Abstract
A novel cellulose acetate–tin (IV) phosphate composite material has been synthesized under varying conditions. The material was characterized by FTIR, SEM, EDX and XRD. The composite material was explored for its use in separation of toxic metal cations present in electroplating waste discharge and similar synthetic fluid by varying the system conditions [temperature (298–313 K), pH (2–12), time (10–420 min), dosage (1–10 g) and initial concentration of metal cations (1.0–15.0 mg/dm3)]. Highest removal rate was achieved for Cr3+, Ni2+ and Zn2+ (99, 98 and 94 %) under optimal conditions. Experimental equilibrium data for Cr3+, Ni2+ and Zn2+ ions fitted well with Langmuir isotherms (R 2 > 0.99). The adsorption capacities for Cr3+, Ni2+ and Zn2+ ions were found to be 31.5, 43.7 and 43.4 mg/g. Pseudo-second-order model explained the kinetics of adsorption (R 2 > 0.99) for all the metal ions undertaken in the study. Thermodynamic parameters such as ΔG, ΔS and ΔH show spontaneity and endothermic nature of the process at all the concentration ranges studied (2–15 mg/dm3). Desorption of metal ion from (CA/TPC) surface was carried out using 0.1 N nitric acid as an eluent under varying system pHs (1–6). Maximum desorption’s for all the metal ions were observed at pH 1. The adsorption efficiencies of CA/TPC for the metal ions were maintained after four consecutive cycles of adsorption/desorption process. The loss in the dry weight of CA/TPC was not more than 10 % after each cycle of process. Due to easy and high regeneration properties of CA/TPC, it was found to be economical and have shown potential benefits in recovery of metal ions from wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Inadequate wastewater management has resulted continuous discharge of heavy metals in the aquatic system. The heavy metals released in excess have significant adverse environmental impacts. These are toxic to many organ systems including the heart, bone, intestine, kidney, reproductive and nervous system, in varying degrees. Consequently, significant research attention has recently been focused on the environmental behavior of heavy metals. The heavy metals are easily concentrated by the food chain, which threatens human health. The heavy metals have entered into the atmosphere, water and soil through mining, electroplating, dyeing, tanning and metal processing industries in large scale [1–4]. In drinking water, the authorized limit set by WHO for the three metals is chromium (0.05 mg/dm3), nickel (0.07 mg/dm3) and zinc (3.0 mg/dm3). Therefore, it is necessary to remove heavy metals from the wastewaters before they are discharged into the natural water system. Various materials, such as (MnO2/CNT) nanocomposite, fertilizer waste activated carbon, waste materials, MWCNT/TiO2 composite, alumina-coated CNT, orange peel/Fe2O3 nanoparticle composite and agriculture waste, have been used to remove hazardous wastes from water [4–16]. Several other techniques have also been developed for the treatment of undesired waste containing heavy metals and dyes [3, 17, 18]. Among these, ion exchange technique is the most common and effective method for the treatment and identification of industrial wastes [19, 20]. The inorganic ion exchange materials are more stable to high temperatures and ionizing radiation than organic exchangers. For this purpose, first inorganic ion exchangers and later on organic resins have been used [21]. However, inorganic ion exchange materials have their own limitations, e.g., these materials in general are reported to be not much reproducible in behavior and fabrication of fine powder of it in rigid-type media suitable for column operations is quite difficult. Organic–inorganic hybrid composite materials, due to its exceptional chemical, mechanical and thermal properties as compared to pure inorganic and organic materials, have attracted a great attention in scientific community. Converting the organic and inorganic materials into an organic–inorganic hybrid composite with better properties is an economic method which has been a hot topic [22–38]. The organic–inorganic hybrid composite minimizes the limitations of organic and inorganic materials by modifying individual properties of each of the organic and inorganic materials. These materials have been obtained by incorporating the organic polymers into the matrix of inorganic precipitates of multivalent metal acid salts [39]. Recently, use of organic–inorganic hybrid composite to separate metal ions has been discussed widely [24, 37, 40]. Therefore, organic–inorganic hybrid composite, cellulose acetate–tin (IV) phosphate, has been preferred to synthesize and use in heavy metal decontamination. This material has better granulometric properties, good ion exchange capacity, reproducibility and possesses good selectivity for heavy metals [21]. However, to our knowledge, there is little or no information about the preparation of cellulose acetate–tin (IV) phosphate composites (CA/TPC) and their use in separation and removal of heavy metals from electroplating wastewater. Thus, this study was aimed at developing a simple and efficient laboratory-scale adsorption technique to remove heavy metals such as chromium, nickel and zinc from synthetic and electroplating wastewater using cellulose acetate–tin (IV) phosphate composite (CA/TPC) material. The material was characterized using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD) and Fourier transform-infrared spectroscopy (FT-IR) techniques. The adsorption mechanism was evaluated from kinetics, isotherms and thermodynamics.
Materials and methods
Chemicals
All solutions were prepared from analytical reagent grade chemicals procured from Merck Specialty Chemicals, (P) Ltd. Mumbai, India, and Millipore Milli-Q grade deionized water (conductivity of 14.7 M cm).
Glasswares
All glasswares of Borosil make glass (A grade) were used in the study. Glasswares were dipped in 10 % (v/v) HNO3 overnight and subsequently rinsed twice with deionized water to remove any possible interference by other metals.
Synthesis of cellulose acetate–tin (IV) phosphate
The composite ion exchanger material was prepared by the sol–gel mixing of cellulose acetate into the inorganic precipitate of tin (IV) phosphate [32]. In this process, tin (IV) phosphates were prepared by adding 0.1 M tin (IV) chloride and 0.1 M sodium dihydrogen phosphate drop wise with continuous shaking in equal volume ratio (1:1). The pH variation was adjusted by adding 1 M nitric acid or 1 M ammonia solutions. The mixture was stirred constantly for 2 h at 303 ± 2 K to obtain precipitate of tin (IV) phosphate. The gel of cellulose acetate was added drop wise into the inorganic precipitate of tin (IV) phosphate and mixed thoroughly with a constant stirring for 4 h. The mixture was kept for 24 h in the mother liquor for digestion. The supernatant liquid was removed, and the precipitates were washed with double distilled water several times to remove excess reagents or impurities (if any). The product was dried at 313 ± 2 K in an oven and then converted to H+ form by placing them in 1 M HNO3 solution. The activated ion exchanger was washed with double distilled water to remove excess acid. The material was finally dried at 313 ± 2 K in a hot air oven and preserved for use in desiccators. A number of samples were prepared using the procedure.
Electroplating wastewater
An over view of electroplating industries chosen for sampling, containing chromium, nickel and zinc plating rinse water, is shown in Table 1. Average physicochemical characteristics of electroplating rinse water are as follows: pH, 7.8, conductivity, 1.54 mS cm−1, COD, 275.6 mg/dm3, TDS, 112.2 mg/dm3. The concentrations of the heavy metals have been found in the four wastewater samples, Table 2. The average concentrations of chromium (16.21 mg/dm3), nickel (15.41 mg/dm3) and zinc (13.23 mg/dm3) were shown to exceed the prescribed legal limits. The COD content (275.6 mg/dm3) in the wastewater samples also exceeds the authorized limit.
Batch adsorption studies
Stock solutions (1,000 mg/dm3) of nickel, chromium and zinc were prepared by dissolving required quantity of Ni(SO4)2·6H2O, Cr2(SO4)3·12 H2O and ZnSO4·7 H2O in (1,000 cm3) deionized water (pH 7). The test solutions of nickel, chromium and zinc were prepared by serial dilution of stock solutions using graduated micropipette and stored at 277 ± 2 K. The concentration ranges of metals varied between 1.0 and 15.0 mg/dm3. To avoid precipitation, the solutions were agitated everytime before use. Batch adsorption experiments (laboratory-scale) were conducted using 1.0–10.0 g of adsorbent with 50 cm3 of solutions containing Cr3+, Ni2+ and Zn2+ ions of desired concentrations, respectively, at constant temperature in 250-cm3 capped conical flasks. To find out the effect of pH on adsorption, the initial pH was adjusted in the range of 2–12 either with (0.1, 1 N) HCl or (0.1, 1 N) NaOH solutions. To avoid the change in pH values with time, the resulting solutions were accurately monitored after regular time intervals. Similarly, the effect of agitation time on initial metal concentrations was carried out. Adsorbent (10.0 g) was suspended in the five conical flasks containing 50 cm3 of metal solutions (15 mg/dm3). The conical flasks were uniformly shaken at optimum speed 160 rpm using thermostatic reciprocating shaker for fixed time period from 10 to 420 min at temperature range from 298 to 313 K. Effects of each factor were determined keeping other variables constant. Samples (5 cm3) were drawn from each flask after a regular time intervals to monitor the progress of adsorption. The sample was then filtered through a Whatman filter paper no. 41 using a vacuum filtration device. The filtrate was analyzed for residual metals concentration determination by (AAS) (AAS-4129, ECIL) using acetylene–air as the fuel–oxidant. The amount of metal adsorbed per unit mass of adsorbent was obtained by using the equation \( q_{\text{e}} = (C_{\text{o}} - C_{\text{e}} )V/m \).
Adsorption isotherms
The equilibrium data were obtained and analyzed using Langmuir, Freundlich and Temkin isotherm models. The general form of Freundlich model for single solute system is expressed in Eq. (1) [41]:
Generally, the logarithmic form of the above equation was used to fit batch equilibrium data. So the linear form of the Freundlich isotherm can be obtained by taking the logarithmic of Eq. (1):
The values of the Freundlich constants such as K f and 1/n can be calculated from intercept and slope of the straight line plot of log (q e) versus log (C e). The linear form of Langmuir isotherm is expressed in Eq. (3) [42]:
The Langmuir constant such as (q m) and (b) values can be obtained from the straight line plot of (1/q e) against (1/C e). A linear form of the Temkin isotherm is expressed in Eq. (4) [43]:
where (A) (mg/g) = (RT/b) ln (a) and (B) (L/mg) = (RT/b). The plot q e against ln C e should be linear wherefrom the values of (A) and (B) can be computed from the intercept and slope, respectively. These values of (A) and (B) were subsequently used for estimating Temkin constants (a) and (b).
Kinetic models
The kinetic data were modelled using Pseudo-second-order and Weber and Morris intraparticle diffusion equations that correlates solute uptake during adsorption process.
The Pseudo-second-order equation
The pseudo-second-order adsorption rate equation is expressed in Eq. (5) [44]
where h is the initial adsorption rate as t → 0 i.e. \( h = k_{2} q_{{{\text{e}} }}^{2} \). According to the above equation, a plot of (t/q t ) against (t) will yield a straight line with slope (1/q e) and intercept (1/h).
Weber and Morris intraparticle diffusion equation
Weber and Morris intraparticle diffusion equation is expressed as follows in Eq. (6) [15]:
Accordingly, the constants (C) and (K id) were interpreted from intercept and slope of the respective plot.
Thermodynamics studies
The thermodynamic constants, entropy change (∆S) and enthalpy change (∆H), were computed from the slope and intercept of the Van’t Hoff plots. The apparent equilibrium constant (k C) of the adsorption is defined as:
The values of ∆S and ∆H were calculated from Eq. (8). The Gibbs free energy was calculated to determine the feasibility of adsorption process using the Eq. (9)
Results and discussion
Structural characterization of CA/TPC
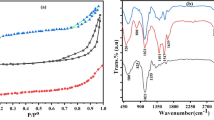
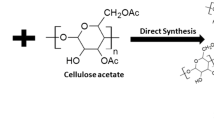
Figure 1 shows the synthesis route of cellulose acetate–tin (IV) phosphate composite material. In order to obtained better ion exchange capacity, a number of samples of CA/TPC were synthesized by sol–gel mixing method. Table 3 illustrates that the ion exchange capacity of CA/TPC was influenced by mixing ratio, concentration of reagents and system pH. As the ratio of anionic part (sodium dihydrogen phosphate) in the reaction mixture was increased, the ion exchange capacity of CA/TPC was increased because the replaceable hydrogen groups were bound to this group (PO4 3− ionogenic group) [40]. Further, the CA/TPC material has shown best synthetic utility at pH 1. Among various samples synthesized, sample-5 has better Na+ ion exchange capacity and good reproducible behavior, (Table 3). Therefore, it was selected for detailed studies. The improvement in ion exchange capacity of CA/TPC may be due to the binding of cellulose acetate with inorganic moiety (tin (IV) phosphate). The surface morphology of CA/TPC before and after Cr3+, Ni2+ and Zn2+ adsorptions was determined by scanning electron microscopy (SEM) and was presented in Fig. 2a–d, respectively. Figure 2a shows semi-crystalline nature of surfaces particles with few sharp edges that become slightly rough after the metal ion adsorptions [32]. Figure 3 shows X-ray diffraction (XRD) pattern of CA/TPC. No sharp peaks were obtained that confirmed the non-crystalline nature of CA/TPC. The infrared spectrum of CA/TPC is shown in Fig. 4a. The broad peak at 3,499 cm−1 was due to the presence of –OH group of water of crystallization. A peak at 1,630 cm−1 is also indicative of presence of strongly bonded –OH groups. The absorption band at 1,710 cm−1 was aroused due to the carbonyl group of cellulose acetate part. The broad peak at 1,020 cm−1 was assigned to the presence of PO4 3− and H2PO4 − groups, respectively [35]. The absorption band at 470 cm−1 may be due to the superposition of metal–oxygen stretching vibrations suggested the combination between cellulose acetate and tin (IV) phosphate [32]. Figure 4b–d show that the intensity and position of absorption band at 1,020 cm−1 has been changed due to Cr3+, Ni2+ and Zn2+ adsorptions. In addition, the peaks at 3,499 cm−1 were also subside after Cr3+, Ni2+ and Zn2+ adsorptions indicating the involvement of –OH, PO4 3− and H2PO4 − groups in the adsorption of Cr3+, Ni2+ and Zn2+ ions. The EDX spectrum of CA/TPC before and after Cr3+, Ni2+ and Zn2+ ions adsorptions was recorded and shown in Fig. 5a–d. Figure 5a illustrates that tin, oxygen and phosphorous were the main constituents present in CA/TPC material. As shown in Fig. 5b–d, some peaks due to chromium, nickel and zinc metals adsorptions were observed in the EDX spectrum of CA/TPC. Figure 5e shows the elemental (wt%) composition of CA/TPC surface.
Schematic depiction of synthesis route for CA/TPC adsorbent [32]
Breakthrough studies
1.0 g of CA/TPC has been taken in glass column with glass wool support at the bottom. One liter each of Cr3+, Ni2+ and Zn2+ solutions of initial concentration 15 mg/dm3 (C o) were passed through a column separately at flow rate of 1 cm3/min. The eluent was collected in 50 cm3 fractions, and the amount of Cr3+, Ni2+ and Zn2+ (C) was determined in each fraction by AAS. The breakthrough curves for Cr3+, Ni2+ and Zn2+ ions were obtained by plotting C/C o versus volume of the eluent, Fig. 6. The observed breakthrough capacities for Cr3+, Ni2+ and Zn2+ in each of the metal solutions were 3.0, 4.0 and 3.5 mg/g., whereas the exhaustive capacity for the same metal ions was 7.5, 9.0 and 8.5 mg/g, respectively.
Effect of adsorbent dosage
The adsorption of heavy metals, as a function of CA/TPC dosage, was studied. Figure 7 shows the effect of CA/TPC dosages on the initial concentrations of Cr3+, Ni2+ and Zn2+ ions. Amount of adsorbent was varied from 1.0 to 10.0 g/50 cm3 (with particle size <20 mesh) and equilibrated for 1 h, keeping a pH of each metal solution of 6. The Cr3+ ion concentration was decreased from 0.99 to 0.15 mg/dm3 with increase in adsorbent loading from 1.0 to 10.0 g/50 cm3 and then became almost constant, whereas the concentrations of Zn2+ and Ni2+ ions were found to be decreased from 0.65 to 0.1 and 0.3 to 0.01 mg/dm3, respectively, for a similar increase in adsorbent dose. The results indicates that 10.0 g of CA/TPC dose was sufficient for the optimum removal of Cr3+, Ni2+ and Zn2+ ions. In order to facilitate analysis and comparison of the experimental data, the remaining experiments were performed with 10.0 g/50 cm3 of CA/TPC dose for each metal ions.
Effect of agitation time
The removal of heavy metals by CA/TPC as a function of contact time was investigated. Contact time was varied in the range of 10–420 min at the given temperatures, (298, 308 and 313 K). As shown in Fig. 8a–c, the value of q was increased as the agitation time and temperature conditions increased, until equilibrium was reached. The adsorptions of Cr3+, Ni2+ and Zn2+ ions on CA/TPC were rapid for the first 50 min and remained almost constant beyond 50 min at all the given temperatures. However, complete equilibrium for Cr3+ adsorption was attained in 60 min, and for Ni2+ and Zn2+, it was attained in 120 min with initial concentrations of each metals 15 mg/dm3. There were no significant changes in Cr3+, Ni2+ and Zn2+ uptake observed by CA/TPC after 420 min. The complete adsorption of Cr3+ ions requires less residence time (60 min) as compared to Ni2+ and Zn2+ ions (120 min). Therefore, the period of 60 min was considered as the optimum time for Cr3+, and for the Ni2+ and Zn2+ ions, adsorptions optimum time was 120 min, respectively. F test analysis illustrates that agitation time and temperature conditions have shown more significant effect on the Ni2+ uptake as compared to Cr3+ and Zn2+ ions due to better F value 15.117 (Tables S1(a-c) shown in supplementary material).
Effect of pH of solution
Hydronium ion concentration is an important parameter affecting the ion exchange process. This is partly because hydrogen ions themselves are strongly competing adsorbate and the solution pH influences the ionization of surface functional groups. Figure 9 shows the effects of pH (in the range of 2–12) on adsorption of Cr3+, Ni2+ and Zn2+ ions by CA/TPC at a fixed concentration (15 mg/dm3) and time of 1 h. The pH of the solution was controlled using buffer solution. The residual concentrations of Ni2+ and Zn2+ ions were found to be ~0.5 mg/dm3 between pH 4–6 and reached to 6.9 and 8.8 mg/dm3 as pH increased to 12 while the residual concentration for Cr3+ ion was ~1.0 mg/dm3 at pH 3 and increased to 12.1 mg/dm3 when pH reached to 12. The results show that CA/TPC has taken maximum Cr3+ from the solution at pH 3 while Ni2+ and Zn2+ ions were effectively adsorbed in the range of pH 4–6. The removal efficiency of CA/TPC for all metals was diminished at higher pH values because the hydrolysis of the metal ions occurs by the formation of metal hydroxides, which compete with the metal ion uptake by the CA/TPC [1, 45]. The effects of solution pH (in the range of 2–12) on the initial metal concentrations (15 mg/dm3) without using CA/TPC dosages under similar conditions were investigated. Figure 10 shows that the residual concentrations of Cr3+, Ni2+ and Zn2+ ions were found to be 14.4, 14.1 and 14.6 mg/dm3 when pH reached to 7.5, 9.0 and 9.5 but decreased sharply to 0.5, 0.2 and 3.7 mg/dm3 as pH was increased to 12. Thus, at higher pH, no more free metals were available for binding with CA/TPC.
Equilibrium study
The adsorption isotherms description for Cr3+, Ni2+ and Zn2+ ions on CA/TPC at given temperatures from 298 to 313 K has been studied. As shown in Fig. 11a–c, the adsorption capacities of CA/TPC for three metals were found to be increased with rise in temperature as well as initial Cr3+, Ni2+ and Zn2+ concentrations. A comparison of adsorption isotherms at 298, 308 and 313 K indicates that Cr3+, Ni2+ and Zn2+ adsorptions by CA/TPC were significantly affected by increase in the temperature. Maximum adsorption capacities of CA/TPC for Cr3+, Ni2+ and Zn2+ ions were found to be 31.5, 43.7 and 43.4 mg/g at higher temperature, 313 K that shows endothermic nature of the process. The initial sharp rise in the isotherm indicates the availability of readily accessible sites for adsorption. However, site saturation occurs as the Cr3+, Ni2+ and Zn2+ concentration increased and a plateau was reached indicating that no more sites remain available for adsorption. F test analysis predicts that initial Ni2+ concentration and temperature conditions have more significantly affected Ni2+ uptake with good F test value, 12.857 as compared to Cr3+ and Zn2+ ions, (Table S2 (a-c) shown in supplementary material). The equilibrium data obtained from Fig. 11a–c were applied to the Eqs. (2), (3) and (4), respectively, for the verification of the Freundlich, Langmuir and Temkin isotherm models, respectively. The results of Freundlich, Langmuir and Temkin linear plots are shown in (Figs. S3,4,5 (a-c) in the supplementary material). The Freundlich, Langmuir and Temkin constants values along with their correlation coefficients (R 2) are compiled in Table 4. Good correlation coefficients (R 2 > 0.99) for Cr3+, Ni2+ and Zn2+ ions at given temperatures indicated the applicability of Langmuir model in the present study. The values of q m calculated by the Langmuir isotherm were all close to experimental values at all given temperatures. These facts suggested that Cr3+, Ni2+ and Zn2+ were adsorbed in the form of monolayer coverage on the surface of the adsorbent. To determine the nature of adsorption process whether favorable or unfavorable, the dimensionless constant separation term (R L) was used and expressed as R L = 1/(1 + bC 0). R L value indicates the nature of adsorption process, and its value should be either unfavorable (R L > 1), linear (R L = 1), favorable (0 < R L < 1) or irreversible (R L = 0). The calculated (R L) values at all the temperatures were found to be in the range of (0 < RL < 1) which indicates that Cr3+, Ni2+ and Zn2+ metals uptake were favorable, (Fig.S2 shown in the supplementary material). The adsorption capacities of CA/TPC for the three metals were compared with other adsorbents and documented in Table 5.

Kinetics study
The Cr3+, Ni2+ and Zn2+ adsorptions dependence on time was studied in the given temperature range from 298 to 313 K for fixed metal concentration 15 mg/dm3. The uptake values for the removal of Cr3+, Ni2+ and Zn2+ are shown in Fig. 8a–c. The data were analyzed using pseudo-second-order kinetic model (Figs. S1(a-c) shown in supplementary material). Kinetic parameters and correlation coefficients (R 2) are summarized in Table 6. The R 2 values for pseudo-second-order model for Cr3+, Ni2+ and Zn2+ were all found to be in the range (0.99–1) at all given temperatures. The calculated q e(cal.) values at given temperatures were all very close to the experimental values q e(exp.) as predicted by Chi-square (χ 2) test. Further, on increasing the temperature, there were no significant differences observed between the experimental as well as calculated adsorption capacities (q e) at 0.99 level of significance for all the metals. Accordingly, the adsorptions of three metals on CA/TPC have been well described by the pseudo-second-order model. The present data were also examined by the Weber and Morris intraparticle diffusion model, Eq. (6) (Figs. S7(a-c) shown in supplementary material), represents plots of (q t ) versus (t 1/2) for three metal ions at given temperatures, whereas the results of the corresponding linear correlation analysis are tabulated in Table 6. The present data exhibit multilinear plots, which shows that the adsorption process was governed by two or more steps. In the given figures, all of the curves for Cr3+, Ni2+ and Zn2+ follow same features, in which the initial steep portion followed by a gradual linear portion was observed. The first linear segment (phase 1) at all the temperature for (t 0.5 = 3.16–7.74) was observed due to the immediate utilization of the most readily available adsorbing sites on the adsorbent surface, and the second phase (phase 2) for (t 0.5 = 7.74–20.4) was attributed to very slow diffusion of the adsorbate from the surface site into the inner pores. The values of k id1 and k id2 (for phase 1 and 2, respectively) obtained from the slope of linear plots are listed in Table 6. Higher values of k id1 as compared to k id2 for Cr3+, Ni2+ and Zn2+ ions at all given temperature indicated the rapid boundary layer diffusion (phase 1) that was followed by a slow intraparticle diffusion (phase 2). Furthermore, the second segment of the lines (phase 2), describing the intraparticle diffusion, does not pass through the origin indicates that the intraparticle diffusion was not only the rate-limiting step. The observed deviations in straight lines in Weber and Morris model were attributed to the difference in the rate of mass transfer in initial and final stages of adsorption [46].
Thermodynamics studies
The experimental data obtained at given temperatures (298–318 K) and concentrations (2–15 mg/dm3) were used in calculating the thermodynamic parameters such as gibbs free energy change (ΔG), enthalpy change (ΔH) and entropy change (∆S). Figs. S6 (a-c) (given in supplementary materials) shows the linear plots of ln k c versus 1/T for different metal concentrations (2–15 mg/dm3). The free energy of the process at all given temperatures and different metal concentrations was found to be negative for all the metal adsorption processes. The negative values of ΔG were attributed due to the fact that all metal adsorption processes were spontaneous and the degrees of spontaneity of the reactions were increased with rise in temperatures in all cases. The positive value of ∆S suggests increased randomness at the solid/solution interface [47]. This may have been aroused by the release of water molecules, ion exchange and/or metal binding to the CA/TPC. The overall Cr3+, Ni2+ and Zn2+ uptake process was endothermic (ΔH > 1) in all the metal concentration ranges studied. Table 7 summarizes the values of calculated thermodynamic parameters for the three metals.
Desorption study
To enable reuse of the adsorbent material and quantitative recovery of the adsorbed species, the metal ions desorption’s from treated CA/TPC (1.0 g) were investigated using 0.1 N HNO3 for four consecutive cycles. In each cycle of desorption, 20 cm3 eluent was passed for a period of 8 h at various pHs ranging from (1–6) and analyzed for given metal concentration in solution, Fig. 12. The highest desorption’s for all of the metals were achieved in first cycle of the elution at pH 1. On increasing the number of cycle and pH value, the metal desorption’s were decreased. At pH 1, amount of metals desorbed were in the order of Ni2+ > Zn2+ > Cr3+. As expected from highest electronegativity value of H+ ions (EN. ~2.20) in comparison with Cr3+ (EN. ~1.6), Ni2+ (EN. ~1.8) and Zn2+ (EN. ~1.6) ions, the H+ ions were able to replace Cr3+, Ni2+ and Zn2+ ions of CA/TPC [48]. This indicates that Cr3+, Ni2+ and Zn2+ adsorptions on CA/TPC were due to the ion exchange mechanism. The adsorption efficiencies of CA/TPC for all the metal ions were maintained after four consecutive cycles of adsorption/desorption process. Figure 13 shows the removal efficiency of CA/TPC for Cr3+, Ni2+ and Zn2+ over four successive adsorption/desorption cycles. In all the cases, the loss in the dry weight of CA/TPC was not more than 10 % after each cycle. It was observed that about ~94, 92 and 91 % removal efficiencies of Cr3+, Ni2+ and Zn2+ were reached in the first cycle and over ~87, 84 and 83 % efficiencies were obtained in the fourth adsorption/desorption cycle, demonstrating that the prepared material is cost-effective, and regeneration of it by 0.1 N HNO3 solution is quite easy. The metals containing regeneration solution after desorption were precipitate out as complex hydroxide from the solution on addition of NH4OH, NaOH and Ca(OH)2 into the solution. The Cr3+, Ni2+ and Zn2+ ions were almost insoluble at pH ≥7.5, 9.5 and ≥9.0 that forms precipitate of Cr(OH)3, Ni(OH)2 and Zn(OH)2 in solution. The chromium, nickel and zinc metals present in the precipitated solid volume were then effectively recovered. The adsorption and desorption process on CA/TPC is shown by the following mechanism:
Competitive adsorption
The competitive adsorption among the chromium, nickel and zinc metal ions was studied, Fig. 14. In the presence of three metals with equal concentrations, the observed adsorption efficiencies of CA/TPC for Cr3+, Ni2+ and Zn2+ were 32.1, 96.1 and 33.6 %, respectively. For Ni2+ and Zn2+ metal binary solution, the efficiency for Ni2+ was 36 % more than its competitive Zn2+ ion while in Ni2+ and Cr3+ mixed solution, the removal for Ni2+ was almost double of Cr3+ ion. These outcomes revealed that in the presence of Ni2+, adsorptions of Zn2+ and Cr3+ were strongly affected. The observed change in adsorption behaviors may be due to the nature of metal ions and the interaction between CA/TPC and adsorbate species [49].
Application to electroplating wastewater
The applicability of the present material for electroplating wastewater system was tested. For the purpose, waste water samples were collected from electroplating units of Jalandhar city of Punjab (India). The concentrations of metals in the effluent sample were analyzed, Table 2. As shown in Table 1, the fresh samples of chromium, nickel and zinc water (100 cm3 each) were collected from electroplating units and mixed in equal volume proportion by volume (1:1:1 v/v). The resultant mixed sample solution was treated with fixed concentration of nitric acid followed by pH adjustment between 3.0 and 6.0 using buffer solution. Batch experiment was carried out by adding 50 cm3 of mixed sample in a conical flask taking 1.0 g of CA/TPC under optimized conditions as described. Percent removal efficiencies of CA/TPC for chromium, nickel and zinc were found to be 87.2, 94.4 and 90.5, respectively. It has been estimated that 1 m3 of effluent discharge containing average 16.21, 15.41 and 13.23 mg/dm3 chromium, nickel and zinc metals required ~1.5 kg of adsorbent to meet the discharge limit imposed by law.
Conclusion
The results from the present study exhibit the application potential of CA/TPC for the effective removal and recovery of chromium, nickel and zinc from simple and electroplating wastewater. The optimum Ni2+ and Zn2+ adsorption was found to occur in pH range of 4–6, whereas best Cr3+ adsorption was achieved at pH 3. The adsorption isotherms for Cr3+, Ni2+ and Zn2+ uptake at given temperatures were best represented by the Langmuir model (R 2 > 0.99) suggesting that the surface was relatively homogenous in terms of functional groups. The kinetics data for Cr3+, Ni2+ and Zn2+ adsorptions were best fitted in pseudo-second-order rate equation as evident from the regression coefficients (R 2 > 0.99). Thermodynamic data illustrate that Cr3+, Ni2+ and Zn2+ adsorption reactions were endothermic and spontaneous. The adsorbed Cr3+, Ni2+ and Zn2+ ions were best recovered using 0.1 N HNO3 solutions at system pH 1, and the regenerated CA/TPC was effectively used up for four consecutive cycles with <10 % loss in weight. The experimental data show that the CA/TPC has reasonable good adsorption capacities for Cr3+, Ni2+ and Zn2+ ions.
Abbreviations
- CA/TPC:
-
Cellulose acetate–tin (IV) phosphate composite
- n :
-
Adsorption intensity (g/dm3)
- b :
-
Affinity of the binding sites (dm3/mg)
- A :
-
Temkin constant (mg/g)
- B :
-
Temkin constant (dm3/mg)
- q t :
-
Adsorption capacity at time (t) (mg/g)
- q e :
-
Adsorption capacity at equilibrium (mg/g)
- h :
-
Initial adsorption rate as t → 0 (mg/g min)
- k 2 :
-
Pseudo-second-order constant (g/mg min)
- α :
-
Initial metal adsorption rate (mg/g min)
- β :
-
Desorption constant (g/mg)
- C :
-
Intercept
- K id :
-
Initial rate of intraparticle diffusion (mg/dm3 min1/2)
- ∆G :
-
Gibbs free energy (kJ/mol)
- ∆H :
-
Enthalpy change (kJ/mol)
- ∆S :
-
Entropy change (J/K mol)
- k c :
-
Equilibrium constant of the adsorption process
- T :
-
Absolute temperature (K)
- R :
-
Universal gas constant (8.314 J/K mol)
- t :
-
Adsorption time (min)
- R L :
-
Dimensionless separation factor
- C 0 :
-
Initial liquid phase concentrations of metal ions (mg/dm3)
- C e :
-
Equilibrium liquid phase concentrations of metal ions (mg/dm3)
- C AD :
-
Equilibrium concentration of metal on the adsorbent (mg/dm3)
- q e,Cal. :
-
Calculated equilibrium adsorption capacity (mg/g)
- q e,exp. :
-
Experimental equilibrium adsorption capacity (mg/g)
- χ 2 :
-
Chi-square
- q m,Cal. :
-
Calculated maximum adsorption capacity (mg/g)
- q m,exp. :
-
Experimental maximum adsorption capacity (mg/g)
- R 2 :
-
Correlation coefficient
- AAS:
-
Atomic absorption spectrophotometer
- k f :
-
Multilayer adsorption capacity (mg/g)
- V :
-
Volume of metal solution (dm3)
- AD:
-
Adsorbent dosages
- m :
-
Mass of adsorbent (g)
- EPU:
-
Electroplating unit
References
Gupta VK, Jain R, Nayak A, Agarwal S, Shrivastava M (2011) Removal of the hazardous dye: tartrazine by photodegradation on titanium dioxide surface. Mater Sci Eng C 31:1062–1067
Khani H, Rofouei MK, Arab P, Gupta VK, Vafaei Z (2010) Multi-walled carbon nanotubes-ionic liquid carbon paste electrode as a super selectivity sensor: application to potentiometric monitoring of mercury ion (II). J Hazard Mater 183:402–409
Owlad M, Aroua MK, Daud WAW, Baroutian S (2008) Removal of hexavelant chromium contaminated water and wastewater: a review. Water Air Soil Pollut 200:59–77
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling: an overview. RSC Adv 2:6380–6388
Saleh TA, Gupta VK (2012) Column with CNT/magnesium oxide composite for lead (II) removal from water. Environ Sci Pollut Res 19:1224–1228
Gupta VK, Srivastava SK, Mohan D, Sharma S (1998) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Manag 17:517–522
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010) Decoloration treatment of a hazardous triarylmethane dye, Light Green SF (Yellowish) by waste material adsorbents. J Colloid Interf Sci 342:518–527
Mittal A, Kaur D, Malviya A, Mittal J, Gupta VK (2009) Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J Colloid Interf Sci 337:345–354
Mittal A, Mittal J, Malviya A, Gupta VK (2009) Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J Colloid Interf Sci 340:16–26
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interf Sci 344:497–507
Saleh TA, Gupta VK (2012) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interf Sci 371:101–106
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Sep Sci Technol 38:463–481
Sharma S, Kothiyal NC (2013) Use of activated dry flowers (ADF) of Alstonia scholaris for chromium (Vl) removal: equilibrium, kinetics, and thermodynamics studies. Environ Sci Pollut Res 20:8986–8995
Sharma S, Kothiyal NC (2013) Study of chromium (Vl) adsorption using Pterospermum-acerifolium fruit capsule activated carbon (FCAC) and commercial activated charcoal (CAC) as a selective adsorbents. Holist Approach Environ 3:63–82
Case OP (1974) Metallic recovery from wastewaters utilizing cementation. EPA-270/2-74-008, US, Washington, DC
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32:12–17
Alyüz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488
Clearfield A (1982) Inorganic ion exchange materials. Florida-CRC Press, Boca Raton
Khan AM, Ganai SA, Nabi SA (2009) Synthesis of a crystalline organic–inorganic composite exchanger, acrylamide stannic silicomolybdate: binary and quantitative separation of metal ions. Colloids Surf A Physicochem Eng Asp 337:141–145
AL-Othman ZA, Naushad M, Nilchi A (2011) Development, characterization and ion exchange thermodynamics for a new crystalline composite cation exchange material: application for the removal of Pb2+ ion from a standard sample (Rompin Hematite). J Inorg Organomet Polym 21:547–559
Khan A, Asiri AM, Rub MA, Azum N, Khan AAP, Khan I, Mondal PK (2012) Review on composite cation exchanger as interdicipilinary materials in analytical chemistry. Int J Electrochem Sci 7:3854–3902
Naushad M, AL-Othman ZA, Islam M (2013) Adsorption of cadmium ion using a new composite cationexchanger polyaniline Sn(IV) silicate: kinetics, thermodynamic and isotherm studies. Int J Environ Sci Technol 10:567–578
Varshney KG, Rafiquee MZA, Somya A, Drabik M (2006) Synthesis and characterization of Hg (ll) selective n-butyl acetate cerium (lV) phosphate as a new intercalated fibrous ion exchanger: effect of surfactants on the adsorption behavior. Ind J Chem 45A:1856–1860
El-Naggar IM, Zakaria ES, Ali IM, Khalil M, El-Shahat MF (2012) Kinetic modeling analysis for the removal of cesium ions from aqueous solutions using polyaniline titanotungstate. Arab J Chem 5:109–119
Khan AA, Khan A, Habiba U, Paquiza L, Ali S (2011) Preparation and characterization of electrically conducting polypyrrole Sn (IV) phosphate cation-exchanger and its application as Mn(II) ion selective membrane electrode. J Adv Res 2:341–349
Khan AA, Habiba U, Khan A (2009) Synthesis and characterization of organic–inorganic nanocomposite poly-o-anisidine Sn(IV) arsenophosphate: its analytical applications as Pb(II) ion-selective membrane electrode. Int J Anal Chem. doi:10.1155/2009/659215
Mispa KJ, Subramaniam P, Murugesan R (2013) Studies on ion-exchange properties of polyaniline Zr (IV) tungstoiodophosphate nanocomposite ion exchanger. J Polym. doi:10.1155/2013/356058
Gupta VK, Agarwal S, Pathania D, Kothiyal NC, Sharma G (2013) Use of pectin–thorium (IV) tungstomolybdate nanocomposite for photocatalytic degradation of methylene blue. Carbohydr Polym 96:277–283
Gupta VK, Pathania D, Singh P, Rathore BS, Chauhan P (2013) Cellulose acetate–zirconium (IV) phosphate nanocomposite with enhanced photo-catalytic activity. Carbohydr Polym 95:434–440
Rathore BS, Sharma G, Pathania D, Gupta VK (2014) Synthesis, characterization and antibacterial activity of celluloseacetate–tin (IV) phosphate nanocomposite. Carbohydr Polym 103:221–227
Khan A, Khan AAP, Asiri AM, Rub MA (2014) Synthesis of silver embedded poly(o-anisidine) molybdophosphate nano hybrid cation-exchanger applicable for membrane electrode. PLoS One. doi:10.1371/journal.pone.0096897
Khan MR (2014) Transport phenomena of inorganic–organic cation exchange nanocomposite membrane: a comparative study with different methods. J Nanostruct Chem. doi:10.1007/s40097-014-0095-0
Nabi SA, Naushad Mu (2008) Synthesis, characterisation and analytical application of a new composite cation exchanger cellulose acetate–Zr (IV) molybdophosphate. Colloids Surf A 316:217–225
Nabi SA, Naushad Mu, Bushra R (2009) Synthesis and characterization of a new organic–inorganic Pb2+ selective composite cation exchanger acrylonitrile stannic (IV) tungstate and its analytical applications. Chem Eng J 152:80–87
Nabi SA, Naushad Mu, Bushra R (2009) A new hybrid EDTA-zirconium phosphate cation exchanger: synthesis, characterization and adsorption behaviour for environmental monitoring. Adsorpt Sci Technol 27:423–437
Siddiqui WA, Khan SA, Inamuddin SA (2007) Synthesis, characterization and ion exchange properties of a new and novel organic-inorganic hybrid cation exchanger: poly (methyl methacrylate) Zr(IV) phosphate. Colloids Surf A 295:193–199
Sanchez C, Julian B, Belleville P, Popall M (2005) Applications of hybrid organic–inorganic nanocomposites. J Mater Chem 15:3559–3592
Naushad M (2014) Surfactant assisted nano-composite cation exchanger: development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem Eng J 235:100–108
Freundlich H (1906) Uber die adsorption in losungen. Zeitschrift fur Physikalische Chemie 57:385–470
Sharma S, Kothiyal NC (2012) Removal of Cr(VI) from aqueous solution by polymer based guar gum-g-poly (AAm) and activated charcoal adsorbents. Holistic Approach Environ 2:3–22
Pandey PK, Sharma SK, Sambi SS (2010) Kinetics and equilibrium study of chromium adsorption on zeoliteNaX. Int J Environ Sci Technol 7:395–404
Ho Y (2006) Review of second-order models for adsorption systems. J Hazard Mater B136:681–689
Sahu SK, Meshrama P, Pandey BD, Kumar V, Mankhand TR (2009) Removal of chromium (III) by cation exchange resin, Indion 790 for tannery waste treatment. Hydrometallurgy 99:170–174
Kumar E, Bhatnagar A, Choi JA, Kumar U, Min B, Kim Y, Songd H, Paeng KJ, Jung YM, Abou-Shanab RAI, Jeon BH (2010) Perchlorate removal from aqueous solutions by granular ferric hydroxide (GFH). Chem Eng J 159:84–90
Karthikeyan S, Gupta VK, Boopathy R, Titus A, Sekaran G (2012) A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J Mol Liquids 173:153–163
Green wood NN, Earnshaw A (2011) Chemistry of the elements (2/e). Elsevier, Butterworth-Heinemann
Pang Y, Zeng G, Tang L, Zhang Y, Liu Y, Lei X, Li Z, Zhang J, Xie G (2011) PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 281:278–284
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interf Sci 261:309–314
Ricou P, Lecuyer L, Le Cloiree P (1998) Influence of operating conditions on heavy metal cation removal by fly ash in aqueous solutions. Environ Technol 19:1005–1016
Malkoc E, Nuhoglu Y (2005) Investigations of nickel (II) removal from aqueous solutions using tea factory waste. J Hazard Mater 127:120–128
Balan C, Bilba D, Macoveanu M (2009) Studies on chromium (III) removal from aqueous solutions by sorption on Sphagnum moss peat. J Serb Chem Soc 74:953–964
Ho YS, John Wase DA, Forster CF (1995) Batch nickel removal from aqueous solution by sphagnum moss peat. War Res 29:1327–1332
Machado R, Carvalho JR, Correia MJN (2002) Removal of trivalent chromium (III) from solution by biosorption in cork powder. J Chem Technol Biotechnol 77:1340–1348
Levine RI, Rubin DS (2005) Statistics for management (7/e). Prentice-Hall, Pearson Education
Acknowledgments
One of the authors Suriti Sharma thanks the authorities of Dr. B. R. Ambedkar National Institute of Technology, Jalandhar, (Punjab) India, for providing all necessary laboratory facilities and infrastructure to carry out the present work.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s40090-015-0035-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sharma, S., Kothiyal, N.C., Rathore, B.S. et al. Use of cellulose acetate–tin (lV) phosphate composite (CA/TPC) in highly effective removal and recovery of heavy metal ions. Int J Ind Chem 6, 43–58 (2015). https://doi.org/10.1007/s40090-015-0031-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-015-0031-2