PCV20 in The Netherlands, we cannot compare our findings to other studies for these vaccination strategies. However, based on the estimated observed reduction in incidence due to disease from Shiri et al. [38], the currently low incidence of serotypes 22F and 33F (PCV15), and the relatively higher 8, 10A, 11A, 12F, and 15B (PCV20), we predicted PCV20 implementation would have a greater impact on disease versus PCV15 [38]. Yet uncertainty remains regarding the true overall impact of novel PCV programs within the Netherlands. Considering the variation in disease reduction observed in five countries that introduced PCV13, we find a range of impacts. Australia saw a relatively modest reduction in disease, and as such the disease burden reduction in our model is smaller when we assume similar trends to Australia. Conversely, Israel Canada and the UK each saw substantially greater proportional reduction in incidence, and as such assuming incidence trends similar to either of these countries would lead to greater disease reduction in the Netherlands.
PCV10 use in the Netherlands has almost eradicated pneumococcal disease caused by PCV10-covered serotypes. In the Netherlands, the overall incidence of PCV13-10 serotype (i.e., serotypes 3, 6A, 19A) IPD cases increased by 44% (from 2.47 to 3.55 cases per 100,000) post-PCV10 introduction and non-PCV13 type incidence increased by 71% (from 5.84 to 9.99 cases per 100,000) by 2016–2018 [4]. Decision makers should consider switching to higher-valent vaccines to prevent disease caused by non-covered serotypes. However, budget impact and financing implications often make investment in newer vaccines challenging. The direct medical costs averted from pneumococcal disease prevention can be cost-effective or even cost-saving, but policymakers often cannot to recognize this given concerns over increasing the fixed immunization budget. This may lead to PCVs being undervalued and underfunded [5].
The limitations of our analysis were primarily related to the availability of data to inform the model parameters. Since IPD incidence during 2004–2019 was used to estimate incidence over the model time horizon (i.e., 2022–2028), increases or decreases in serotype-specific incidence may not be precisely estimated. Additionally, because PCV15 and PCV20 have not been licensed for children yet, data limitations prohibit us from applying real-world evidence from these vaccines to predict future serotype behavior. As a result, we may be over- or underestimating PCV15 and PCV20 impact. However, we ran multiple sensitivity analyses on the potential impact of these higher-valent PCVs from countries worldwide, and conclusions regarding overall vaccination strategies remained the same. We also made an assumption regarding the proportion of disease that is pneumococcal since limited information is available on the etiology of pneumonia and AOM. However, we tested this assumption with a scenario analysis reducing the assumed proportion of non-invasive disease that is pneumococcal and observed similar impact. This study does not consider the impact of sequelae following cases of IPD or the long-term impact of pneumonia on future pneumonia risk. This approach is conservative, as we would expect this benefit to only augment the value of the higher-valent vaccines. Another limitation was that we could not consider the impact of adult vaccination within our current model structure; therefore, it is not possible to determine to what extent the adult program impacts the benefits of the infant program. Since PCV vaccination in adults may occur before the use of higher-valent PCVs in the pediatric NIP, we included a scenario analysis in which we only considered disease incidence in those < 18 years of age and still observed significant reductions in disease under a higher-valent vaccination program. Lastly, the distribution of serotypes among non-invasive disease was assumed to vary proportionately to future IPD cases, which may not be true; however, data regarding serotype distribution of pneumococcal pneumonia and AOM are limited.
Conclusion
In the Netherlands, there is minimal PCV10-serotype pneumococcal disease circulating in the population and a substantial proportion of remaining pneumococcal disease burden is attributable to serotypes contained in higher-valent vaccines. As demonstrated in this analysis, switching from PCV10 to PCV13 in the near-term can reduce pneumococcal disease burden across the entire Dutch population. However, higher-valent vaccines are estimated to have greater impact by protecting against currently uncovered serotypes, and switching to PCV20 in 2024 (after its expected licensure in the Netherlands) would provide the greatest public health impact.
References
Australian Government Department of Health. The national notifiable disease surveillance system (NNDSS): pneumococcal disease (invasive). Notifications in Australia 2009 to 2019. NNDSS data current as at 03/12/2020. 2020. https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-pub-datasets.htm. Accessed 28 Oct 2021.
Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the Post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5): e0177113.
Ben-Shimol S, Regev-Yochay G, Givon-Lavi N, Van Der Beek BA, Brosh-Nissimov T, Peretz A, et al. Dynamics of invasive pneumococcal disease in Israel in children and adults in the PCV13 era: a nationwide prospective surveillance. Clin Infect Dis. 2021.
Bertran M, Amin-Chowdhury Z, Sheppard C, Eletu S, Zamarreño DV, Ramsay ME, et al. Increased incidence of invasive pneumococcal disease in children in England: July to December 2021, compared to pre-pandemic years (2017–2019). 2022.
Bloom DE, Kirby PN, Pugh S, Stawasz A. Commentary: why has uptake of pneumococcal vaccines for children been so slow? The perils of undervaluation. Pediatr Infect Dis J. 2020;39(2):145–56.
Casanova C, Küffer M, Leib SL, Hilty M. Re-emergence of invasive pneumococcal disease (IPD) and increase of serotype 23B after easing of COVID-19 measures, Switzerland, 2021. Emerg Microbes Infect. 2021;10(1):2202–4.
CBS StatLine. Deaths; underlying cause of death (shortlist), sex, age. 2019. https://opendata.cbs.nl/statline/#/CBS/en/dataset/7052eng/table?ts=1629076604711. Accessed 24 Aug 2021.
CBS StatLine. Population; sex, age and marital status, 1 January; 1950–2019. 2019. https://opendata.cbs.nl/statline/#/CBS/en/dataset/7461eng/table?ts=1656413471348. Accessed 28 Jun 2022.
CDC. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate baccine on incidence of invasive pneumococcal disease—United States, 1998–2003. Morb Mortal Wkly Rep. 2005;54(36):893–7.
CDC. Centers for Disease Control and Prevention (CDC). Pneumococcal disease: clinical features. 2022. https://www.cdc.gov/pneumococcal/clinicians/clinical-features.html. Accessed 24 Aug 2021.
Cohen O, Knoll M, O’Brien K, Ramakrishnan M, Constenla D, Privor-Dumm L, et al. Pneumococcal conjugate vaccine (PCV) product assessment. Balt MD Johns Hopkins Bloom Sch Public Heal. 2017.
Dagan R, Givon-Lavi N, Shkolnik L, Yagupsky P, Fraser D. Acute otitis media caused by antibiotic-resistant Streptococcus pneumoniae in Southern Israel: implication for immunizing with conjugate vaccines. J Infect Dis. 2000;181(4):1322–9.
De Gier B, Nijsten D, Duijster J, Hahne S. State of infectious diseases in the Netherlands, 2016. 2017.
EMA. European Medicines Agency (EMA). Apexxnar pneumococcal polysaccharide conjugate vaccine (20-valent, adsorbed) summary product characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/apexxnar-epar-product-information_en.pdf. Accessed 27 Jun 2022.
EMA. European Medicines Agency (EMA). Vaxneuvance pneumococcal polysaccharide conjugate baccine (adsorbed) summary product characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/vaxneuvance-epar-product-information_en.pdf. Accessed 27 Jun 2022
Government of Canada. National Laboratory Surveillance of Invasive Streptococcal Disease in Canada - Annual Summary 2019. 2021. https://www.canada.ca/en/public-health/services/publications/drugs-health-products/national-laboratory-surveillance-invasive-streptococcal-disease-canada-annual-summary-2019.html. Accessed 29 Apr 2022.
Hullegie S, Schilder AGM, Marchisio P, de Sévaux JLH, van der Velden AW, van de Pol AC, et al. A strong decline in the incidence of childhood otitis media during the COVID-19 pandemic in the Netherlands. Front Cell Infect Microbiol. 2021;11: 768377.
Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51.
Løchen A, Croucher NJ, Anderson RM. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci Rep. 2020;10(1):18977.
Mangen MJ, Rozenbaum MH, Huijts SM, van Werkhoven CH, Postma DF, Atwood M, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–16.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14.
Melegaro A, Edmunds WJ, Pebody R, Miller E, George R. The current burden of pneumococcal disease in England and Wales. J Infect. 2006;52(1):37–48.
National Institute for Public Health and the Environment. The national immunisation programme in the Netherlands: surveillance and developments in 2018–2019. 2019. https://rivm.openrepository.com/handle/10029/623614. Accessed 28 Jun 2022.
National Institute for Public Health and the Environment. The national immunisation programme in the Netherlands: surveillance and developments in 2019–2020. 2020. https://rivm.openrepository.com/handle/10029/624530. Accessed 28 Jun 2022.
Naucler P, Galanis I, Morfeldt E, Darenberg J, Örtqvist Å, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65(11):1780-90.e1.
Peckeu L, van der Ende A, de Melker H, Sanders E, Knol M. Impact and effectiveness of the 10-valent pneumococcal conjugate vaccine on invasive pneumococcal disease among children under 5 years of age in the Netherlands. Vaccine. 2021;39(2):431–7.
Perdrizet J, Horn E, Hayford K, Grant L, Barry R, Huang L, et al. Historical population-level impact of infant 13-valent pneumococcal conjugate vaccine (PCV13) national immunization programs on invasive pneumococcal disease in Australia, Canada, England & Wales, Israel, and the United States. Infect Dis Ther. 2023 (in press).
Perdrizet J, Lai YS, Williams S, Struwig VA, Wasserman M. Retrospective impact analysis and cost-effectiveness of the pneumococcal conjugate vaccine infant program in Australia. Infect Dis Ther. 2021;10(1):507–20.
Perdrizet J, Santana CFS, Senna T, Alexandre RF, Sini de Almeida R, Spinardi J, et al. Cost-effectiveness analysis of replacing the 10-valent pneumococcal conjugate vaccine (PCV10) with the 13-valent pneumococcal conjugate vaccine (PCV13) in Brazil infants. Hum Vaccin Immunother. 2021;17(4):1162–72.
Perniciaro S, van der Linden M, Weinberger DM. Reemergence of invasive pneumococcal disease in Germany during the spring and summer of 2021. Clin Infect Dis. 2022;75(7):1149–53.
Pfizer. Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) Data. Data on File. October 2020.
Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41.
Pugh S, Wasserman M, Moffatt M, Marques S, Reyes JM, Prieto VA, et al. Estimating the impact of switching from a lower to higher valent pneumococcal conjugate vaccine in Colombia, Finland, and the Netherlands: a cost-effectiveness analysis. Infect Dis Ther. 2020;9(2):305–24.
RIVM. The National Institute for Public Health and the Enviroment (RIVM). The national immunisation programme in the Netherlands: surveillance and developments in 2017–2018. 2018. https://www.rivm.nl/publicaties/national-immunisation-programme-in-netherlands-surveillance-and-developments-in-2017. Accessed 4 Apr 2023.
Rozenbaum MH, Mangen MJ, Huijts SM, van der Werf TS, Postma MJ. Incidence, direct costs and duration of hospitalization of patients hospitalized with community acquired pneumonia: a nationwide retrospective claims database analysis. Vaccine. 2015;33(28):3193–9.
Schurink-van't Klooster T, De Melker H. The national immunisation programme in the Netherlands: surveillance and developments in 2018–2019. 2019.
Shafie AA, Ahmad N, Naidoo J, Foo CY, Wong C, Pugh S, et al. Estimating the population health and economic impacts of introducing a pneumococcal conjugate vaccine in Malaysia—an economic evaluation. Hum Vaccin Immunother. 2020;16(7):1719–27.
Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(1):e51–9.
Thorrington D, van Rossum L, Knol M, de Melker H, Rumke H, Hak E, et al. Impact and cost-effectiveness of different vaccination strategies to reduce the burden of pneumococcal disease among elderly in the Netherlands. PLoS One. 2018;13(2): e0192640.
van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M. The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine. 2012;30(50):7205–13.
Vestjens SMT, Sanders EAM, Vlaminckx BJ, de Melker HE, van der Ende A, Knol MJ. Twelve years of pneumococcal conjugate vaccination in the Netherlands: impact on incidence and clinical outcomes of invasive pneumococcal disease. Vaccine. 2019;37(43):6558–65.
Wasserman M, Palacios MG, Grajales AG, Baez/Revueltas FB, Wilson M, McDade C, et al. Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico. Hum Vaccin Immunother. 2019;15(3):560–9.
Wilson M, Wasserman M, Jadavi T, Postma M, Breton MC, Peloquin F, et al. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71.
Wilson MR, McDade CL, Perdrizet JE, Mignon A, Farkouh RA, Wasserman MD. Validation of a novel forecasting method for estimating the impact of switching pneumococcal conjugate programs: evidence from Belgium. Infect Dis Ther. 2021;10(3):1765–78.
Wilson MR, Wasserman MD, Breton MC, Peloquin F, Earnshaw SR, McDade C, et al. Health and economic impact of routine pediatric pneumococcal immunization programs in Canada: a retrospective analysis. Infect Dis Ther. 2020;9(2):341–53.
Acknowledgements
Funding
This study, including the Journal’s Rapid Service Fee, was sponsored by Pfizer Inc.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Disclosures
This study was funded by Pfizer Inc. Johnna Perdrizet and Vishalini Sundaram were employees of Pfizer Inc. at the time of this study. Vishalini Sundaram is now affiliated with Illumina. Anna Trisia Beby-Heijtel and Angela Waterval-Overbeek were employees of Pfizer BV at the time of this study.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wilson, M., McDade, C., Beby-Heijtel, A.T. et al. Assessing Public Health Impact of Four Pediatric Pneumococcal Conjugate Vaccination Strategies in the Netherlands. Infect Dis Ther 12, 1809–1821 (2023). https://doi.org/10.1007/s40121-023-00828-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00828-8