Abstract
Introduction
Hospital-acquired infections (HAIs) and growing antimicrobial resistance (AMR) represent a significant healthcare burden globally. Especially in Greece, HAIs with limited treatment options (LTO) pose a serious threat due to increased morbidity and mortality. This study aimed to estimate the clinical and economic value of introducing a new antibacterial for HAIs with LTO in Greece.
Methods
A previously published and validated dynamic model of AMR was adapted to the Greek setting. The model estimated the clinical and economic outcomes of introducing a new antibacterial for the treatment of HAIs with LTO in Greece. The current treatment pathway was compared with introducing a new antibacterial to the treatment sequence. Outcomes were assessed from a third-party payer perspective, over a 10-year transmission period, with quality-adjusted life years (QALYs) and life years (LYs) gained considered over a lifetime horizon.
Results
Over the next 10 years, HAIs with LTO in Greece account for approximately 1.4 million hospital bed days, hospitalisation costs of more than €320 million and a loss of approximately 403,000 LYs (319,000 QALYs). Introduction of the new antibacterial as first-line treatment provided the largest clinical and economic benefit, with savings of up to 93,000 bed days, approximately €21 million in hospitalisation costs and an additional 286,000 LYs (226,000 QALYs) in comparison to the current treatment strategy. The introduction of a new antibacterial was linked to a monetary benefit of €6.8 billion at a willingness to pay threshold of €30,000 over 10 years.
Conclusion
This study highlights the considerable clinical and economic benefit of introducing a new antibacterial for HAIs with LTO in Greece. This analysis shows the additional benefit when a new antibacterial is introduced to treatment sequences. These findings can be used to inform decision makers to implement policies to ensure timely access to new antibacterial treatments in Greece.
Plain Language Summary
Antimicrobial resistance is a major issue for the Greek healthcare system. The overuse of antibacterial agents contributes to the growing resistance levels, making currently available treatment options less effective. As a result, there is an imperative need to address antimicrobial resistance in Greece. This study developed a mathematical model to investigate the clinical and economic benefits of introducing a new antibacterial to current treatment practice. The model uses regression equations to describe the relationships between inputs and outputs from a published and validated model, which describes the transmission and treatment of infections. The model is used to estimate the impact of a new treatment in Greece, considering differing treatment sequence scenarios. The largest health and financial benefits were seen when a new antibacterial was introduced at first line prior to currently used treatments. Over 10 years, savings of up to 93,000 hospital bed days and €21 million in hospitalisation costs could be achieved, as well as a gain of 286,000 patient life years and 226,000 patient quality-adjusted life years (QALYs), a measure of a patient’s quality and length of life, over their remaining lifetime. The introduction of a new antibacterial into the current treatment pathway resulted in an overall monetary benefit of €6.8 billion over 10 years, when additional QALYs are valued at €30,000. This study demonstrates considerable health economic benefits of introducing a new antibacterial in Greece and can help inform decision makers when developing a national action plan to combat resistance and improve access to treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Increasing antimicrobial resistance (AMR) in hospital-acquired infections (HAIs) with limited treatment options (LTO) represents an important challenge for the healthcare system in Greece; rates of HAIs are 50% higher in Greece, with a significantly higher burden of multidrug-resistant infections than the rest of Europe. |
To combat HAIs with LTO there is a need to both preserve the effectiveness of current treatments by decreasing AMR emergence and spread, and to improve access to new effective treatments. |
This study aimed to assess the current burden of HAIs with LTO and to estimate the clinical and economic value of introducing a new antibacterial agent in Greece. |
What was learned from the study? |
The analysis showed that introduction of a new antibacterial agent in the management of HAIs with LTO in Greece could generate considerable clinical and economic benefits, representing a monetary benefit to healthcare providers of up to €6.8 billion, over 10 years; the greatest benefits were recognised when the new antibacterial agent was introduced to the treatment sequence as the first line. |
This analysis can support decision makers on implementing an effective AMR national action plan to alleviate AMR burden and encourage access to new treatments in Greece. |
Introduction
Hospital-acquired infections (HAIs) are a major public health concern, representing a threat to patient safety and a substantial economic burden globally [1]. Patients with HAIs have an 80% increased 90-day risk of death after admission to the hospital compared to those without (HR 1.8%; 95% CI 1.3–2.6) [2]. Greece is amongst the European countries worst affected by HAIs [2,3,4,5,6,7,8,9]. According to a study from the European Center for Disease Prevention and Control (ECDC) in which 33 European countries participated, it was estimated that the rate of HAIs in public hospitals in Greece is 50% higher than the European average (9.1% vs 6%) [2, 3, 5, 10]. Further compounding this issue, Greece, alongside Italy, has a significantly higher burden of infections caused by multidrug-resistant (MDR) bacteria than the rest of Europe. In Greece, a high proportion of this infection burden is caused by gram-negative pathogens resistant to carbapenems or colistin [5].
In Greece, gram-negative MDR pathogens present a major threat for both clinical medicine and public health by causing infections for which few effective antimicrobials are available [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. In addition, the SARS-CoV-2 pandemic has further complicated the epidemiological situation, as it is associated with increased morbidity and mortality of patients with COVID-19 due to secondary bacterial infections [15].
Some of the most frequently isolated pathogens in hospitals in Greece, with particularly high rates of resistance, are Klebsiella spp., E. coli, Acinetobacter and Pseudomonas aeruginosa [8]. Resistance developed amongst these pathogens against established antibiotics (cephalosporins, carbapenems, colistin, tigecycline, fosfomycin, aminoglycosides) is highly concerning to the medical community. Especially against carbapenems, which are considered the last treatment option for many infections, the resistance of these pathogens (with the exception of E. coli) in Greece in 2020 ranged between 36% and 95% [16]. Meanwhile, the limited available treatment options are associated with increased adverse effects, off-label administration and use as salvage therapies that have limited effectiveness and increased treatment costs [6,7,8,9, 11,12,13,14,15, 17, 18]. Thus, these pathogens are considered by the World Health Organization (WHO) as the most dangerous resistant bacteria and are ranked as top priority for research and development of new antimicrobials [19].
Therefore, a multilevel strategy is important to curb the entry and spread of these highly resistant bacteria in hospitals. This is outlined in the European Union (EU) Council Recommendation on patient safety, including the prevention and control of HAIs [20]. Furthermore, in order to tackle increasing AMR, the WHO published the Global Action Plan on Antimicrobial Resistance in 2015, calling for an integrated “one health” response, involving international organisations, with the aim of optimising the health of people, animals and the environment by coordinated actions [21]. The Global Action Plan called on supporting countries to implement national action plans to achieve the objectives of increased awareness, generating evidence, infection control, stewardship and developing an economic case for investment [21]. In 2017, the EU devised a European One Health Action Plan against AMR, centred around the one health approach [1]. Its aim was to preserve the effectiveness of current treatments for infections in both humans and animals by decreasing AMR emergence and spread, and to increase new effective antimicrobial deployment and availability [1].
In that context, the Ministry of Health in Greece has developed a series of actions and policies that show advancements in the prioritisation of AMR and recognition by policy makers of the crucial nature of AMR reduction. The establishment of a stewardship group per hospital that monitors the implementation of prescribing guidelines and evaluates the consumption of antibiotics in comparison with the relevant AMR levels has been a critical step in the AMR battle [22]. Moreover, in 2020, the Ministry of Health created the Agency for Quality Assurance in Health S.A. (AQAH SA), which is responsible, among others, for the implementation of educational programmes on (nosocomial) infection prevention and control [23]. However, Greece is yet to fully implement a country-specific AMR national action plan that ensures a homogenous uptake of these measures [24]. Limited availability of local data makes it difficult to conduct robust estimations of the economic burden of HAIs and of AMR in general in Greece and may have contributed to the delays and the hesitancy in policy-making.
To provide valuable insights to decision makers on the benefits of addressing AMR and highlight the imperative need and value of new antibacterial agents, the present study sought to assess the current burden of HAIs with LTO and estimate the clinical and economic value of introducing a new antibacterial agent in Greece.
Methods
Overview
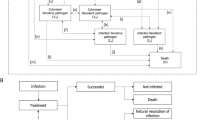
On the basis of a previously published and validated dynamic model of AMR [25], we developed a simplified model that aims to demonstrate the value of a new antibacterial. Regression equations were used to describe the relationships between key inputs and outputs that characterise the transmission dynamics of HAIs, described in the original model, and allowed the estimation of outcomes, in the Greek setting, whilst reducing the complexity and data requirements of the analysis [25]. The regression equations were derived by running over 1 million simulations of the original dynamic transmission model [25], including varying key model inputs (population, baseline resistance, treatment strategy, treatment duration and treatment efficacy) to generate a data set of input–output relationships. Outputs captured included time on treatment and mortality. Input values used to run the simulations in the original model to generate the regression equations are provided in Supplementary Table 1. In the current model, inputs were sourced from the literature and were validated from two local infectious diseases experts to ensure that they fully reflect the current clinical Greek practice (Tables 3 and 4). These local inputs were used in the regression equations to estimate the clinical and economic impact of introducing a new antibacterial as compared to the current treatment strategy in Greece. The underlying transmission dynamics and prevalence of infection in the original model were calibrated to UK data and were assumed to be applicable to Greece for the generation of regression equations. The model considered outcomes associated with treating HAIs with LTO, responsible for a considerable burden in Greece; the HAIs with LTO and responsible pathogens considered in the model are summarised in Table 1. Since it is less likely for a new treatment to be effective against all pathogens, the analysis followed a more realistic and moderate approach and, therefore, excludes infections caused by Acinetobacter from the model.
Model Structure
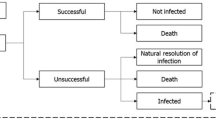
Infected patients are assigned to either current treatment pathway or to an alternative treatment pathway which includes the introduction of a new antibacterial. The current treatment pathway includes meropenem as the first-line treatment and colistin as the second-line treatment; this was assumed to be a best representation of current clinical practice in Greece when considering a simplified two-line treatment strategy and was informed by local expert clinical opinion.
Patients receiving first-line treatment are either cured (via successful treatment or natural resolution of the infection), remain infected or die. If treatment fails to resolve the infection, patients move to the next line of treatment (Fig. 1). It was assumed that patients who have exhausted all available treatment lines without achieving a successful resolution of the infection die from infection 3 days after receiving their last treatment. The model accounts for the development of resistance to all modelled treatments over time as a consequence of increased treatment exposure. Changes to resistance rates are captured implicitly within the regression equations based on the dynamic AMR model.

Source: Gordon et al. (2020) [25]
Deterministic treatment pathway.
As a result of differences in treatment characteristics (efficacy and resistance levels), treatment sequence may impact patients’ clinical course and population-level AMR levels. Thus, we explored three alternative strategies, where the new antibacterial’s use is varied, i.e. it is introduced either as a third-, second- or first-line treatment (Table 2). Additionally, the impact of treatment diversification was also explored to demonstrate the impact of stewardship practices, yielding in total six alternative treatment scenarios. In treatment diversification, the patient population was split evenly between all three treatments, as their first-line treatment (i.e. 33% patients received meropenem, 33% received colistin and 33% received the new antibacterial as their first-line treatment). Following unsuccessful treatment patients move to the next available treatment in the defined treatment sequence, regardless of which treatment they started with, until all available treatments are exhausted.
Model Inputs
The incidence rates of HAIs with LTO due to antibiotic-resistant bacteria in Greece were extracted from Cassini et al. 2019 [5] (calculation was based on EARS-Net data collected during 2015). Overall, the number of annual HAIs with LTO due to E. coli, Klebsiella spp. and P. aeruginosa in Greece was estimated to be 11,292. The number and relative proportion of infections according to causing pathogen are shown in Table 3. Pathogen-specific resistance levels for each treatment were informed by WHONET data [26], published literature [14, 15] and expert opinion (Table 3). It was assumed that the new antibacterial would have a resistance level of 0%. Additional key model inputs are described in Table 4. Indication-specific inputs and inputs weighted by indication are presented in Supplementary Table 2.
Analysis
A 10-year time horizon was utilised, to capture the value provided by a new antibacterial over time but to limit the uncertainty associated with modelling over long time horizons [25]. The model estimates clinical and economic outcomes (hospital length of stay [LOS], total days on treatment [TDT], quality-adjusted life years [QALYs] and life years [LY], hospitalisation costs and monetary benefit [MB]). For the purpose of this analysis, TDT is defined as the total number of days patients are on treatment. QALYs and LYs were considered over the lifetime of the patient, based on the number of infections modelled over the 10-year time horizon. Therefore, a lifetime horizon considers the number of infections over the next 10 years. A lifetime is based on the life expectancy of a successfully treated member of the general population in Greece (20.12 years). Outcomes were considered from the perspective of a third-party payer in Greece.
Monetary benefit was estimated according to the following equation:
In Greece, no standard willingness-to-pay (WTP) threshold or discount rate is applied; therefore, a WTP threshold of €30,000 per QALY gained and a 3.5% discount rate were utilised to align with health technology assessment guidance in Europe and with WHO recommendation [30, 31].
Sensitivity Analyses
One-way sensitivity analyses were performed to assess the impact of uncertainty around key model input parameters. Key model inputs, outlined in Table 4, were varied by ± 20%. An additional scenario excluded the 3.5% discount rate for costs and benefits, in this scenario the discount rate was set at 0%. The impact was assessed on model estimates of hospitalisation costs and QALYs gained, of introducing the new antibacterial as a first-line treatment and diversifying all treatment lines equally between patients.
Ethics Approval
Ethics approval was not required for this study; the analysis in this article is based on previously publicly available data and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Absolute health economic outcomes are estimated over the 10-year time horizon. These are based on treating the modelled HAIs using the current treatment strategy in Greece, and in the alternative scenarios, treatment with a new antibacterial. These are presented in Fig. 2 and Table 5. The burden of treating the infected population with the current treatment strategy was estimated at 1.4 million hospital bed days resulting in an expenditure of €320.4 million in hospitalisation costs, as well as substantial LYs and QALYs lost (403,489 and 318,873, respectively; Table 5).
Over a 10-year time horizon, introducing a new antibacterial resulted in substantial savings, reducing both bed days, and hospitalisation costs. The introduction of a new antibacterial also yielded considerable LYs and QALYs gains (Fig. 2 and Table 5). The introduction of a new antibacterial as a first-line treatment with diversification was associated with the greatest economic and clinical benefit. Compared to the current treatment strategy, this alternative strategy was associated with 92,957 bed day saved, hospitalisation cost savings of €21.6 million, 661 fewer TDT and 285,597 LYs gained (corresponding to 225,630 QALYs), over 10 years (Fig. 2).
Based on a WTP threshold of €30,000 per QALY gained, the monetary benefit associated with introducing a new antibacterial to the current treatment strategy was estimated to be up to €6.8 billion (when the antibacterial was introduced as a first-line treatment with diversification), over the 10-year time horizon (Fig. 3).
Whilst the overall health economic benefits of introducing a new antibacterial are apparent, when considering individual pathogens, we observed an increase in bed days and up to €1.8 million and €4.5 million in additional hospitalisation costs associated with E. coli and P. aeruginosa infections, respectively, as compared to the current treatment pathway. This is due to the relatively low baseline resistance levels of these pathogens and the dynamics of resistance gain. Nevertheless, the use of the new antibacterial for the treatment of E. coli and P. aeruginosa infections was still associated with substantial LY and QALY gains, regardless of the position of the new antibacterial treatment in the treatment pathway. In addition, the savings obtained when introducing a new antibacterial treatment for Klebsiella spp. infections largely offset hospitalisation costs accrued for the treatment of E. coli and P. aeruginosa infections, resulting in net overall savings (Table 5). Importantly, across all pathogens, introducing a new antibacterial resulted in gains in LYs and QALYs. In Klebsiella spp. infections, over 230,000 and 180,000 additional LYs and QALYs, respectively, over a lifetime (Table 5). The key driver of monetary benefit was the increase in QALY gains in all pathogens considered.
Sensitivity Analysis
Varying LOS, treatment efficacy and utility (resolution of infection) by ± 20%, and excluding discounting were shown to have the greatest impact on hospitalisation costs and QALYs, in all scenarios explored (Fig. 4). Treatment efficacy had the greatest influence on hospitalisation cost savings (from €11.2 million to €26.1 million). Excluding discounting impacted on QALYs estimates, resulting in QALY gains of 364,613.
Discussion
The findings of the present analysis highlight the urgent need and the potential benefits of introducing new effective antibacterial agents to existing treatment pathways for HAIs, as a tool to counteract the continuously evolving AMR threat in Greece. Consistent with previous analyses [5, 32], this study confirms that HAIs with LTO are responsible for a significant clinical and economic burden to the Greek healthcare system. The addition of a new antibacterial for the treatment of HAIs with LTO would provide considerable benefit to healthcare providers in Greece when compared with currently available antibacterial treatment strategies.
With the rise of LTO infections [33], Greece is heading towards an era of pan-drug resistance [34]. Since the mid-1980s, no new classes of antimicrobial treatments have been approved for use [35]. The limited diversity of antimicrobial classes has resulted in increased exposure to treatments with shared mechanisms of action, increasing selection pressure and AMR levels in the population [19]. With an inadequate clinical pipeline and many pharmaceutical companies withdrawing investment [36, 37], push and pull incentives have been proposed, to reduce the economic risk, to incentivise research and development of new antibacterial agents [38]. Push incentives aim to reduce preclinical research costs while pull incentives are intended to reward pharmaceutical companies’ efforts for antibiotic development, following the introduction of new antibacterial agents [38, 39]. Pull incentives include novel reimbursement models that de-link revenue from volume of sales and provide financial assurance to companies that bring new treatments to market, such as the PASTEUR act in the USA (introduced to Congress 2021) [40, 41], and the first ‘subscription-style’ model launched by the National Institute for Health and Care Excellence (NICE) and National Health Service (NHS) England [42, 43]. These mechanisms aim to stimulate antimicrobial research and development whilst enabling the appropriate use of antimicrobials. For such initiatives to be effective in providing an economic incentive capable of reinvigorating research and development efforts globally, a proportional worldwide response is required [42]. In order to develop adequate reimbursement mechanisms that promote patient access to new treatments, there is a need to assess the societal value of antimicrobials specific to individual countries.
Development of new antimicrobials needs to be accompanied by optimal use of existing ones, to slow and reduce AMR, through the enforcement of stewardship programmes. This is still a significant issue in Greece, where consumption in both community and hospital settings ranks amongst the highest in Europe [10, 44], contributing to the increasing burden of MDR infections. In order to reduce the significant burden of AMR in Greece, a series of measures have been implemented based on the WHO Global Action Plan on Antimicrobial Resistance 2015 [21] and the European One Health Action Plan against AMR 2017[1]. These include the introduction of prescribing guidelines and antibiotic consumption monitoring by hospital stewardship groups [22], and infection prevention and control education programmes [23]. Despite these attempts, Greece is yet to fully implement a national AMR action plan [24]. The lack of local data practicality and actionability issues have likely contributed to policy-making delays and uneven uptake of these measures in the country. A strong political commitment and unified efforts are needed to implement the multifaceted interventions required to both reduce the burden of AMR in Greece and enable prompt access to new treatments.
The analysis presented in this study is associated with a number of limitations as with any study of its kind. Firstly, the model only considered the impact of resistance in the hospital environment and not in the community within a select number of pathogens and indications; therefore, our analysis may under-represent the actual burden in Greece. Additionally, it must also be noted that the assumptions for Greek transmission dynamics and prevalence of infection were based on UK data, which may not be fully reflective of the Greek context. Furthermore, as with all economic models, the model presented is subject to the uncertainty associated with two key factors: (1) extrapolation of outcomes beyond the available data and (2) necessary simplification of the underlying disease pathology and interpatient variability in natural disease course, response to treatment, mechanisms of treatment failure, and other relevant phenomena. Variation in resistance levels between sources may also be considered a limitation. Additionally, while this analysis is specific to the Greek setting and may not be generalisable between countries, the findings from this study may also be informative to other countries with a high AMR burden.
Conclusion
This study provides a quantitative estimation of the potential clinical and economic benefits that may be realised through the introduction of a new antibacterial in Greece, as well as highlighting the significant burden of HAIs with LTOs. There is a significant and urgent need for a coordinated response from both healthcare and political leaders to tackle the antibiotic crisis. The findings presented in this study can be used to inform decision makers in Greece about the potential benefits of implementing a national action plan that incentivises and improves access to new antibacterial agents, alongside improving education, preventing infection and enhancing stewardship measures.
References
European Commission. A European One Health Action Plan against Antimicrobial Resistance (AMR). 2017. https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf. Accessed 17 Oct.
Kritsotakis EI, Kontopidou F, Astrinaki E, Roumbelaki M, Ioannidou E, Gikas A. Prevalence, incidence burden, and clinical impact of healthcare-associated infections and antimicrobial resistance: a national prevalent cohort study in acute care hospitals in Greece. Infect Drug Resist. 2017;10:317–28.
Suetens C, Latour K, Kärki T, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46):1800516.
Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) working group. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill. 2015;20(45):30062.
Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66.
Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR gram-negative bacteria. Front Med (Lausanne). 2019;6:74.
Grundmann H, Glasner C, Albiger B, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017;17(2):153–63.
Polemis M, Tryfinopoulou K, Giakkoupi P, WHONET-Greece study group, Vatopoulos A. Eight-year trends in the relative isolation frequency and antimicrobial susceptibility among bloodstream isolates from Greek hospitals: data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance - WHONET-Greece, 2010 to 2017. Euro Surveill. 2020;25(34):1900516.
Bassetti M, Carnelutti A, Peghin M. Patient specific risk stratification for antimicrobial resistance and possible treatment strategies in gram-negative bacterial infections. Expert Rev Anti-infect Ther. 2017;15(1):55–65.
European Center for Disease Control (ECDC). Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals, 2011–2012. ECDC Surveillance Report. 2013.
Galani I, Karaiskos I, Karantani I, et al. Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Euro Surveill. 2018;23(31):1700775.
Galani I, Souli M, Nafplioti K, et al. Correction to: In vitro activity of imipenem-relebactam against non-MBL carbapenemase-producing Klebsiella pneumoniae isolated in Greek hospitals in 2015–2016. Eur J Clin Microbiol Infect Dis. 2019;38(6):1151–2.
Maltezou HC, Kontopidou F, Dedoukou X, et al. Action plan to combat infections due to carbapenem-resistant, Gram-negative pathogens in acute-care hospitals in Greece. J Global Antimicrob Resist. 2014;2(1):11–6.
Galani I, Papoutsaki V, Karantani I, et al. In vitro activity of ceftolozane/tazobactam alone and in combination with amikacin against MDR/XDR Pseudomonas aeruginosa isolates from Greece. J Antimicrob Chemother. 2020;75(8):2164–72.
Polemis M, Mandilara G, Pappa O, et al. COVID-19 and antimicrobial resistance: data from the greek electronic system for the surveillance of antimicrobial resistance-WHONET-Greece (January 2018–March 2021). Life (Basel). 2021;11(10):996.
WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022–2020 data. Copenhagen: WHO Regional Office for Europe; 2022.
Barber KE, Ortwine JK, Akins RL. Ceftazidime/avibactam: who says you can’t teach an old drug new tricks? J Pharm Pharm Sci. 2016;19(4):448–64.
European Center for Disease Control (ECDC). Antimicrobial resistance surveillance in Europe 2015. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2017.
World Health Organization. 2020 Antimicrobial agents in clinical and preclinical development and overview and analysis. 2020. https://www.who.int/publications/i/item/9789240021303. Accessed 17 Oct.
Council of the European Union. Council Recommendation of 9 June 2009 on Patient Safety, Including the Prevention and Control of Healthcare Associated Infections. Off J Eur Union (OJ C 151, 372009). https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32009H0703%2801%29. Accessed 17 Oct.
World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/publications/i/item/9789241509763. Accessed 17 Oct.
Hellenic Republic Ministry of Health Directorate-General for Public Health and Quality of Life Directorate of Public Health Department of Contagious and Non-Contagious Communicable diseases. Guidelines for the proper management of antimicrobial agents (older and new) in the hospital space. 2019. https://eody.gov.gr/wp-content/uploads/2019/08/ma-egkyklios_xrisi_antiviotikon_nosokomeia.pdf Accessed 17 Oct.
Ephemerida of the Government of the Hellenic Republic. Arrangements to ensure access to quality health services - Establishment and statute of the Organization for Quality Assurance in Health S.A. (O.DI.P.Y. S.A.), other urgent provisions of the Ministry's competence Health and other provisions. 2020. https://odipy.gov.gr/wp-content/uploads/2022/06/149a-20.pdf Accessed 17 Oct.
World Health Organization. 2021 TrACSS Country Report on the Implementation of National Action Plan on Antimicrobial Resistance (AMR). 2021. https://www.who.int/publications/m/item/antimicrobial-resistance-tracss-grc-2021-country-profile. Accessed 17 Oct.
Gordon J, Darlington O, McEwan P, et al. Estimating the value of new antimicrobials in the context of antimicrobial resistance: development and application of a dynamic disease transmission model. Pharmacoeconomics. 2020;38(8):857–69.
WHONET Greece. The Greek System for the Surveillance of Antimicrobial Resistance is a Public Health initiative operating in the framework of the scientific alliance between the National School of Public Health and the Hellenic Center for Disease Control and Prevention. 2021. http://www.mednet.gr/whonet/. Accessed 18 Nov 2021.
Hellenic Statistical Authority. Demographic characteristics. 2011. https://www.statistics.gr/el/statistics/-/publication/SAM03/. Accessed 17 Nov 2021.
Hellenic Statistical Authority (ELSTAT). PRESS RELEASE Health Expectancy 2013. 2016. https://www.statistics.gr/en/statistics?p_p_id=documents_WAR_publicationsportlet_INSTANCE_qDQ8fBKKo4lN&p_p_lifecycle=2&p_p_state=normal&p_p_mode=view&p_p_cacheability=cacheLevelPage&p_p_col_id=column-2&p_p_col_count=4&p_p_col_pos=1&_documents_WAR_publicationsportlet_INSTANCE_qDQ8fBKKo4lN_javax.faces.resource=document&_documents_WAR_publicationsportlet_INSTANCE_qDQ8fBKKo4lN_ln=downloadResources&_documents_WAR_publicationsportlet_INSTANCE_qDQ8fBKKo4lN_documentID=196504&_documents_WAR_publicationsportlet_INSTANCE_qDQ8fBKKo4lN_locale=en Accessed 17 Oct.
Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Dordrecht (NL): Springer; 2014. https://doi.org/10.1007/978-94-007-7596-1.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/process/pmg9. Accessed 16 Feb 2022.
McDougall JA, Furnback WE, Wang BCM, Mahlich J. Understanding the global measurement of willingness to pay in health. J Mark Access Health Policy. 2020;8(1):1717030.
Tacconelli E, Pezzani MD. Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis. 2019;19(1):4–6.
Feretzakis G, Loupelis E, Sakagianni A, et al. A 2-year single-centre audit on antibiotic resistance of Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae strains from an intensive care unit and other wards in a general public hospital in Greece. Antibiotics (Basel). 2019;8(2):62.
Nowak J, Zander E, Stefanik D, et al. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother. 2017;72(12):3277–82.
Pew Charitable Trusts. A scientific roadmap for antibiotic discovery: a sustained and robust pipeline of new antibacterial drugs and therapies is critical to preserve public health. 2016. https://www.pewtrusts.org/-/media/assets/2016/05/ascientificroadmapforantibioticdiscovery.pdf Accessed 17 Oct.
Projan SJ. Why is big pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6(5):427–30.
Towse A, Sharma P. Incentives for R&D for new antimicrobial drugs. Inte J Econ Bus. 2011;18(2):331–50.
Dutescu IA, Hillier SA. Encouraging the development of new antibiotics: are financial incentives the right way forward? A systematic review and case study. Infect Drug Resist. 2021;14:415–34.
So AD, Shah TA. New business models for antibiotic innovation. Ups J Med Sci. 2014;119(2):176–80.
Rothery C, Woods B, Schmitt L, Claxton K, Palmer S, Sculpher M. Framework for value assessment of new antimicrobials. Sheffield: EEPRU; 2018.
S.4760 - 116th Congress: The PASTEUR Act. USA 2020. https://www.congress.gov/bill/116th-congress/senate-bill/4760?s=1&r=14. Accessed 17 Oct.
GOV.UK. World-first scheme underway to tackle AMR and protect UK patients. 2022. https://www.gov.uk/government/news/world-first-scheme-underway-to-tackle-amr-and-protect-uk-patients. Accessed 17 Oct 2022.
National Institute for Health and Care Excellence. Models for the evaluation and purchase of antimicrobials. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials. Accessed 17 Oct 2022.
European Centre for Disease Prevention and Control (ECDC). Prevention and Control (ECDC). Technical document: point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals, Protocol version 5.3 2016–2017. 2016. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/PPS-HAI-antimicrobial-use-EU-acute-care-hospitals-V5-3.pdf. Accessed 17 Oct.
Acknowledgements
Funding
This work was supported by Pfizer, Hellas SA who provided support for the model development/analysis and medical writing for this study. Pfizer, Hellas SA also sponsored the journal’s rapid service fee.
Medical Writing Assistance
The authors thank Chloe Hembury of Health Economics and Outcomes Research Ltd. for providing medical writing support/editorial support, which was funded by Pfizer, Hellas SA in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author Contributions
Jason Gordon, Amer Al-Taie and Myrto Barmpouni conceptualised and designed the study. Clive Pritchard and Ryan Miller were responsible for data analysis. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Disclosures
Amer Al-Taie is an employee and stockholder of Pfizer R&D UK. Myrto Bampouni, Vassilis Grammelis, , and Aris Rousakis are employees of Pfizer Hellas SA. Jason Gordon, , Ryan Miller and James Dennis are employees of Health Economics and Outcomes Research Ltd. Health Economics and Outcomes Research Ltd. received fees from Pfizer Hellas SA in relation to this study. Clive Pritchard was employed by Health Economics and Outcomes Research Ltd. at the time of the study and is currently employed by ICON plc. ICON plc received no fees in relation to this study. Kyriakos Soulioti has received a research grant from Pfizer, Hellas, unrelated to this study. Garyphallia Poulakou has received consultancy fees and speaker’s honoraria by Gilead, Menarini, MSD, Pfizer and SOBI. George L. Daikos has received consulting Fees from Pfizer.
Compliance with Ethics Guidelines
The analysis in this article is based on previously publicly available data and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Barmpouni, M., Gordon, J.P., Miller, R.L. et al. Estimating the Clinical and Economic Impact of Introducing a New Antibacterial into Greek Clinical Practice for the Management of Hospital-Acquired Infections with Limited Treatment Options. Infect Dis Ther 12, 527–543 (2023). https://doi.org/10.1007/s40121-022-00743-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00743-4