Abstract
Introduction
Antimicrobial resistance (AMR) is a global public health challenge requiring a global response to which Australia has issued a National Antimicrobial Resistance Strategy. The necessity for continued-development of new effective antimicrobials is required to tackle this immediate health threat is clear, but current market conditions may undervalue antimicrobials. We aimed to estimate the health-economic benefits of reducing AMR levels for drug-resistant gram-negative pathogens in Australia, to inform health policy decision-making.
Methods
A published and validated-dynamic health economic model was adapted to the Australian setting. Over a 10-year time horizon, the model estimates the clinical and economic outcomes associated with reducing current AMR levels, by up to 95%, of three gram-negative pathogens in three hospital-acquired infections, from the perspective of healthcare payers. A willingness-to-pay threshold of AUD$15,000—$45,000 per quality-adjusted life-year (QALY) gained and a 5% discount rate (for costs and benefits) were applied.
Results
Over ten years, reducing AMR for gram-negative pathogens in Australia is associated with up to 10,251 life-years and 8924 QALYs gained, 9041 bed-days saved and 6644 defined-daily doses of antibiotics avoided. The resulting savings are estimated to be $10.5 million in hospitalisation costs, and the monetary benefit at up to $412.1 million.
Discussion
Our results demonstrate the clinical and economic value of reducing AMR impact in Australia. Of note, since our analysis only considered a limited number of pathogens in the hospital setting only and for a limited number of infection types, the benefits of counteracting AMR are likely to extend well beyond the ones demonstrated here.
Conclusion
These estimates demonstrate the consequences of failure to combat AMR in the Australian context. The benefits in mortality and health system costs justify consideration of innovative reimbursement schemes to encourage the development and commercialisation of new effective antimicrobials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study: |
Antimicrobial resistance (AMR) is a global public health challenge requiring a global response, to which Australia has issued a National Antimicrobial Resistance Strategy. |
The necessity for continued development of new effective antimicrobials is required to tackle this immediate health threat is clear, but current market conditions may undervalue antimicrobials. |
This study aimed to assess the current burden of gram-negative hospital-acquired infections (HAIs) in Australia and to estimate the clinical and economic value of reducing AMR. |
What was learned from the study: |
Over 10 years, a theoretical reduction in resistance by 95%, in the three modelled pathogens, is associated with a monetary benefit of $412.1 million to healthcare providers in Australia. |
Significant economic savings associated with reductions in AMR support spending in policy, such as novel reimbursement incentives, aiming to address the threat of AMR. |
Introduction
Antimicrobial resistance (AMR) represents a significant global public health crisis. The increasing prevalence of AMR and subsequent scarcity of treatments for bacterial infections means that more than 700,000 people per year die from antimicrobial-resistant bacterial infections globally [1]. If current trends are not slowed, 2016 estimates suggest that this figure could rise to 10 million lives per year by 2050 [1], with an associated cumulative cost of 100 trillion US dollars (USD). Data from model predictions estimate that, in Australia, resistant bacterial infections kill 290 people annually. This number is predicted to increase to 10,430 people by 2050, with health care costs predicted to reach $521 million Australian dollars (AUD) (~ $370 million USD at the time of submission) [2]. Such modelled predictions of the burden of AMR are important for comparing countries, allocating resources and developing interventions; however, they are limited by uncertainty in the best approach to measure burden, and the availability of appropriate national or local data [3]. Nevertheless, these estimates are supported by a global study of systematic literature reviews and hospital and surveillance systems which highlighted that, in 2019, 1.27 million deaths were due to AMR [4].
In 2015, the World Health Organisation (WHO) published the Global Action Plan on Antimicrobial Resistance, calling for a “one health” response [5]. The Australian government responded by publishing Australia’s First National Antimicrobial Resistance Strategy 2015–2019 [6]. This was then reviewed and replaced by Australia’s First National Antimicrobial Resistance Strategy 2020 and beyond [7]. The 2020 strategy approach broadly aligns with those recommended by the WHO, including the pivot to one health, focussing on education, antimicrobial stewardship (AMS), AMR surveillance, infection control, and governance [6, 7].
While AMR arises whenever and wherever antimicrobials are used, overuse of antimicrobials exerts additional selection pressure on organisms to amplify AMR [8]. The use of heterogeneous antimicrobial agents has been widely documented to lower selection pressure, thus reducing the rate of AMR [9, 10]. Therefore, to tackle the AMR health crisis, there is a requirement for the continued-development of new antimicrobials to provide effective treatment against resistant infections. However, current market conditions have slowed. Despite increasing global use of antibiotics, global revenues have fallen by $13 billion (USD) since 2001, due to a shift towards the increased use of generic antibiotics over branded antibiotics in combination with lower costs for branded antibiotics [11]. The loss of branded antibiotic sales has reduced the spending power for pharmaceutical companies to finance research and development (R&D) of new antimicrobials, resulting in a clinical pipeline that is inadequate to address the increasing emergence and spread of AMR, with multiple reports calculating fewer than 50 antibiotics in all stages of clinical development [12, 13]. Furthermore, as AMS promotes the reduction of unnecessary antimicrobial use, effective treatments are often reserved as a last resort for multi-resistant organisms. This, combined with low prices, high development costs and the short product life of antimicrobials, results in limited economic returns for manufacturers [12, 14]. Consequently, pharmaceutical companies have been abandoning the market or, in some cases, forced into bankruptcy [15, 16]. Even when new antimicrobial agents are introduced to the market, low commercial viability has led to delayed or withdrawn commercialisation, even in high income countries [17].
It is important for policy and decision-makers to recognise the value of reducing AMR, when allocating resources to address these issues. The objective of this study was to quantify the clinical and economic value of reducing projected AMR levels for healthcare payers in Australia, in the management of serious gram-negative hospital-acquired infections (HAI).
Methods
Overview
In this study, we have adapted the deterministic treatment pathway component of a previously published and validated dynamic model of AMR [18] to an Australian setting. Health economic outcomes were assessed as a function of varying AMR projections of three gram-negative pathogens (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa) in treating three of the most common HAIs: complicated urinary tract infections (cUTIs), complicated intra-abdominal infections (cIAIs), and hospital-acquired pneumonia including ventilator-associated pneumonia (HAP/VAP). The pathogens and HAIs were selected as they account for a significant proportion of deaths associated with AMR globally [4]. Analyses were conducted over a 10-year time horizon, from a healthcare payer’s perspective. A time horizon of 10 years was considered to appropriately demonstrate the benefits of reduced AMR that continually accrue over time, when balanced with the uncertainty associated with estimating outcomes over the long-term.
Model Structure
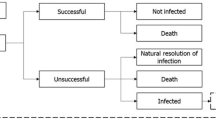
The model utilised the deterministic treatment pathway component of a previously developed AMR cost-effectiveness model to evaluate input–output relationships [18]. The deterministic treatment pathway (Fig. 1) evaluates the clinical and economic impact of a specified treatment strategy, whereby all infected patients are treated according to a pre-determined treatment sequence. Patients are either cured (successfully treated or the infection naturally resolves), die from infection or remain infected. Patients who are unsuccessfully treated remain infected and progress onto the second-line antimicrobial in the treatment pathway. Patients who remain infected and have exhausted all available treatment options, and fail to clear the infection naturally, are assumed to die 3 days after their last treatment as a result of their infection. The probability of successful treatment is determined by the efficacy of the antimicrobial treatment received and the susceptibility of the pathogen. The duration of unsuccessful treatment is assumed to be 2 days for all pathogens and indications. After this time, it is likely that the efficacy of a treatment in an individual patient will be known, and patients not responding to treatment would discontinue to a subsequent treatment line if available. The model considered a simplified treatment strategy with piperacillin/tazobactam used as the first-line treatment and meropenem as the second-line treatment; this was considered to most closely represent the current treatment approach for the modelled HAIs and pathogens in Australia [19]. All treatments were considered to have equivalent efficacy against susceptible pathogens, and resistant infections may only resolve naturally or result in unsuccessful treatment.

Source: Gordon et al. [18]
Model deterministic treatment pathway.
Australian-specific inputs were applied to estimate the clinical and economic value [hospital length of stay (LOS), defined-daily dose (DDD) of antibiotics, quality-adjusted life-years (QALYs)], life-years (LY), hospitalisation costs and monetary benefit] associated with reducing AMR levels, from the perspective of healthcare payers in Australia. LYs and QALYs were assessed over the lifetime of the infected patient population, based on the infections gained-during the 10-year model time horizon. The life expectancy of the modelled population after successful treatment is based on an average age of 67 years among those with HAIs, estimated using life tables for general population in Australia [20, 21]. Monetary benefit was estimated according to the following equation:
Model Settings
Pathogen-specific resistance levels for each treatment in Australia, presented in Table 1, were estimated using the data from the 2021 Antimicrobial Use and Resistance in Australia (AURA) Surveillance System report of antimicrobial use and resistance [22].
The total annual number of hospital-acquired cUTI, cIAI and HAP/VAP infections and the distribution of E. coli, K. pneumoniae and P. aeruginosa HAIs are presented in Table 1. As no national surveillance of HAIs has been conducted in Australia [23], the annual number of infections was estimated through published literature. A systematic literature review in 2017 suggested that the overall incidence of HAIs may be close to 165,000 per year [23]. The distribution of cUTI, cIAI and HAP/VAP infections and the frequency of pathogens (E. coli, Klebsiella spp. and P. aeruginosa) responsible for each indication were estimated from a point prevalence investigation of the prevalence of HAIs in Australia [20] and a study of microorganisms found in cIAIs in Australia [24]. These estimates were applied to the annual number of HAIs in Australia to estimate the annual infection incidence of hospital-acquired cUTI, cIAIs and HAP/VAP. Further details on the calculations of the annual number of infections can be found in Supplementary Table 1.
Where possible, Australian-specific sources were used for key model inputs; inputs and sources are presented in Table 2. Indication-specific model inputs for on-treatment utility, mortality and hospitalisation costs are presented in Supplementary Table 2. Local clinical experts provided assumptions based on their clinical experience in Australia for inputs relating to clinical practice and where no appropriate published-data could be sourced.
Analysis
Clinical and economic outcomes were evaluated over a 10-year time horizon based on current antimicrobial resistance levels in Australia as well as under four alternative scenarios where resistance levels were reduced by 10%, 20%, 50% and 95%. These scenarios were selected to reflect achievable reductions in AMR (10–50%) and a perfect-world scenario where all resistance is removed; clinical experts suggested 95% rather than 100% as resistance is inherent and cannot be completely eradicated. The pooled resistance of modelled treatments to organisms of interest under the alternative AMR scenarios is presented in Supplementary Table 3. Analyses were evaluated with a willingness-to-pay (WTP) threshold of $15,000—$45,000 per QALY gained and a 5% discount rate, in line with thresholds and discounting used in Australia [25, 26]. Absolute outcomes were assessed for each resistance level scenario, and incremental costs are presented for the alternative scenarios versus current AMR levels. Costs are expressed as AUD (2021).
Sensitivity Analysis
One-way sensitivity analyses (OWSA) were conducted on key model input parameters, listed in Table 2. Each input was adjusted by ± 20% to assess the impact on monetary benefit (according to a WTP threshold of $45,000), in the scenario where current AMR levels were reduced by 50%. Two further scenarios were explored. One applied a 1.5% discount rate, anticipating potential changes to the HTA guidance in Australia. The second adjusted the probability of infections naturally resolving (up to 30%), to assess the uncertainty of this assumption informed by clinical experts. A range up to 30% was selected based on published literature [27, 28].
Ethics Approval
The analysis in this article is based on publicly available data and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Absolute outcomes based on the current AMR levels in Australia are presented in Table 3. It is estimated that, under current AMR conditions, the burden of the infected patient population is associated with the utilisation of 510,520 hospital bed-days, equating to $594.4 million in hospitalisation costs, and 28,223 LYs lost due to infection, corresponding to 24,860 QALYs lost, over 10 years.
Clinical and economic outcomes in scenarios where current resistance levels are reduced by 10%, 20%, 50% and 95% are presented in Table 3. Over a 10-year period, reducing AMR levels by 95% is estimated to save up to 9041 hospital bed-days, corresponding to $10.5 million in hospitalisation costs (Fig. 2). This is associated with up to 10,251 LYs and 8924 QALYs gained over a lifetime (based on the number of infections per year over a 10-year period) (Fig. 3). The associated monetary benefit achieved over a lifetime (by reducing current AMR levels by 10–95%) ranged from $15.9 million to $412.1 million based on a WTP threshold ranging from $15,000 to $45,000 per QALY gained (Fig. 4).
Sensitivity Analysis
In the OWSA, variation in estimates for treatment efficacy (i.e. the probability of successful treatment to non-antimicrobial resistant infections) had the greatest impact on health economic outcomes, with the monetary benefit varying from $94.7 million to $372.2 million, in response to a ± 20% change in efficacy. Other key drivers include the probability of naturally resolving infection, annual infected population size and utility (not infected); results of the OWSA are presented in Fig. 5.
Discussion
To the authors’ knowledge, this is the first study of its kind to assess the economic and clinical value of reducing the impact of AMR in Australia. Our results indicate that reducing AMR in Australia can provide considerable clinical and economic benefits from a healthcare system perspective. A theoretical reduction in AMR of 95% would translate into monetary benefits of $412.1 million over a 10-year period; a potentially achievable reduction of 10–50% would still lead to a monetary benefit between $15.9 million and $222.2 million. This estimation was based on a WTP threshold of $45,000 and represents the maximum investment it is worth the health system making to support decision-making on AMR strategies. Our results are conservative when compared to a 2022 analysis of the health economic burden of AMR in Australian hospitals, which estimated that AMR infections result in 27,705 QALYs being lost and in $72 million in hospitalisation costs, over a 1-year period [29]. Our conservative estimate can be explained by two key differences in approach. Firstly, our model only considers an annual incidence of 9653 compared to 21,663, due to the 2022 analysis including a broader range of infections, and alternative approaches in estimating national level incidence. Secondly, and more importantly, the 2022 analysis assumed an alternative scenario in which all AMR infections were replaced by no infection, whereas we assumed that infections would still occur but only the infection resistance status changed [29]. Based on the results presented here, Australia could gain substantially from contributing to the global efforts against AMR; in addition, the COVID-19 pandemic has highlighted the broader economic impact of infectious diseases beyond healthcare systems, including considerable societal costs. This analysis can be used to support decision-makers in Australia investigating alternative approaches to encourage the commercialisation of new antibiotics, such as novel reimbursement schemes that aim to capture the full value of antimicrobials in the Australian market.
It is worth highlighting that, if reducing AMR levels is linked to substantial benefit, AMR increase according to current trends would translate into substantial economic cost. In an exploratory scenario where AMR rates of Indonesia (39.5% in 2015) [2] were used in the Australian setting, an additional monetary burden of $8.8 billion (at a WTP threshold of $45,000/QALY) over 10 years, was estimated, compared to the current burden.
Our conclusions must be interpreted in the context of the study limitations. Recent surveys show that, in Australia, appropriateness of prescribing in hospitals is approximately 70%, indicating that there is still room for improvement [22]. However, the dire consequences of antimicrobial failure means that situations such as sepsis require initial and immediate use of broad spectrum antibiotics [30]. Due to this complexity, it is not possible to estimate the exact contribution of AMS strategies. In addition, while this analysis is intended to demonstrate the value in reducing the risk of antibiotic failure due to AMR, it does not consider the additional costs, associated with both human and health resources, of implementing approaches such as AMS to achieve this. Furthermore, future health costs associated with improved survival are not included, and the feasibility of achieving the reductions in AMR analysed have not been validated. Additional caveats to the study include that inputs for the number of annual infections had to be calculated using data from the literature and expert opinion, due to the limited available data on the indications and organisms of interest in Australia (Supplementary Table 1). Two other model assumptions, both informed by expert clinical opinion, include the use of an average treatment efficacy for all treatments and indications, and an assumption that patients who are unsuccessfully treated and have exhausted all available treatments die after 3 days. Resistance does not confer increased mortality, although it may be a mechanism for increased virulence and therefore mortality, but some case–control trials have shown no direct impact from antimicrobial susceptibility [31, 32]. As with all modelling studies, such assumptions should be taken into consideration when interpreting model estimates. In the current model, such assumptions may introduce uncertainty in the absolute outcomes; however, incremental outcomes comparing scenarios of alternative AMR will remain broadly consistent. The sensitivity of the model to key inputs was tested in sensitivity analyses. The model only considered a limited number of HAIs and pathogens; thus, it is likely that the analysis underestimates the true benefit of reducing AMR in the Australian system. However, due to limited availability of data, estimating the broader impact of AMR reduction would have required substantial assumptions and increased model complexity, that could hinder the robustness of our estimation. Modelling AMR is complex, this analysis takes a deterministic approach and does not capture the transmission dynamics of infectious diseases; changes to resistance and incidence of infection rates are likely to change throughout the modelled time horizon. For this reason, the time horizon was not extended beyond 10 years as the uncertainty associated with these assumptions may impact the suitability of the analysis to support decision-making; however, analysis over 10 years is appropriate to inform medium- to long-term policy decisions. A two-line treatment strategy was used as a simplifying assumption, although in practice there may be additional treatment options available, such as cephalosporins. Including a third-line treatment with similar resistance levels to current options to the model would alter the absolute outcomes, causing an increase in hospital resource use and costs, but reducing deaths; however, the incremental outcomes comparing alternative AMR scenarios are more robust to this change. Average daily hospitalisation costs specific to each indication were applied to patient LOS; however, patients experience one-off costs at the start of their treatment, including diagnostic tests. Therefore, hospitalisation costs for patients in the model with shorter LOS may be conservative, as these up-front costs are spread over the average LOS and not fully accounted for. Finally, assumptions around benefits of treatment are difficult, as they are heavily influenced by both host and pathogen factors [33]; due to data constraints, the model could not account for variations in treatment efficacy in different treatment lines.
Successful initial antibiotic treatment, as opposed to no effective treatment in the first 36 h, yielded a 16% survival benefit in comparable real-world studies of British hospitalised pneumonia [34], and antimicrobial resistance is thought to approximately double infection mortality overall [1]. Complete antibiotic failure in severe sepsis has long been recognised as resulting in very high mortality in controlled models [35], as well as in human look-back studies of patients presenting to US and Canadian hospitals [36]. Sepsis causes up to half of all in-hospital deaths, and the three pathogens are the agent of sepsis in 58.8% of Australian bacteraemias [37]. The value of antibiotics is equally hard to define, and antimicrobials are still being considered based on traditional criteria which may not capture their full value to healthcare payers. A framework has been proposed to capture the unique value elements associated with antimicrobials, described with the acronym STEDI (spectrum, transmission, enablement, diversity, and insurance). However, the attributes of new and narrow spectrum antibiotics and how these impact on future transmission, resistance development (diversity) and outbreak readiness (insurance), and how these impact the enablement of other medical procedures, such as chemotherapy and surgery, are not considered in the current HTA process in Australia. Therefore value beyond QALYs are not captured and does not adequately inform funding decisions [38]. Furthermore, current reimbursement schemes fail to consider that the use of antimicrobials needs to be restricted through stewardship strategies to preserve their effectiveness, thus leading to inadequate return on investment for most pharmaceutical companies. It is important to note that the research presented here does not attempt to estimate the value of a new antimicrobial, so the STEDI framework is not fully applicable; however, we could have considered the impact of reducing AMR on enablement value. There have been previous efforts to estimate the value of antimicrobials taking into consideration transmission and diversity value, and future research efforts are required to estimate the enablement, spectrum and insurance value [18, 39, 40]. This highlights the need to explore alternative reimbursement frameworks that could encourage their commercialisation, once approved within Australia. “Push” and “pull” incentives are being considered to encourage R&D efforts from the pharmaceutical industry. “Push” incentives directly fund research into the development of new antibiotics, whilst reward-based “pull” incentives are associated with providing revenue-generating benefits to companies for successfully introducing a new drug to the market.
Novel reimbursement mechanisms aim to act as “pull” incentives by ensuring revenue for the new treatment by de-linking revenue from the volume of sales, thus reducing the risk to returns from AMS that protects a valuable new antimicrobial. Subscription-based reimbursement mechanisms have been explored in the NICE and NHS England in the UK [16, 41, 42], and in the PASTEUR act introduced to Congress in the USA [43]. It is estimated globally that subscription-based-de-linked “pull” incentives should be valued between $2.2 and $4.8 billion (USD) over 10 years to sufficiently incentivise R&D [44, 45]. The development of a “pull” incentive, similar to that trialled in the UK and the PASTEUR act in the US, would help to re-incentivise R&D of novel antimicrobials, while promoting the appropriate use of new antimicrobials, giving clinicians the ability to prescribe the most appropriate treatment. Such a reimbursement scheme would require a collaborative approach between both Federal and State health systems to address current barriers. Action would position Australia as one of the leading nations in addressing the burgeoning AMR crisis, just as coordinated global efforts are required to limit carbon dioxide emissions.
Conclusion
This analysis demonstrates the substantial clinical and economic benefits of reducing AMR in Australia, as well as the dangers of not addressing the issue, which can be used by policy and decision-makers to develop novel methods to incentivise R&D efforts and commercialisation, alongside the Australian National AMR strategy, in Australia.
References
Jim O'Neill (Chair). Tackling drug-resistant infections globally: final report and recommendations. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
OECD. Stemming the Superbug Tide. 2018. https://www.oecd.org/health/stemming-the-superbug-tide-9789264307599-en.htm.
Wernli D, Jørgensen PS, Harbarth S, Carroll SP, Laxminarayan R, Levrat N, et al. Antimicrobial resistance: the complex challenge of measurement to inform policy and the public. PLoS Med. 2017;14(8): e1002378.
Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles-Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2019;399(10325):629–55.
World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1. Accessed 15 Jan 2021.
Australian Government. Australia’s first national antimicrobial resistance strategy 2015–2019. 2015. https://www.amr.gov.au/resources/national-amr-strategy.
Australian Government. Australia's national antimicrobial resistance strategy 2020 and beyond. 2020. https://www.amr.gov.au/resources/australias-national-antimicrobial-resistance-strategy-2020-and-beyond.
Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–83.
Piper GL, Kaplan LJ. Antibiotic heterogeneity optimizes antimicrobial prescription and enables resistant pathogen control in the intensive care unit. Surg Infect (Larchmt). 2012;13(4):194–202.
Sandiumenge A, Diaz E, Rodriguez A, Vidaur L, Canadell L, Olona M, et al. Impact of diversity of antibiotic use on the development of antimicrobial resistance. J Antimicrob Chemother. 2006;57(6):1197–204.
Madden J, Outterson K. Trends in the global antibiotics market. Nat Rev Drug Discov. 2023;22(3):174.
World Health Organization. Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. 2019. https://www.who.int/publications/i/item/9789240000193. Accessed Sep 2021.
McCall B. New fund stimulates the ailing antibiotic pipeline. Lancet Infect Dis. 2020;20(9):1017.
McKenna M. The antibiotic paradox: why companies can’t afford to create life-saving drugs. Nature. 2020;584(7821):338–41.
AMR Industry Alliance. AMR Industry Alliance, 2020 Progress Report. Geneva, Switzerland. 2020.
Rothery C, Woods B, Schmitt L, Claxton K, Palmer S, Sculpher M. Framework for value assessment of new antimicrobials. Sheffield: EEPRU. 2018.
Outterson K, Orubu ESF, Rex J, Årdal C, Zaman MH. Patient access in fourteen high-income countries to new antibacterials approved by the FDA, EMA, PMDA, or Health Canada, 2010–2020. Clin Infect Dis. 2021;74(7):1183–90.
Gordon J, Darlington O, McEwan P, Lumley M, Taie A, Hicks M, et al. Estimating the value of new antimicrobials in the context of antimicrobial resistance: development and application of a dynamic disease transmission model. Pharmacoeconomics. 2020;38(8):857–69.
Therapeutic Guidelines [digital]. Antibiotic Melborne. 2021. https://tgldcdp.tg.org.au/guideLine?guidelinePage=Antibiotic&frompage=etgcomplete.
Russo PL, Stewardson AJ, Cheng AC, Bucknall T, Mitchell BG. The prevalence of healthcare associated infections among adult inpatients at nineteen large Australian acute-care public hospitals: a point prevalence survey. Antimicrob Resist Infect Control. 2019;8(1):114.
Australian Bureau of Statistics. Statistics about life tables for Australia, states and territories and life expectancy at birth estimates for sub-state regions (2017–2019). 2020. https://www.abs.gov.au/statistics/people/population/life-tables/latest-release.
Australian Commission on Safety and Quality in Health Care. AURA 2021: fourth Australian report on antimicrobial use and resistance in human health. Sydney. 2021. https://www.safetyandquality.gov.au/sites/default/files/2021-09/aura_2021_-_report_-_final_accessible_pdf_-_for_web_publication.pdf.
Mitchell BG, Shaban RZ, MacBeth D, Wood CJ, Russo PL. The burden of healthcare-associated infection in Australian hospitals: a systematic review of the literature. Infect Dis Health. 2017;22(3):117–28.
Tan A, Rouse M, Kew N, Qin S, La Paglia D, Pham T. The appropriateness of ceftriaxone and metronidazole as empirical therapy in managing complicated intra-abdominal infection-experience from Western Health. Austr PeerJ. 2018;6: e5383.
The Pharmaceutical Benefits Scheme Australia. Public summary document—July 2019 PBAC Meeting 7.02 BEZLOTOXUMAB. 2019. https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2019-07/files/bezlotoxumab-psd-july-2019.pdf. Accessed Sep 2021.
The Pharmaceutical Benefits Scheme Australia. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee (Version 5.0). 2016. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf. Accessed Sep 2021.
Henderson A, Bursle E, Stewart A, Harris PNA, Paterson D, Chatfield MD, et al. A systematic review of antimicrobial susceptibility testing as a tool in clinical trials assessing antimicrobials against infections due to gram-negative pathogens. Clin Microbiol Infect. 2021;27(12):1746–53.
Tong SYC, Lewis RJ, Morpeth SC. The tension between clinical and microbiological relevance in applying clinical trial results for Gram negative bacterial infections. Clin Microbiol Infect. 2021;27(12):1733–5.
Wozniak TM, Dyda A, Merlo G, Hall L. Disease burden, associated mortality and economic impact of antimicrobial resistant infections in Australia. Lancet Regional Health Western Pac. 2022;2022:27.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:11.
Rottier WC, Deelen JWT, Caruana G, Buiting AGM, Dorigo-Zetsma JW, Kluytmans JAJW, et al. Attributable mortality of antibiotic resistance in gram-negative infections in the Netherlands: a parallel matched cohort study. Clin Microbiol Infect. 2021;27(5):742–9.
Lee XJ, Stewardson AJ, Worth LJ, Graves N, Wozniak TM. Attributable length of stay, mortality risk, and costs of bacterial health care-associated infections in australia: a retrospective case-cohort study. Clin Infect Dis. 2021;72(10):e506–14.
Peeters P, Ryan K, Karve S, Potter D, Baelen E, Rojas-Farreras S, et al. The impact of initial antibiotic treatment failure: real-world insights in patients with complicated, health care-associated intra-abdominal infection. Infect Drug Resist. 2019;12:329–43.
Daniel P, Rodrigo C, McKeever TM, Woodhead M, Welham S, Lim WS. Time to first antibiotic and mortality in adults hospitalised with community-acquired pneumonia: a matched-propensity analysis. Thorax. 2016;71(6):568.
Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193(2):251–8.
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:6.
Coomb G, Bell J, Daley D, Collignon P, Cooley L, Gottlieb T, et al. Australian Group on Antimicrobial Resistance Sepsis Outcomes Programs: 2019 Report. In: Australian Commission on Safety and Quality in Health Care. 2021.
Hillock NT, Merlin TL, Karnon J, Turnidge J, Eliott J. Value assessment of antimicrobials and the implications for development, access, and funding of effective treatments: Australian stakeholder perspective. Int J Technol Assess Health Care. 2021;37(1): e28.
Barmpouni M, Gordon JP, Miller RL, Pritchard CRJ, Dennis JW, Grammelis V, et al. Estimating the clinical and economic impact of introducing a new antibacterial into Greek clinical practice for the management of hospital-acquired infections with limited treatment options. Infect Dis Therapy. 2023;12(2):527–43.
Matsumoto T, Yuasa A, Miller R, Pritchard C, Ohashi T, Taie A, et al. estimating the economic and clinical value of introducing ceftazidime/avibactam into antimicrobial practice in Japan: a dynamic modelling study. PharmacoEconomics Open. 2023;7(1):65–76.
Adler A, Cooper S, Crabb N. New ways of evaluating and purchasing antimicrobials. Lancet Infect Dis. 2022;22(11):1542.
Glover RE, Singer AC, Roberts AP, Kirchhelle C. The antibiotic subscription model: fostering innovation or repackaging old drugs? The Lancet Microbe. 2023;4(1):e2–3.
S.4760—116th Congress: the PASTEUR Act. 2020. https://www.congress.gov/bill/116th-congress/senate-bill/4760/text?r=2&s=1. Accessed 3 Sep 2021.
Outterson K. Estimating The Appropriate Size Of Global Pull Incentives For Antibacterial Medicines. Health Aff. 2021;40(11):1758–65.
Towse A, Bonnifield RS. An ambitious USG advanced commitment for subscription-based purchasing of novel antimicrobials and its expected return on investment. Center for Global Development [Internet]. 2022. https://www.cgdev.org/publication/ambitious-usg-advanced-commitment-subscription-based-purchasing-novel-antimicrobials.
Pfizer Austalia. Australian Product Information—TAZOCIN EF (PIPERACILLIN/TAZOBACTAM) Powder for injection. 2021. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-06048-3. Accessed Sept 2021.
McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. 2016;14(1):133.
Acknowledgements
Funding
This work was supported by Pfizer Australia who provided support for the model development/analysis and medical writing for this study. Pfizer Australia are also funding the journal’s Rapid Service fee.
Author Contributions
Jason Gordon and Amer Al-Taie conceptualised and designed the study. Ryan Miller was responsible for data analysis. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Disclosures
David. C. Grolman and Jacqueline Youssef are employees of Pfizer Australia. Amer Al-Taie is an employee of Pfizer R&D UK. Jason Gordon, Ryan Miller and James Dennis are employees of Health Economics and Outcomes Research Ltd. Health Economics and Outcomes Research Ltd. received fees from Pfizer, Australia in relation to this study. Mark A. T. Blaskovich receives government and industry funding for antibiotic discovery and development, and has attended conferences sponsored by Pfizer, Australia. Jonathan Iredell receives government funding for antibiotic resistance research and has attended conferences sponsored by Pfizer, Australia. John Turnidge and Geoffrey W. Coombs have no conflicts of interest.
Compliance with Ethics Guidelines
The analysis in this article is based on publicly available data and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
The model itself that was used in this study is not available as it is proprietary, but the datasets generated-during and/or analysed-during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gordon, J.P., Al Taie, A., Miller, R.L. et al. Quantifying the Economic and Clinical Value of Reducing Antimicrobial Resistance in Gram-negative Pathogens Causing Hospital-Acquired Infections in Australia. Infect Dis Ther 12, 1875–1889 (2023). https://doi.org/10.1007/s40121-023-00835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00835-9