Abstract
Purpose
This study aimed to develop a transdermal delivery system for fluocinolone acetonide (FLA), a corticosteroid used in treating inflammatory conditions like rheumatoid arthritis (RA), to overcome the limitations of oral administration, such as poor solubility and bioavailability.
Methods
FLA-loaded PEG decorated hyalurosomes (FLA-PHs) were fabricated using ethanol injection, incorporating various Brij® surfactants and different amounts of hyaluronic acid (HA) based on a full factorial design. The impact of independent variables, HA amount (mg) (X1) and Brij type (X2) were inspected for entrapment efficiency (EE%), particle size (PS), and zeta potential (ZP). The optimum FLA-PHs were then incorporated into ε-polycaprolactone (PCL) and cellulose acetate (CA) nanofibers to enhance sustained transdermal delivery (FLA-NFs).
Results
The optimum FLA-PHs exhibited EE% of 83.58 ± 0.69%, PS of 169.00 ± 1.41 nm, and ZP of -22.90 ± 0.14 mV. Morphological assessment of FLA-NFs showed promising results in terms of surface roughness. In a Freund-induced rat model of adjuvant-induced arthritis, transdermal treatment with FLA-NFs significantly improved joint histopathological analyses. Furthermore, it suppressed inflammatory markers such as mTORC1, TNF-α, and NF-κB while upregulating TRIM24 and the anti-inflammatory IL-10.
Conclusion
FLA-NFs present a promising strategy for enhancing the transdermal delivery of FLA for managing RA, offering potential improvements in efficacy and reduced systemic side effects compared to conventional oral administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The autoimmune disease known as rheumatoid arthritis (RA) is characterized by symmetric polyarthritis that is both severe and progressive. A pathological feature of RA is abnormal hyperplasia of the synovium. Fibroblast-like synoviocytes (FLS) emit a range of chemokines, proinflammatory factors like interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), matrix metalloproteinases (MMPs), and cathepsin to degrade the cartilage and extracellular matrix (ECM) (Pulik et al. 2023). According to (Elhabal et al. 2024c), the regulation of cell growth, proliferation, differentiation, and death is significantly influenced by the mammalian target of rapamycin (mTOR) signaling cascade (Elhabal et al. 2024a; Fathy Elhabal et al. 2024b). Laragione and Gulko (2010) discovered that the mTOR signaling system controlled the invasion of RA-FLS. While inflammation is an immune response to insults, it also aids in maintaining tissue homeostasis (Laragione and Gulko 2010). Hundreds of genes encoding mediators and suppressors of inflammation, including Tripartite motif (TRIM) proteins, are activated by various signaling cascades during stressful situations (Yang and Xia 2021; Koumadorakis et al. 2023). It has been demonstrated that the large family of E3 ligases known as TRIM proteins regulate inflammation (Koumadorakis et al. 2023). According to Jeon et al. (2020), TRIM24 is a significant transcriptional coregulator that controls nuclear factor kappa B (NF-κB)-mediated TNF-α signaling (Jeon et al. 2020). According to Lo et al (2021), roughly 1% of people in the general population have RA (Lo et al. 2021). In addition to being an extremely debilitating condition, RA causes substantial economic burdens because of its propensity to cause distant tissue damage (Schett et al. 2021; Elhabal et al. 2024a). RA has no known cure; therefore, creating a therapeutic regimen that may minimize the genesis of the illness is crucial.
Fluocinolone acetonide (FLA) is a synthetic hydrocortisone derivative that, due to its immunosuppressive, anti-inflammatory, and antiproliferative characteristics, serves as a promising arthritis treatment. However, because FLA is a BCS class II medication with low solubility, bioavailability, and dose-dependent side effects, its traditional topical formulation is limited (Pradhan et al. 2020; Fathy Elhabal et al. 2023). In line with Talebi et al (2023), transdermal nanofiber (NFs) systems for FLA can therefore be created to improve the solubility and dissolution kinetics while maintaining its sustained release that fosters higher compliance (Talebi et al. 2023).
Brij® are amphiphilic surfactants consisting of a hydrophobic alkyl or aryl group and a hydrophilic polyoxyethylene (PEG) chain. In an aqueous environment, Brij® surfactants can self-assemble into micelles or other nanostructures, with the hydrophobic tails inward and the hydrophilic PEG chains outward. PEG chains increase the hydrophilicity of the nanoparticles, providing a steric stabilization effect which helps prevent aggregation (Patel and Thakkar 2023). PEGylation, which involves attaching PEG chains, is recognized for its ability to improve biocompatibility and prolong circulation duration in biological systems (Albash et al. 2021b; Dhandapani et al. 2022). Sodium hyaluronate, also known as hyaluronan, is the sodium salt derivative of hyaluronic acid. It is naturally found in various human organs, including connective tissues, lungs, synovial fluid and muscle tissue. Hyaluronan is a negatively charged, high molecular weight carbohydrate glycosaminoglycan. Hyalurosomes (HS) are nanovesicles made of hyaluronan polymer and surfactant. They have specific properties such as biocompatibility, gelling capabilities, and the ability to enhance penetration with high physical stability, drug deposition, and load and retain drugs effectively (Elhabal et al. 2024b). To overcome the low solubility of FLA, HS composed of Brij, PEG, and hyaluronic acid can be formulated. The hydrophobic center of the HS can trap FLA, enhancing its ability to dissolve in water-based surroundings. The nanoparticle formulation provides stability to the medicine, preventing its degradation and improving its shelf-life and effectiveness. Wsoo et al. (2020) reported cellulose acetate (CA) is extremely soluble, biocompatible, semi-biodegradable, non-irritating, and non-toxic. Since ε-polycaprolactone (ε-PCL) degrades slowly, it is a good option for long-term medication administration (Wsoo et al. 2020). Thus, using coaxial CA and ε-PCL NFs membranes with FLA-loaded PEG decorated hyalurosomes (FLA-PHs) might produce composite NFs with a uniform and smooth surface, good mechanical strength, hydrophilicity, and improved biocompatibility qualities for possible use in a variety of biomedical fields (Pradhan et al. 2020). It is worth noting that Brij® was utilized in the fabrication of the nano-system to obtain the merit of PEG as its hydrophilic PEG moieties in surfactants could increase the water uptake in stratum corneum (SC), which leads to SC swelling and it might also cause structural alterations in SC lipids and/or corneocytes which lead to broadening of the intercellular junctions in the barrier, and results in enhanced FLA retention and deposition (Fathy Elhabal et al. 2023).
Thus, the current research work aimed at the development of FLA- loaded PEG decorated hyalurosomes (FLA-PHs) and electrospun CA/ε-PCL NFs loaded FLA-PHs (CA/PCL loaded FLA-HS) a novel nano-transdermal drug delivery system for targeting RA in rats. A Complete Freund’s Adjuvant (CFA) induced arthritis rat model was established, and FLA delivery and anti-arthritic efficacy using FLA-PHs and CA/PCL loaded FLA-PHs were compared with FLA suspension. Also, study effect of different formulations of FLA on inflammatory mTOR, TNF-α and NF-Κb and histopathological examination using Hematoxylin and Eosin (H&E) and Safranin O stain was studied.
Materials and methods
Materials
The electrospinning polymers were cellulose acetate (CA, Mn = 30,000, 180,955) and poly ɛ-caprolactone (PCL, Mn = 80,000). Organic solvents such as acetone (99%), N, N-dimethylacetamide (99%), N, N-dimethylformamide (99.9%), absolute ethanol, fluocinolone acetonide (FLA), hyaluronic acid (HA) with a molecular weight (400–800 k. Dalton), and cholesterol (CH), were purchased from Sigma Aldrich, Methanol. Diethyl ether, ketamine, and xylazine were supplied by Boehringer Ingelheim in Germany and acetonitrile (HPLC-grade) was obtained from Merck and Brij93, Brij58, Brij30 and Hydroxypropyl methylcellulose K4m (HPMC) from Alpha Chemika in Andheri, India. Complete Freund’s adjuvant (CFA) was acquired from Sigma-Aldrich (St. Louis, MO).
Preparation of fluocinolone acetonide-loaded PEG decorated hyalurosomes (FLA-PHs)
An ethanol injection approach was employed to prepare the FLA-PHs. In brief, 2 ml of absolute ethanol were used to dissolve various kinds of Brij® (10 mg), CH (100 mg), and FLA (10 mg). A 10-ml hydration medium solution containing different amounts of HA in water was mixed continuously with a magnetic stirrer (Model MSH-20D, GmbH, Germany) for 30 min at room temperature at 900 rpm. A 23-G syringe was used to administer the aqueous phase dropwise. Finally, the dispersion was further described after being hermetically sealed and stabilized for the following day at 4◦C in the refrigerator (Elhabal et al. 2024a).
In vitro characterization of the prepared fluocinolone acetonide-loaded PEG decorated hyalurosomes (FLA-PHs):
Determination of entrapment efficiency (EE%)
The estimation of PHs EE% was obtained by an indirect method of determining free FLA (unentrapped FLA). The resultant formula (1 mL) was processed for 60 min at 4°C using a cooling centrifuge (Beckman Optima®, Ultracentrifuge, California, USA) running at 21,000 rpm. After separating the clear supernatant, the amount of drug in the filtrate was evaluated using a spectrophotometer (UV-1800 240 V, Shimadzu Corporation, Kyoto, Japan) set to measure wavelengths of 240 nm to calculate the quantities of unentrapped FLA. The EE% was computed using the subsequent formula (Elhabal et al. 2022; Al-Shoubki et al. 2024).
The total amount of FLA is the real weighed quantity, and the total amount of free FLA is the quantity of FLA in the supernatant.
Measurements of particle size (PS), distribution, and Zeta potential (ZP)
The FLA-PHs 1 mL were diluted with bi-distilled water (ten-folds), and the Zetasizer 3600 (Malvern Instruments, Malvern, U.K.) was used to measure the PS using the light scattering technique (DLS) at 25 °C. The light scattering angle was fixed at 90°, and the laser’s obscuration range was 10 to − 0%. Every sample received three cycles. ZP was determined using the same equipment by tracking the mobile particles in an electric field (Elhabal et al. 2022, 2024a).
Selection of the optimum FLA-loaded PHs
The study explored the effects of various independent parameters using the full factorial design. Two factors were assessed: the amount of hyaluronic acid (HA) (mg) (X1) and the type of Brij® (X2). The chosen dependent variables were EE%, PS, and ZP (Table 1). Each parameter was subjected to an ANOVA test using the Design-Expert® software (Version 7, Stat-Ease Inc., Minneapolis, MN). A cutoff point of p < 0.05 was utilized for significance. After optimization, the system recommended the formulation with the lowest (PS) and highest (EE% and ZP) values. The desirability function, which enables simultaneous examination of all restrictions, served as the foundation for the recommendation. The formulation that produced the most desirable result was selected. To ensure the model performed accurately, the suggested ideal formula was created and tested, and its results were compared to the anticipated data (Al-Shoubki et al. 2023).
Freeze-drying of FLA-loaded PHs solutions
FLA-loaded PHs underwent freezing and lyophilization using the Novalyphe-NL 500 lyophilizer (Savant Instruments; NY, USA) for 24 h at 45 °C under a pressure of 7 mbar. Subsequently, solid-state investigations were conducted on the resulting lyophilized FLA-loaded PHs (Fathy Elhabal et al. 2023).
Fabrication of cellulose acetate/polycaprolactone nanofibers loaded FLA-HS
Electrospinning fabrication
The polymer solution was added to the electrospinning device using a syringe pump (NE-300) from New Era Pump Systems, which produced webs of NFs covered in foil. To introduce the polymer solutions into an electrospinning apparatus, a 5-mL syringe was utilized. Coaxial needles (inner 21 gauge, exterior 15 gauge) were used for coaxial electrospinning. The tip-collector distance was established at 15 cm, and the electrospinning voltage (8.5 kV) was employed (E-Spin Nanotech, Electrospinning Unit ES-II Super). The syringe was horizontally positioned on the syringe pump. The meal was administered at a controlled rate of 0.1–0.5 ml/hr using a syringe pump. Electrospinning was done inside the chamber with continuous UV light. Aluminum foil was used to gather nanofibers and was encircled by a plate collector. At 40 °C, the NFs were dried in a vacuum oven. The CA/PCL-NFs were inserted into a tiny cartridge until additional atmospheric analysis (Permyakova et al. 2024).
Characterization of synthesized nanofibers:
Nanofibers morphology investigation
An energy-dispersive X-ray detector (EDX) equipped with a scanning electron microscope (SEM; JSM-6510LV, JEOL, Tokyo, Japan) was used to perform the elemental analysis (El-Nawawy et al. 2024). After cutting them into squares with a sharp knife, scaffolds were covered with gold using a sputter coating. The gold-coated scaffolds were then examined at magnifications of 1000 and 5000 (Mohammed et al. 2023a). The average diameter of the Electro spun fibers was computed using SEM micrographs. Fifty to two hundred NFs were selected and examined in each SEM micrograph obtained during the measuring procedure (Mohammed et al. 2023b). Using an atomic force microscope (AFM) on a Dimension Icon microscope (Bruker, Massachusetts, USA), surface morphology and roughness measurements were made. Following the attachment of the NFs membrane, a glass slide was subjected to tapping mode scanning (2 m scan size, 0.894 Hz scan rate, 512 samples per line) (Elhabal et al. 2023).
Chemical analysis
To record the NFs’ infrared spectra, a double-beam dispersive Fourier transform infrared (FTIR) spectrometer (Nicolet iS20, Thermo Electron Corporation, Warwickshire, UK) was used. It used a selected range of 500–4000 (cm−1), with 2 cm−1 resolution, aggregating 100 scans (Shoman et al. 2023).
Mechanical tensile testing
A 20 N load cell on an Autograph AG–X series tabletop tensile testing machine made in Shimadzu, Japan, was used to evaluate the mechanical strengths of the CA/PCL -FLA loaded NFs and the blank NFs that were constructed. For the tested NFs, the mechanical characteristics, such as breaking strain (%), maximum displacement (mm), and tensile stress (KPa), were calculated (Barbosa et al. 2023).
In-vitro release study
In-vitro release was determined using the United States Pharmacopeia (USP) dissolution apparatus (Pharma Test, Hainburg, Germany) for 72 h at 37 °C. Samples of 1 mL (containing 1 mg FLA) from the optimum FLA-PHs and FLA-PHs loaded NFs were placed in plastic cylindrical tubes which have a permeation area of 3.14 cm2 with one end tightly covered with a cellulose membrane and the other end attached to the shaft of the USP dissolution apparatus instead of the baskets. The formulae were immersed in 50 mL PBS (pH 7.4) (Albash et al. 2019). The sink condition was maintained in this volume. Aliquots were withdrawn at 1, 2, 3, 4, 5, 6, 8, 10, 24, 48 and 72 h. FLA in aliquots was analyzed by a UV spectrophotometer at λmax 240 nm, and the experiment was performed in triplicate.
Freund’s adjuvant-induced rheumatoid arthritis in vivo model in rats
Animals
Thirty-two adults female Wistar rats weighing (200–250) gm were obtained from the Faculty of Pharmacy at Cairo University in Cairo, Egypt, and were adjusted to laboratory conditions over two weeks. Before the trial began, the animals were kept in a pathogen-free, controlled, air-conditioned environment. The animals were housed in polyacrylic cages with a 12/12-h light and dark cycle at 25 ± 2°C. They also have unrestricted access to regular food and tap water. The ARRIVE guidelines were adhered to throughout the entire trial (experimental) process. The Faculty of Pharmacy, Cairo University, Egypt’s Research Ethics Committee endorsed this experimental study (PI: 2942). It is worth mentioning that for better application of the nanoformulation and to enhance its retention, the optimum PHs were converted into gel to enhance the retention where a specified amount of HPMC was mixed with the assistance of a magnetic stirrer to the optimum PHs to have a final FLA gel loaded PHs concentration of 2% w/w.
Experimental design
Rats were randomly categorized into four groups, each consisting of eight. Group I (Negative Control): a typical control group that received only vehicles. Induction of RA caused by Complete Freund’s Adjuvant (CFA) was in groups II, III, and IV. Group II (Positive Control): rats were injected by CFA for induction of RA using three subcutaneous doses of CFA, each of 0.4 ml, injected in three different limbs with three days intervals between every two doses, separated by three days to induce RA (Salem et al. 2020). Group III (FLA-gel loaded optimum PHs) gel (2% (w/w) HPMC): Rats were given topical and were applied daily. The gel (1 g) was applied on the knee joint surface of each test animal to an area of 4 cm2. Group IV (FLA-NFs): FLA-NFs were applied daily to the rats as a treatment. The test animal’s knee joint surface received one application of the NFs patch 4 cm2 area where hair was shaved off. All therapies were administered for 27 days, starting on the 13th day. Rats were starved for 12–14 h the previous night, and 8 ml of blood was drawn from the retro-orbital plexus, where blood is found under anaesthesia, followed by 60 mg/kg ketamine plus 6 mg/kg xylazine (Sotoudeh and Namavar 2022). The sample was gathered and centrifuged for 15 min at 3000 rpm. The clear, non-hemolyzed supernatant sera were extracted right away, aliquoted in Eppendorf tubes, and stored at – 20 °C until they were needed for biochemical tests to measure the levels of mTOR, TNF-α, NF-κB, TRIM 24/TIF1α, and IL-10. Rats were rapidly terminated by cervical dislocation as soon as the blood was extracted. The right knee joint with the distal femur and proximal tibia were dissected and preserved in neutral buffered formalin at ten percent dilution for 72 h. Knee joint samples were carefully cut off from surrounding soft tissue and muscles. They were decalcified in a weekly change of a 10% ethylene-diamine tetra-acetic acid (EDTA) solution at 7.4 pH buffered at 4 ˚ degrees for 4 weeks for each experimental group of rats, eight of their knee joints were processed for histological pathological evaluation (Saleem et al. 2020).
Histopathological examination using hematoxylin and eosin (H&E) and safranin O stain
Under a light microscope, softened decalcified formalin-fixed knee joints were trimmed from Group I (Negative Control); Group II (RA, positive control), Group III (RA + FLA-gel loaded optimum PHs) and Group IV (FLA-NFs). Joint tissues were trimmed from the extra bone of the tibia and femur, washed with tap water, dehydrated in ascending ethanol grades, cleared in xylene, and processed for paraffin blocks. Four µm thick paraffin knee joint sagittal sections were processed for general histopathological evaluation and scoring and proteoglycan content of extracellular matrix of cartilage by H&E and Safranin orange staining, respectively, graded the osteoarthritis cartilage histopathology using the grading approach displayed. This grading approach was done on 24 microscopic fields, 3 sections from each rat from each group (8 rats /group) of the following groups: (RA, positive control), (RA + FLA-gel loaded optimum PHs) and (FLA-NFs) as shown in Table 2 (Pritzker et al. 2006).
Results and Discussion
Optimization of PEGylated hyalurosomes (PHs) using full factorial design
The preliminary trials aimed to ascertain the independent variables’ effective ranges (data not shown). Using Design-expert® software, the full factorial design was applied to optimize the created PHs, resulting in nine experimental runs. In the cases of EE%, PS, and ZP, the 2FI model was chosen. Sufficient accuracy is employed to claim that the model could be chosen to move across the design area. Table 3 shows that a ratio larger than four is preferred for all dependent variables. For every dependent variable, the expected R2 and adjusted R2 agreed as intended (Fouad et al. 2023).
Model analysis of EE%
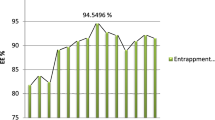
The range of EE% for PHs loaded with FLA was 43.85 ± 3.37 to 98.53 ± 1.10%. The plausible EE% of FLA within PHs may be associated with CH, which gave the bilayers stiffness and consequently increased FLA EE% inside PHs (Yasser et al. 2024).Table 4 presents the impact of the independent variables, HA amount (X1) and Brij type (X2), on the EE% of FLA in PHs. This effect is visually represented in Fig. 1a as three-dimensional (3-D) surface plots. EE% was significantly impacted by HA amount (mg) (X1) (p < 0.0001). It was discovered that increasing the amount of HA from 5 to 15 mg increased EE%, which may be connected to the fact that HA is known to promote vesicle rigidity, which prevents drug leakage into aqueous media. Our investigation’s results are consistent with those of Sadeghi-Ghadi et al. (2021) (Sadeghi-Ghadi et al. 2021), who found that niosomes containing HA produced an adequate EE% of quercetin (Albash et al. 2021a, b; Elhabal et al. 2024a). Furthermore, EE% was significantly affected by Brij type (X2) (p < 0.0001); EE% rose when Brij93 was utilized in comparison to other kinds since it had the lowest HLB value among the Edge activators tested. Brij 58, Brij 30, and Brij 93 had HLB values of 15.7, 10.7, and 4.9, respectively. Our results were in line with those of Aboud et al. because it is widely known that SAAs with lower HLB values are more lipophilic and would encourage the EE% of lipophilic medicines. This helps to explain why utilizing low HLB value SAAs resulted in a higher EE% of FLA, a highly lipophilic medication.
Model analysis of PS
PS varied from 108.40 ± 2.64 to 764.60 ± 46.28 nm in the prepared PHs. Table 4 displays the effect of the independent factors, HA amount (mg) (X1) and Brij type (X2), on the PS of FLA-loaded PHs. This effect is visually represented in Fig. 1b as three-dimensional (3-D) surface plots. PS of FLA-loaded PHs was substantially impacted by HA amount (mg) (X1) (p < 0.0001). It was discovered that when the amount of HA was raised, the PS also rose. This may be because HA is a hydrophilic molecule that can absorb water and swell, increasing its size in aqueous conditions several times, leading to an increase in the PS of PHs. Regarding Brij type (X2), using Brij 93 produced the greatest PS (p < 0.0001), followed by Brij 30 and Brij 58. Based on previously stated HLB values, previous literature stated that using SAA with lower HLB might produce larger vesicles and vice versa (Saady et al. 2024).The results show that the PS outcomes and the EE% findings were correlated. Thus, the decline in EE% could offer an additional rationale for the reduced PS of PHs.
Evaluation of PDI
A zero value of PDI demonstrates monodisperse dispersion. On the contrary, a PDI value of 1 resembles polydisperse vesicles (Elhabal et al. 2024a). PDI values ranged from 0.32 ± 0.04 to 0.54 ± 0.02 (Table 4). This viewed the fabricated PHs as polydisperse but within an acceptable range (Fig. 1c).
Model analysis of ZP
ZP is utilized to evaluate the surface charge that PHs acquire. A stable nano-system is resembled by a ZP value greater than ± 15 mV, which provides a good indication of the physical stability of the PHs based on its magnitude. as Table 4 and Fig. 1d’s response 3-D plots reveal. The range of ZP values was − 17.00 ± 0.20 to − 24.40 ± 0.56 mV. It was discovered that the negative value of ZP dropped linearly when the concentration of HA was increased from 5 to 15 mg, considering the HA amount (mg) (X1). As was previously mentioned, employing a large amount of HA raised the PS of the prepared PHs. According to Xu et al., higher PS in nanovesicles may result in vesicles with lower ZP values and a low dielectric constant, meaning they won’t have enough electrostatic repulsion to keep particles from clumping together (Xu et al. 2023).
It was discovered that the PHs, generated by Brij 58, produced the lowest ZP values for Brij type (X2) compared to Brij 30 and Brij 93. This may be because Brij 58 (20 PEG units) had a higher concentration of hydrophilic PEG steric shield, which somewhat screened the carboxyl group surface charge at the vesicle surface and resulted in lower ZP values than Brij 30 and Brij 93, which had four and two PEG units, respectively.
Determination of the optimum PH
The non-significant effect on the response (PDI) was to be excluded from the optimization (PHs) by the limits indicated in Table 1. Using the Design-Expert®-7 program, numerical optimization based on the desirability criterion was used to determine the optimal values of the variables. A recommended optimum formula (PH2) with Brij 30 serving as SAA and 10 mg of HA. Table 4 shows the observed and expected values of PHs (PH2). The strong correlation between the PHs’ observed and anticipated outcomes may indicate that the response prediction design is valid. The hydrophilic PEG chains of Brij SAA extend into the surrounding aqueous medium, providing a steric barrier that prevents aggregation and stabilizes the PHs, the hydrophobic center of the PHs can trap FLA, enhancing its ability to dissolve in water-based surroundings. The nanoparticle formulation provides stability to the medicine, preventing its degradation and improving its shelf-life and effectiveness. Cell surface adhesion receptor (CD44) receptors mediate cell adhesion by binding to hyaluronic acid (HA), which is found in higher amounts in different inflammatory and malignant tissues. The HA shell enables the precise distribution of FLA to specific tissues, enhancing therapeutic effectiveness and minimizing systemic side effects. The formulation enables a controlled and continuous release of FLA, ensuring that effective levels of the medicine are maintained for a prolonged duration and minimizing the need for frequent administration. Implementing controlled release mechanisms can result in improved patient compliance and adherence to the prescribed treatment regimen.
Nanofibers morphology investigation
The separate electrospinning of CA/PCL and CA/PCL loaded FLA-PHs was accomplished, and it was an effective fabrication scaffold. When the intact CA/PCL membrane was morphologically characterized, it revealed a uniformly distributed, randomly distributed NFs mat with beads for CA/PCL loaded FLA and without beads for PCL loaded FLA, with a significant diameter distribution between 400 and 600 nm (Fig. 2a, b). Figure 2c, which displays the percentage of elements like C, O, Na, and S on the surfaces of FLA-NFs loaded with CA/PCL, illustrates the EDX mapping. The weight of the elements for CA/PCL loaded FLA-NFs was determined to be 47.8% carbon (C) atom at 0.37 keV, 39.5% oxygen (O) atom at 0.59 keV, 9.2% sodium (Na) at 2 keV, and 3.5% sulfur (S) at 3.89 keV. The estimations for surface roughness are shown in Fig. 2d. Because of the surfactant’s absorption, the CA/PCL-loaded FLA-NFs smooth to slightly rough appearance can change. Ionization of carboxylic end groups on the scaffold surface causes hydrophobic poly-oxy-propylene chains to surface bond with the solvent polymer, while protrusion of the hydrophilic poly-oxy-propylene chains in the surrounding media helps to skin the surface negative charge formed on the nanoparticles (NPs). The edge activator and the positive charge of the active molecules to be encapsulated (FLA) work together to counteract the negative charge of the polymeric material. Drugs can penetrate and release over time on the skin’s epithelium if they have a positive charge, which lengthens their residence time (Aly and Ahmed 2021; Barbosa et al. 2023). The presence of sulfur (S) at 2.32 keV and sodium (Na) at 1.04 keV serves as evidence for this. The electrospun CA/PCL nanofibers loaded with FLA-PHs exhibit superior morphology, enhanced elemental composition, and surface characteristics that support sustained drug release. These properties make the FLA-PHs loaded NFs a promising scaffold for transdermal drug delivery systems, offering extended therapeutic benefits and improved patient outcomes.
Morphology investigation of nanofiber, SEM of CA/PCL loaded FLA-PHS nanofibers a before and b after loading of lyophilized FLA hyalurosomes, Energy-dispersive X-ray (EDX) chemical element analysis of the scaffold (c), Atomic Force Microscopy (AFM) images of CA/PCL loaded FLA-HS nanofibers (d). CA/PCL ε-polycaprolactone/cellulose acetate nanofibrous, FLA-PHs fluocinolone acetonide- PEGylated hyalurosomes
Chemical analysis
Figure 3a illustrates how FTIR analysis was carried out to confirm that the formulation’s constituents did not interact chemically. A notable absorption band was seen at 1750 cm 1, most likely resulting from the ester groups’ carbonyl stretching. The NFs- PCL showed distinct peaks at 3000 cm−1, associated with the vibrations of methylene groups. However, the PCL main absorption band at 1750 cm−1 changed slightly to 1740 cm−1 in the active uniaxial mats. This change signifies a hydrogen bond between PCL’s carbonyl and FLA hydroxyl groups. Furthermore, unique peaks were seen in CA-based fibers at 1750 cm−1, linked to the stretching of C = O groups, 1150 cm−1, related to the bending of methylene groups C–H, and 1100 and 1030 cm−1, due to the stretching vibration of the backbone’s C–O–C linkage. Notably, distinctive bands from PCL and CA were seen in active FLA-NFs, and their peaks were altered because of their chemical interactions with FLA (Abdelmonem et al. 2021). In comparison to the control polymers, the most noticeable peaks in the FLA-NFs spectra were seen at 1750, 1150, 1100, and 1030 cm−1, which were associated with carbonyl stretching, methylene group C–H vibrations, and vibrations in C–O–C groups like those in FlA-NFs, bands associated with FLA may be hidden by polymer peaks or may not be seen at all (El-Gizawy et al. 2018).
a Fourier transform infrared (FTIR) spectra of coaxial CA/PCL -FLA hyalurosomes scaffold and PCL and CA control and b In vitro release of FLA from FLA-PHS and cellulose CA/PCL NFs-loaded FLA-HS compared to FLA suspension. CA/PCL ε-polycaprolactone/cellulose acetate nanofibrous, FLA fluocinolone acetonide
Mechanical tensile testing
Tensile testing is a characteristic examination indicating the strength and flexibility of a formulation. The mechanical properties of the electro-spun CA/PCL and FLA-PHsNFs were evaluated by measuring the breaking strain (%), maximum displacement (mm), and tensile strain (%). Intriguingly, adding FLA-PHs did not significantly change the NF’s maximum displacement (p > 0.05) compared to the blank CA/PCL NFs. However, as depicted in Table 5, the NFs’ breaking and tensile strain values decreased notably (p < 0.05) after incorporating FLA-PHs. These results suggest that the FLA-PHs may have clustered together, creating areas of concentrated stress in the NFs mats, thus potentially impacting their mechanical properties negatively. Previous research has also shown a reduction in NFs strength following the integration of FLA-PHs (Aly and Ahmed 2021).
In-vitro release study
Figure 3b compares the in vitro release of FLA from FLA-PHs and cellulose CA/PCL NFs-loaded FLA-PHS to pure FLA in PBS pH 7.4. The figure demonstrates each formulation’s effectiveness in suppressing FLA release. In the first release phase, the CA/PCL NFs-loaded FLA-PHs exhibit a modest amount of release in the beginning (2% after 1 h), suggesting a controlled release during the early stages. Conversely, the FLA suspension that is not restricted or limited has a greater initial discharge (2.1% within 1 h), which may result in a quick reduction of the medication. Over a period, the FLA-PHs formulation consistently releases the medicine in a regulated manner, achieving a 98% release rate after 72 h. This exhibits a continuous release pattern, which is essential for preserving therapeutic concentrations for a prolonged duration. The FLA suspension has a release rate of only 45% after 72 h, indicating a limited and short-lived release. The FLA-PHs formulation achieves a near-complete release (98%) of the medicine, ensuring optimal usage of the encapsulated molecule. The FLA suspension, available at no cost, has a significantly reduced total release, perhaps leading to less effective therapeutic results. In a previous study, FLA release from simple FLA suspension and FLA-loaded nanostructured lipid carriers (NLCs) suspension was measured in vitro for 24 h. FLA-loaded NLCs suspension showed higher initial burst release and delayed drug release, with 66.91 ± 2.90% FLA released at 24 h. However, ordinary FLA suspension drug release was faster, with 97.12 ± 4.90% release within 7 h (Pradhan et al. 2015). In another study, FLA-transfersomes (NTs) exhibited a biphasic release pattern, with initial burst release of ~ 29% up to 3 h and sustained release of 68.43 ± 3.2% at 24 h. FLA dispersion showed 21.02 ± 2.5% release until 24 h. Initially, 55–60% drug dissolution was responsible for burst release in phases 1–12 h, while continuous release of ~ 65% was detected in phases 12–24 h (Dhavale et al. 2021). This study confirms that the CA/PCL nanofiber system delivers FLA with the most regulated and sustained release, making it a promising long-term drug delivery option. Amorphous drug formation upon release from the FLA-PHs, the medication undergoes a transition to an amorphous state, which improves its solubility and absorption rates (Sultanova et al. 2016). The amorphous form does not possess the crystalline structure that frequently restricts the solubility of drugs. Increased solubility leads to improved bioavailability, guaranteeing a greater amount of the medication enters systemic circulation. This study confirms that the CA/PCL nanofiber system delivers fluocinolone acetonide with the most regulated and sustained release, making it a promising long-term drug delivery option. The addition of an FLA-loaded nanosystem to the fabricated NFs was based on enhanced solubility and absorption, the amorphous form encapsulating the drug within PHs converts it into an amorphous form upon release, significantly enhancing its solubility and absorption rates. This leads to improved bioavailability compared to the crystalline form of the free drug loaded directly into NFs. Release profile, the data indicates that CA/PCL NFs loaded with FLA-PHs exhibit a more controlled and sustained release over time compared to the free drug-loaded NFs and FLA suspension (Sultanova et al. 2016). This sustained release ensures a consistent therapeutic level of the drug in the bloodstream, minimizing peaks and troughs that can lead to side effects or reduced efficacy. Stability and protection could be obtained by PHs by shielding the drug from environmental factors such as pH and enzymatic degradation (Elhabal et al. 2024b). This protection is crucial for maintaining the integrity and therapeutic effectiveness of the drug until it reaches the target site. Reduction in dose frequency, the extended release provided by PHs means that the dosing frequency can be reduced, improving patient compliance and convenience. This is particularly beneficial for chronic conditions requiring long-term medication. The synergistic effects, of hyalurosomes can be loaded with multiple drugs or therapeutic agents, allowing for combination therapies that can provide synergistic effects. This multi-drug delivery system can enhance overall treatment efficacy (Sadeghi-Ghadi et al. 2021).
Biochemical results
Effect of fluocinolone nanofibers on inflammatory mTOR, TNF-α and NF-κB
Figure 4, induction of RA in rats provoked inflammation, signified by a marked elevation in mTOR, TNF-α and NF-κB tissue levels by 8, 3 and 4 folds, respectively, compared to control rats. However, treatment of arthritic rats with FLA significantly mitigated these markers by 48.7, 19.6, and 64.4%, respectively, compared to the RA group. Moreover, RA rats treated with FLA-PHs showed further remarkable mitigation by 65.2, 60, and 78.2%, respectively, compared to untreated RA rats, attaining levels more significant than that caused by topical FLA-free nanoparticles.
Rats’ articular tissue levels of mTOR, TNF-α & NF-κB in Group I (Negative Control); Group II (RA, positive control), Group III (RA + FLA-gel loaded optimum PHs) and Group IV (FLA-NFs). Data is presented as mean ± SD (n = 8/group). ****p < 0.0001, ***p < 0.001, and **p < 0.01. FLA-NF fluocinolone acetonide nanofibers, FLA-PHs FLA-loaded PEG decorated hyalurosomes, RA rheumatoid arthritis, FLA-PHs FLA-loaded PEG decorated hyalurosomes, mTOR mammalian target of rapamycin, RA rheumatoid arthritis, TNF-α tumor necrosis factor-α, NF-κB nuclear factor kappa B
Effect of fluocinolone nanofibers on anti-inflammatory TRIM 24/TIF1α and IL-10
Figure 5 revealed that RA induction in rats declined TRIM 24/TIF1α and IL-10 levels by 74.3, and 60%, respectively, compared to control rats. Conversely, treatment of arthritic rats with FLA considerably amplified these levels by 2 and 1.3 folds, respectively, when compared to untreated RA rats, with an additional elevation by 3 and 2.2 folds when RA rats were treated with CA/PCL loaded FLA-HSNFs.
Rats’ articular tissue levels of TRIM 24/TIF1α & IL-10 in Group I (Negative Control); Group II (RA, positive control), Group III (RA + FLA-gel loaded optimum PHs) and Group IV (FLA-NFs). Data is presented as mean ± SD (n = 8/group). ****p < 0.0001, ***p < 0.001, and **p < 0.01. FLA-NF fluocinolone acetonide nanofibers, FLA-PHs FLA-loaded PEG decorated hyalurosomes, RA rheumatoid arthritis, FLA-PHs FLA-loaded PEG decorated hyalurosomes, IL-10 interleukin-10, RA rheumatoid arthritis, TRIM 24/TIF1α tripartite motif-24/transcriptional intermediary factor 1α
RA is regarded as an autoimmune illness and a chronic inflammatory condition. In addition to greater local fibroblast activation, the pathogenesis of RA involves elevated levels of surface adhesion molecules and chemokines, which, in concert with local hypoxia, drive angiogenesis and lymphangitic dysfunction, establishing the early features of synovitis. Nevertheless, the complete etiology of RA remains unclear (Pulik et al. 2023). Consequently, this work aimed to investigate the potential underpinning pathogenic mechanism of RA induced by Freund’s adjuvant.
Our study showed an upregulation of mTOR, NF-κB, and TNF-α concomitant with the downregulation of TRIM24 and IL-10 in the RA group. Wu et al. (2017) and Feng and Qiu (2018) demonstrated that the PI3K/Akt/mTOR signaling pathway has a role in RA pathogenesis, which is consistent with our findings (Wu et al. 2017; Feng and Qiu 2018).
The mTOR signaling pathway influences numerous pathological conditions, including growth, proliferation, angiogenesis, metabolism, and autophagy. mTORC1 regulates hypoxia-inducible factor1α (HIF1α) to promote the switch from oxidative phosphorylation to glycolysis, thereby fostering the differentiation of Effector T cells (Teffs) by regulating the activity of RhoA and restricting the development and functioning of regulatory T cells (Tregs) therefore causing detrimental alterations (Zhang et al. 2023). Additionally, glycolysis impacts Treg function by activating mTORC1 to inhibit FOXP3 expression (Gerriets et al. 2016).
B cells contribute to the pathophysiology of RA by generating autoantibodies, proinflammatory cytokines, and chemokines, as well as serving as effective antigen-presenting cells and controlling T cell activation and differentiation (Dehnavi et al. 2023). The differentiation of B cell lineages depends critically on mTOR. To suppress BACH2 transcription, activated mTORC1 collaborates with Syk to stimulate EZH2 expression. This leads to a rise in the expression of Blimp-1 and XBP1, which are crucial for plasma B cell development and antibody production (Zhang et al. 2023).
The upregulation of mTORC1 facilitates the polarization of macrophage M1, which secretes pro-inflammatory cytokines like TNF-α and activates osteoclasts (OC). Moreover, mTORC1 promotes angiogenesis and tissue remodeling in the synovia of RA by decreasing the polarization of M2, generating anti-inflammatory cytokines, including IL-10 and transforming growth factor-β (TGF-β) (Siouti and Andreakos 2019; Cheng et al. 2023).
During RA, dendritic cells (DCs) release chemokines that draw neutrophils, T cells, and macrophages to the synovium, enhancing the ensuing immunological reactions. Tregs express neuropilin-1, lymphocyte-activating gene-3, and cytotoxic T-lymphocyte-associated protein-4 to inhibit DC functions. Thus, the flaring up of DCs in RA was brought on by the downregulation of Tregs by mTOR (Zhang et al. 2023).
Furthermore, increased expression of the anti-apoptosis genes decreased autophagy, and ongoing FLS proliferation is caused by aberrant activation of PI3K/Akt/mTOR in RA (Cao et al. 2020). Additionally, mTOR controls the autophagy pathway to participate in OC differentiation and formation, destroying bone in RA (Zhang et al. 2023; Abdelfattah et al. 2024).
Kim et al. (2017) found that RA patients’ synovial fluid had higher levels of TNFα and IL-1β (Kim et al. 2017). NF-κB may be activated in RA-FLS in response to TNF-α, resulting in the production of proinflammatory cytokines, chemokines, and MMPs that worsen arthritic joint degeneration, neovascularization, and chronic inflammation (Zhai et al. 2017; Liang et al. 2020).
Necroptosis is a type of cell death in which the ECM degrades, and the cell membrane bursts, releasing molecules linked to damage that produce strong inflammatory reactions. The primary mechanism of necroptosis is the formation and activation of the complex known as receptor-interacting protein kinase 1 (RIP1)-receptor-interacting protein kinase-3 (RIP3). This complex phosphorylates mixed lineage kinase domain-like protein (MLKL), which translocate to the cell membrane and causes disruption. RIP3 facilitates the development of arthritis, even in the absence of MLKL, through the TLR-TRIF-RIP3-IL-1β and p55TNFR-IKK2-RIP3 axes, which coordinate the arthritogenic and cell death processes of synovial fibroblasts. In the RA model, it was observed that TRIM24 knockdown increased RIP3 expression (Jeon et al. 2020).
Treatment for RA is challenging since the exact etiology of the condition is unknown. The medications that are currently on the market have a lot of adverse effects and little patient compliance. Recently, biological treatments have been used for RA more frequently. However, their high cost has severely constrained their usage (Novella-Navarro et al. 2020).
FLA is a hydrocortisone derivative because its anti-inflammatory, immunosuppressive, and anti-proliferative properties might be used as a possible RA therapy (Hall and Banerjee 1967). However, due to dose-related adverse effects like skin irritation, burning sensation, and steroidal acne, a standard topical formulation of FLA is constrained. Thus, the distribution of FLA nano formulations could overcome the associated limits of its application (Pradhan et al. 2020). Our findings corroborated this, demonstrating the downregulation of mTOR, NF-KB, and TNF-α concomitant with the upregulation of TRIM24 and IL-10 in the FLA-NFs treated group(Elhabal et al. 2024a).
Our results were reinforced by Allander and Hellgren (1964) report of a considerable decrease in hand volume, along with decreased morning discomfort and pain when moving and increased mobility when applying FLA topically to treat arthritis (Allander and Hellgren 1964).
One negative modulator of mTOR/Akt signaling is REDD1. Several cellular stressors, such as hypoxia, DNA damage, and glucocorticoids, induce the expression of REDD1 (Lesovaya et al. 2018). In contrast, Hara et al. (2015) discovered that, in an in vivo cartilage defect model in the knee joints, FLA extremely boosts transforming growth factor beta 3 (TGF-β3)-induced phosphorylation of Smad2 and Smad3, as well as mTORC1/AKT pathway-mediated chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells (hBMSCs) and could entirely restore the articular surface (Hara et al. 2015). These contentious results emphasize how crucial it is to conduct more studies in this field of research.
Elevated levels of proinflammatory cytokines, such as TNF-α and IL-6, have a substantial association with synovitis and the extent of joint degeneration in patients with RA, and preceding the circadian rhythm of pain, stiffness, and inflammatory edema of the synovium and the periarticular structures (Cutolo 2019). Neutrophils, monocytes, endothelial cells, and bone-derived cells are attracted to and more active when exposed to IL-6. TNF-α activates the IKK complex, allowing NF-KB to enter the nucleus and affect the targeted genes’ expression (Millar et al. 2022). Therefore, these humoral and cellular inflammatory processes implicated in the pathogenesis of RA can be targeted by glucocorticoids.
Furthermore, it was discovered that FLA, in addition to reducing mechanical hyperalgesia and thereby slowing the progression of the disease, blocked NF-κB and MMP-13 activity, receptor activator of NF-κB ligand (RANKL)-mediated osteoclast genesis and decreased the percentage of chondrocyte necrosis and proteoglycan degeneration throughout the tibial and femoral cartilage while minimizing bone changes in RA degenerative joints (TenBroek et al. 2016).
Notably, NF-κB-driven transcriptional expression of MMP-1, MMP-3, TNF, IL-6, IL-8, monocyte chemotactic protein 1, and IL-1 are down-regulated by other glucocorticoid receptor (GR)–activating compounds and Compound A (CpdA) to an equivalent degree as that following therapy with dexamethasone (DEX) in RA FLS (Gossye et al. 2009).
TNF α, IL-17, and IL-22 levels were significantly reduced by topical administration of FLA-integrated nanostructured lipid carriers (FLA-NLC), according to S. Uprit et al. (2013) (Uprit et al. 2013) and Pradhan et al. (2020) (Pradhan et al. 2020). The FLA could explain this result of burst release, which happens when a drug that isn’t entrapped and has a small lPS stick to the NLC’s surface. This is followed by the medication being released from the core, which causes a gradual, prolonged release.
Due to the efficient fusion of lipid nanocarriers with the skin, a dermal pharmacokinetic study revealed significantly greater skin retention of FLA. In contrast, a penetration investigation revealed minimal absorption of FLA into the bloodstream, a finding that ultimately helps to minimize the systemic adverse reactions of the drug. Furthermore, the skin pharmacokinetic study’s findings revealed that FLA-loaded NLCs were only present in the skin’s dermal and epidermal layers, which boosted the efficiency of the FLA found in a unique gel formulation (Pradhan et al. 2020).
Necroptosis mediated by RIP3 in arthritis has attracted increasing attention. Since TRIM24 is an unfavourable upstream regulator of RIP3 transcription, joint necroptosis can be inhibited by overexpressing TRIM24. Moreover, Jeon et al. (2020) stated that TRIM24 is an important transcriptional coregulator that regulates TNF-α signaling via NF-κB (Jeon et al. 2020). According to Lv et al. (2017), TRIM24 regulates NF-κB in GBM cells differently from STAT3, even though it does not connect directly to the NF-κB p65 unit. In EGFR vIII-driven GBM cells, TRIM24 also markedly altered the expression of NF-κB target genes (Lv et al. 2017; Hamza et al. 2022).
Th2 cells were the main cell type that expressed Trim24. Trim24-/T cells produced fewer cytokines and chemokines, including IL-10, indicating Trim24’s anti-inflammatory capabilities (Yang and Xia 2021). Further investigation is needed to evaluate the FA-TRIM24 relationship because, as far as we know, no studies have examined the influence of FA on TRIM24 in RA models.
Literature has studied the co-electrospinning of PCL/CA polymers with varying ratios, resulting in composite nanofibers with a smooth and uniform surface, superior mechanical strength, hydrophilicity, and better biocompatibility. Blending CA (high solubility) with PCL (long-term drug delivery) as conducted in our study and applying electrospinning successfully created PCL/CA composite nanofibers, increasing their physicochemical and biological properties for prospective use in diverse biomedical applications (Azari et al. 2022).
Histopathological examination results (H&E staining)
The results of the histopathological examination of rats’ knee joints stained by H&E are expressed in Fig. 6 as follows: The control (group I), as presented in (a & b), showed the normal histological structure of knee joint cartilage formed of articular cartilage (AC), which was separated from underlying calcified cartilage (CC) by a tidal mark followed by bone. The cartilage is a form of rounded chondrocytes found in the deep layer with a homogeneous extracellular matrix (ECM). The exposed surface of the articular cartilage is smooth and has a regular pattern. However, RA (group II), as displayed in Fig. 6c–f, showed damaged normal histological knee joint structure in the form of articular surface disruption, deformity, and small occasional fragmentation in the articular surface. The AC showed an apparent focal decrease in the thickness and deep zoon erosion with subcartilaginous bone upward invading. Articular chondrocytes were decreased in number with pyknotic nuclei or depletion of chondrocytes in certain areas. However, a collection of chondrocyte hyperplasia that was irregularly arranged in clusters appeared in the articular cartilage. Also, the tidemark as a demarcation line between AC and CC is disrupted and discontinuous, and the zones of the AC were not well demarcated compared to those of the control group. However, FLA gel-treated RA (group III), as displayed in Fig. 6g, h was found to improve the histological structure of the knee joint in the form of a smooth articular surface with restoration of articular cartilage except for minor superficial erosion and small surface fragments. Also, a well-demarcated tidal mark separates the AC from calcified cartilage. Chondrocytes also developed in a well-organized and homogeneous matrix. Only a few chondrocytes are small. The FLA-NFs RA (group IV), as illustrated in (Fig. 6i, j), showed an almost normal histological structure of the knee joint with a smooth, free surface of articular cartilage, well-developed articular cartilage separated by a sharply demarcated tidal mark from calcified cartilage. Chondrocytes grow and divide in a rounding pattern in a well-developed cell nest surrounded by an extra cell matrix. After inducing RA, both groups demonstrated remarkable improvement; however, the outcomes were considerably more favorable for the group treated with FLA-NFs than the FLA-gel-loaded optimum PHs treatment group. This formula had good qualities, which might reduce the arthritic sequences and indications in all studied joints. According to the OARSI grading system, there was a difference between FLA-NFs and FLA-gel loaded optimum PHs groups.
Collected light microscopic photographs of knee joint sections stained by H&E for different experimental groups (a, b): Reveal the articular cartilage (AC) separated from calcified cartilage (CC) by tidal mark (zigzag arrow), rounded chondrocytes (bifid arrow), and smooth surface of the articular cartilage (hollow arrow) (H&E: a, and b, scale bar; 40 µm), (c–f): Showing superficial erosion and deformity and loss of almost all articular cartilage (AC) in the deep zone and loss (arrow) with upward invasion of the subcartilaginous bone. Articular cartilage chondrocyte zonal configuration loss with areas of increased number of chondrocytes in irregular manner (irregular chondrocytes clustering), the articular cartilage (black arrowhead), the tidemark line is disturbed (zigzag), focal disruption with decreased chondrocyte numbers with small pyknotic nuclei (*), simple fragment (cleft) (arrowhead). Articular cartilage (AC), calcified cartilage (CC), joint space (JS), and bone marrow (BM). (H&E: c–f, scale bar;100& 40 µm), (g, h): The RA + fluocinolone-treated RA group sections show a smooth articular surface (hollow arrow) of the articular cartilage (AC) surface and a Tipeline (zigzag arrow) that separates the AC from the calcified cartilage (CC). Organized growing round chondrocytes (bifid arrow) with a few chondrocytes that are reduced in size (curved arrow) and (I, j): The CA/PCL loaded FLA-HS nanofibers (Group IV) treated RA group show almost normal histological structure, with the smooth articular surface (hollow arrow) of articular cartilage (AC) separated from calcified cartilage (CC) by the Tide line (zigzag arrow). Chondrocytes divide in a round pattern in a cell nest (bifid arrow) (H&E: a, b, scale bar; 40 µm). Small superficial erosion (fraying arrowhead) (H&E: g, h, scale bar; 40 µm)
In parallel with the histopathological findings, Table 6 and Fig. 7 displayed the osteoarthritic cartilage histopathology grading of 24 fields from each experimental group scoring among the different study groups as follows:
Graphical presentation of rheumatoid arthritis cartilage histopathology (OA) scoring among the experimental groups. The graph represents the number of fields (from a total of 24 fields) that positively express each grade value (from 0 to 4.5)/for each experimental group. RA group (positive control), RA + FLA-gel loaded optimum PHs group and FLA-NFs group
The RA group’s cartilage histopathology score revealed the greatest degree of cartilage damage with the following scores: 4 fields (0; Intact surface and cartilage), 2 fields (1; Intact surface, cells, and superficial zone), 5 fields (1.5; Intact surface, cell death, and superficial fibrillation only;), 2 fields (2; Surface discontinuity and fibrillation through the superficial zone), 2 fields (2.5; Surface discontinuity and abrasion with matrix loss within the superficial zone), 2 fields (3; vertical and simple fissures), 3 fields (4; Erosion and superficial zone delamination) and 1 field (4.5; Erosion and mid-zone excavation). The RA + FLA-gel loaded optimal PHs group indicated positive effects on cartilage preservation and showed improved cartilage histopathology scores: 15 fields (0; Intact surface and cartilage), 1 field (1; Intact surface, cells, and superficial zone), 4 fields (1.5; Intact surface, cell death, and superficial fibrillation only), 1 field (3; Vertical and simple fissures), 1 field (4; Erosion and superficial zone delamination) and 2 fields with a score of 4.5; Erosion and mid-zone excavation). However, as compared to the positive control, the RA + FLA-NFs group showed marked improved cartilage histopathology scores, with 16 fields scoring (0; Intact surface and cartilage, normal), 4 fields scoring (1; Intact surface, cells, and superficial zone), 1 field scoring (1.5 Intact surface, cell death, and superficial fibrillation only), 1 field scoring (4; erosion and superficial zone delamination) and only 1 field scoring (4.5; Erosion and mid-zone excavation). this scoring indicates that both FLA-PHs and RA + FLA-NFs improved articular cartilage histopathology with higher improvement with RA + FLA-NFs treatment in reducing the histopathological changes associated with RA.
Histopathological examination results (Safranin O)
As presented in Fig. 8, in the control (Group I), the cartilage matrix proteoglycan of articular cartilage appeared uniformly intensely stained red by Safranin O, and the chondrocytes were regularly distributed and arranged in the cell nest. The subarticular calcified area was sharply demarcated in a bluish colour. RA (Group II) in comparison to the control group, the articular cartilage area was thinner, the surface of cartilage appeared irregular, and the cartilage matrix proteoglycan degenerated, not stained by Safranin O and replaced by a thin layer of fibrous tissue. Both RAs treated with FLA-gel loaded optimum PHs group and RAs treated with FLA-NFs showed almost regeneration. Almost restoration of normal articular cartilage matrix proteoglycan intensely stained ECM proteoglycan, chondrocytes were regularly distributed and arranged in cell nest, and the subarticular calcified area was sharply demarcated in bluish colour, although in RA treated with FLA group area of articular cartilage loss still not regenerated.
Collected light microscopic photographs of knee joint sections stained by Safranin O: Showing the control group’s knee joints with normal articular cartilage (AC) with intensely stained red ECM proteoglycan (a); however, in the RA group, AC appears degenerated with fibrous tissue (black arrow) (b). RA treated with the FLA group showed regeneration of AC with red-stained ECM proteoglycan, but an area of cartilage loss was still not regenerated (black star) (c). RA treated by CA/PCL loaded FLA-HS nanofibers showed almost regeneration and almost restoration of normal AC intensely stained ECM proteoglycan (d). (Safranin O stain; a, b, c& d scale bar: 25 µm)
Conclusion
When considered collectively, the data provides credence to the idea that utilizing a modified-release nano-formula Fluocinolone acetonide (FLA) loaded PEG decorated Hyalurosomes loaded into nanofibers (NFs) to optimize the release of glucocorticoid administration enhances the risk-to-benefit ratio for prolonged glucocorticoid therapy for RA patients. Potential treatment targets for FLA in RA patients may include mTOR and TRIM24. Through various methods, synergistic mixes of multiple medicinal substances guarantee a multi-target treatment strategy, eventually enhancing therapeutic benefit. The NFs’ high surface area and tunable porosity improved the permeation of FLA through the skin. The NFs-based transdermal delivery systems offered flexibility in formulation, allowing for the incorporation of FLA. Moreover, the NFs were designed to mimic the skin closely, potentially promoting better compatibility and reducing irritant effects.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abdelfattah DSE, Fouad MA, Elmeshad AN et al (2024) Anti-obesity effect of combining white kidney bean extract, propolis ethanolic extract and crpi3 on sprague-dawley rats fed a high-fat diet. Nutrients 16:310. https://doi.org/10.3390/NU16020310
Abdelmonem R, Elhabal SF, Abdelmalak NS et al (2021) Formulation and characterization of acetazolamide/carvedilol niosomal gel for glaucoma treatment: in vitro, and in vivo study. Pharmaceutics 13:221. https://doi.org/10.3390/PHARMACEUTICS13020221
Albash R, Abdelbary AA, Refai H, El-Nabarawi MA (2019) Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: in vitro, ex vivo, and in vivo evaluation. Int J Nanomed 14:1953–1968. https://doi.org/10.2147/IJN.S196771
Albash R, Fahmy AM, Hamed MIA et al (2021a) Spironolactone hyaluronic acid enriched cerosomes (HAECs) for topical management of hirsutism: in silico studies, statistical optimization, ex vivo, and in vivo studies. Drug Deliv 28:2289–2300. https://doi.org/10.1080/10717544.2021.1989089
Albash R, Yousry C, Al-Mahallawi AM, Alaa-Eldin AA (2021b) Utilization of PEGylated cerosomes for effective topical delivery of fenticonazole nitrate: in-vitro characterization, statistical optimization, and in-vivo assessment. Drug Deliv 28:1–9. https://doi.org/10.1080/10717544.2020.1859000
Allander E, Hellgren L (1964) topical treatment of rheumatoid arthritis with fluocinolone acetonide under occulsive dressing. Acta Rheumatol Scand 10:305–312. https://doi.org/10.3109/rhe1.1964.10.issue-1-4.34
Al-Shoubki AA, Teaima MH, Abdelmonem R et al (2023) Sucrose acetate isobutyrate (SAIB) and glyceryl monooleate (GMO) hybrid nanoparticles for bioavailability enhancement of rivaroxaban: an optimization study. Pharm Dev Technol 28:928–938. https://doi.org/10.1080/10837450.2023.2274944
Al-Shoubki AA, Teaima MH, Abdelmonem R et al (2024) Potential application of sucrose acetate isobutyrate, and glyceryl monooleate for nanonization and bioavailability enhancement of rivaroxaban tablets. Pharm Sci Adv 2:100015. https://doi.org/10.1016/J.PSCIA.2023.100015
Aly AA, Ahmed MK (2021) Nanofibers of cellulose acetate containing ZnO nanoparticles/graphene oxide for wound healing applications. Int J Pharm. https://doi.org/10.1016/J.IJPHARM.2021.120325
Azari A, Golchin A, Mahmoodinia Maymand M et al (2022) Electrospun polycaprolactone nanofibers: current research and applications in biomedical application. Adv Pharm Bull 12:658–672. https://doi.org/10.34172/apb.2022.070
Barbosa F, Garrudo FFF, Marques AC et al (2023) Novel electroactive mineralized polyacrylonitrile/PEDOT:PSS electrospun nanofibers for bone repair applications. Int J Mol Sci. https://doi.org/10.3390/IJMS241713203
Cao F, Cheng M-H, Hu L-Q et al (2020) Natural products action on pathogenic cues in autoimmunity: efficacy in systemic lupus erythematosus and rheumatoid arthritis as compared to classical treatments. Pharmacol Res 160:105054. https://doi.org/10.1016/j.phrs.2020.105054
Cheng J-W, Yu Y, Zong S-Y et al (2023) Berberine ameliorates collagen-induced arthritis in mice by restoring macrophage polarization via AMPK/mTORC1 pathway switching glycolytic reprogramming. Int Immunopharmacol 124:111024. https://doi.org/10.1016/j.intimp.2023.111024
Cutolo M (2019) Circadian rhythms and rheumatoid arthritis. Joint Bone Spine 86:327–333. https://doi.org/10.1016/j.jbspin.2018.09.003
Dehnavi S, Sadeghi M, Tavakol Afshari J, Mohammadi M (2023) Interactions of mesenchymal stromal/stem cells and immune cells following MSC-based therapeutic approaches in rheumatoid arthritis. Cell Immunol 393–394:104771. https://doi.org/10.1016/j.cellimm.2023.104771
Dhandapani G, Wachtel E, Patchornik G (2022) Detergent micelle conjugates containing amino acid monomers allow purification of human IgG near neutral pH. J Chromatogr B Analyt Technol Biomed Life Sci. https://doi.org/10.1016/J.JCHROMB.2022.123358
Dhavale RP, Nadaf SJ, Bhatia MS (2021) Quantitative structure property relationship assisted development of fluocinolone acetonide loaded transfersomes for targeted delivery. J Drug Deliv Sci Technol 65:102758. https://doi.org/10.1016/j.jddst.2021.102758
El-Gizawy SA, Osman MA, Ibrahim SF (2018) Effect of cosolvents on the absorptive clearance of ketotifen fumarate from rabbit intestine, in-situ. J Adv Pharm Res 2:168–179. https://doi.org/10.21608/APRH.2018.3450.1055
Elhabal SF, Elwy HM, Hassanin S et al (2022) Biosynthesis and characterization of gold and copper nanoparticles from Salvadora persica fruit extracts and their biological properties. Int J Nanomed 17:6095. https://doi.org/10.2147/IJN.S385543
Elhabal SF, Ghaffar SA, Hager R et al (2023) Development of thermosensitive hydrogel of amphotericin-B and Lactoferrin combination-loaded PLGA-PEG-PEI nanoparticles for potential eradication of ocular fungal infections: in-vitro, ex-vivo and in-vivo studies. Int J Pharm X 5:100174. https://doi.org/10.1016/J.IJPX.2023.100174
Elhabal S, Abdelaal N, Saeed Al-Zuhairy S et al (2024a) Green synthesis of zinc oxide nanoparticles from althaea officinalis flower extract coated with chitosan for potential healing effects on diabetic wounds by inhibiting TNF-α and IL-6/IL-1β signaling pathways. Int J Nanomed 19:3045–3070. https://doi.org/10.2147/IJN.S455270
Elhabal SF, Abdelaal N, Al-Zuhairy SAS et al (2024b) Revolutionizing psoriasis topical treatment: enhanced efficacy through ceramide/phospholipid composite cerosomes co-delivery of cyclosporine and dithranol: in-vitro, ex-vivo, and in-vivo studies. Int J Nanomed 19:1163–1187. https://doi.org/10.2147/IJN.S443812
Elhabal SF, Abdelmonem R, El Nashar RM, et al (2024c) Enhanced Antibacterial Activity of Clindamycin Using Molecularly Imprinted Polymer Nanoparticles Loaded with Polyurethane Nanofibrous Scaffolds for the Treatment of Acne Vulgaris. Pharmaceutics 16:947. https://doi.org/10.3390/PHARMACEUTICS16070947
El-Nawawy TM, YomnaA A, Teaima M et al (2024) Intranasal bilosomes in thermosensitive hydrogel: advancing desvenlafaxine succinate delivery for depression management. Pharm Dev Technol. https://doi.org/10.1080/10837450.2024.2376067
Fathy Elhabal S, El-Nabarawi MA, Abdelaal N et al (2023) Development of canagliflozin nanocrystals sublingual tablets in the presence of sodium caprate permeability enhancer: formulation optimization, characterization, in-vitro, in silico, and in-vivo study. Drug Deliv 30:2241665. https://doi.org/10.1080/10717544.2023.2241665
Fathy Elhabal S, Abdelaal N, Kareem SA et al (2024) Green synthesis of zinc oxide nanoparticles from Althaea officinalis flower extract coated with chitosan for potential healing effects on diabetic wounds by inhibiting TNF-α and IL-6/IL-1β signaling pathways. Int J Nanomed 19:3045–3070. https://doi.org/10.2147/IJN.S455270
Feng FB, Qiu HY (2018) Effects of artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis. Biomed Pharmacother 102:1209–1220. https://doi.org/10.1016/j.biopha.2018.03.142
Fouad SA, Teaima MH, Gebril MI et al (2023) Formulation of novel niosomal repaglinide chewable tablets using coprocessed excipients: in vitro characterization, optimization and enhanced hypoglycemic activity in rats. Drug Deliv. https://doi.org/10.1080/10717544.2023.2181747
Gerriets VA, Kishton RJ, Johnson MO et al (2016) Foxp3 and toll-like receptor signaling balance T(reg) cell anabolic metabolism for suppression. Nat Immunol 17:1459–1466. https://doi.org/10.1038/ni.3577
Gossye V, Elewaut D, Bougarne N et al (2009) Differential mechanism of NF-kappaB inhibition by two glucocorticoid receptor modulators in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 60:3241–3250. https://doi.org/10.1002/art.24963
Hall L, Banerjee NR (1967) Topical fluocinolone in rheumatoid arthritis. Ann Rheum Dis 26:260–261. https://doi.org/10.1136/ard.26.3.260
Hamza AA, Khasawneh MA, Elwy HM et al (2022) Salvadora persica attenuates DMBA-induced mammary cancer through downregulation oxidative stress, estrogen receptor expression and proliferation and augmenting apoptosis. Biomed Pharmacother 147:112666. https://doi.org/10.1016/J.BIOPHA.2022.112666
Hara ES, Ono M, Pham HT et al (2015) Fluocinolone acetonide is a potent synergistic factor of TGF-β3-associated chondrogenesis of bone marrow-derived mesenchymal stem cells for articular surface regeneration. J Bone Miner Res 30:1585–1596. https://doi.org/10.1002/jbmr.2502
Jeon J, Noh HJ, Lee H et al (2020) TRIM24-RIP3 axis perturbation accelerates osteoarthritis pathogenesis. Ann Rheum Dis 79:1635–1643. https://doi.org/10.1136/annrheumdis-2020-217904
Kim M, Yoo S-J, Kang SW et al (2017) TNFα and IL-1β in the synovial fluid facilitate mucosal-associated invariant T (MAIT) cell migration. Cytokine 99:91–98. https://doi.org/10.1016/j.cyto.2017.07.007
Koumadorakis DE, Dimitrakopoulos GN, Krokidis MG, Vrahatis AG (2023) Gene regulatory network reconstruction using single-cell RNA-sequencing. In: Vlamos P, Kotsireas IS, Tarnanas I (eds) Handbook of computational neurodegeneration. Springer International Publishing, Cham, pp 181–195
Laragione T, Gulko PS (2010) mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol Med 16:352–358. https://doi.org/10.2119/molmed.2010.00049
Lesovaya E, Agarwal S, Readhead B et al (2018) Rapamycin modulates glucocorticoid receptor function, blocks atrophogene REDD1, and protects skin from steroid atrophy. J Invest Dermatol 138:1935–1944. https://doi.org/10.1016/j.jid.2018.02.045
Liang T, Song M, Xu K et al (2020) TRIM32 promotes inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. Scand J Immunol 91:e12876. https://doi.org/10.1111/sji.12876
Lo J, Chan L, Flynn S (2021) A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch Phys Med Rehabil 102:115–131. https://doi.org/10.1016/j.apmr.2020.04.001
Lv D, Li Y, Zhang W et al (2017) TRIM24 is an oncogenic transcriptional co-activator of STAT3 in glioblastoma. Nat Commun 8:1454. https://doi.org/10.1038/s41467-017-01731-w
Millar MW, Fazal F, Rahman A (2022) Therapeutic targeting of NF-κB in acute lung injury: a double-edged sword. Cells. https://doi.org/10.3390/cells11203317
Mohammed MHH, Hamed ANE, Elhabal SF et al (2023a) Metabolic profiling and cytotoxic activities of ethanol extract of Dypsis leptocheilos aerial parts and its green synthesized silver nanoparticles supported by network pharmacology analysis. S Afr J Bot 161:648–665. https://doi.org/10.1016/J.SAJB.2023.08.026
Mohammed MHH, Hamed ANE, Elhabal SF et al (2023b) Chemical composition and anti-proliferative activities of Hyophorbe lagenicaulis aerial parts and their biogenic nanoparticles supported by network pharmacology study. S Afr J Bot 156:398–410. https://doi.org/10.1016/J.SAJB.2023.03.018
Novella-Navarro M, Plasencia C, Tornero C et al (2020) Clinical predictors of multiple failure to biological therapy in patients with rheumatoid arthritis. Arthritis Res Ther 22:284. https://doi.org/10.1186/s13075-020-02354-1
Patel B, Thakkar H (2023) Formulation development of fast dissolving microneedles loaded with cubosomes of febuxostat: in vitro and in vivo. Pharmaceutics. https://doi.org/10.3390/pharmaceutics15010224
Permyakova ES, Solovieva AO, Sitnikova N et al (2024) Polycaprolactone nanofibers functionalized by fibronectin/gentamicin and implanted silver for enhanced antibacterial properties, cell adhesion, and proliferation. Polymers (basel). https://doi.org/10.3390/POLYM16020261
Pradhan M, Singh D, Murthy SN, Singh MR (2015) Design, characterization and skin permeating potential of Fluocinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis. Steroids 101:56–63. https://doi.org/10.1016/J.STEROIDS.2015.05.012
Pradhan M, Yadav KS, Singh DK, Singh MR (2020) Topical delivery of fluocinolone acetonide integrated NLCs and salicylic acid enriched gel: a potential and synergistic approach in the management of psoriasis. J Drug Deliv Sci Technol. 61:102282
Pritzker KPH, Gay S, Jimenez SA et al (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14:13–29
Pulik Ł, Łęgosz P, Motyl G (2023) Matrix metalloproteinases in rheumatoid arthritis and osteoarthritis: a state of the art review. Reumatologia 61:191–201. https://doi.org/10.5114/reum/168503
Saady M, Shoman NA, Teaima M et al (2024) Fabrication of gastro-floating sustained-release etoricoxib and famotidine tablets: design, optimization, in-vitro, and in-vivo evaluation. Pharm Dev Technol. https://doi.org/10.1080/10837450.2024.2343320
Sadeghi-Ghadi Z, Ebrahimnejad P, Talebpour Amiri F, Nokhodchi A (2021) Improved oral delivery of quercetin with hyaluronic acid containing niosomes as a promising formulation. J Drug Target 29:225–234. https://doi.org/10.1080/1061186X.2020.1830408
Saleem A, Saleem M, Akhtar MF et al (2020) Polystichum braunii extracts inhibit complete Freund’s adjuvant-induced arthritis via upregulation of I-κB, IL-4, and IL-10, downregulation of COX-2, PGE2, IL-1β, IL-6, NF-κB, and TNF-α, and subsiding oxidative stress. Inflammopharmacology 28:1633–1648. https://doi.org/10.1007/s10787-020-00688-5
Salem HF, Nafady MM, Kharshoum RM et al (2020) Mitigation of rheumatic arthritis in a rat model via transdermal delivery of dapoxetine HCl amalgamated as a nanoplatform: in vitro and in vivo assessment. Int J Nanomed 15:1517–1535. https://doi.org/10.2147/IJN.S238709
Schett G, Tanaka Y, Isaacs JD (2021) Why remission is not enough: underlying disease mechanisms in RA that prevent cure. Nat Rev Rheumatol 17:135–144. https://doi.org/10.1038/s41584-020-00543-5
Shoman NA, Saady M, Teaima M et al (2023) Merging konjac glucomannan with other copolymeric hydrogels as a cutting-edge liquid raft system for dual delivery of etoricoxib and famotidine. Drug Deliv. https://doi.org/10.1080/10717544.2023.2189630
Siouti E, Andreakos E (2019) The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol 165:152–169. https://doi.org/10.1016/j.bcp.2019.03.029
Sotoudeh N, Namavar MR (2022) Optimisation of ketamine-xylazine anaesthetic dose and its association with changes in the dendritic spine of CA1 hippocampus in the young and old male and female Wistar rats. Vet Med Sci 8:2545–2552. https://doi.org/10.1002/VMS3.936
Sultanova Z, Kaleli G, Kabay G, Mutlu M (2016) Controlled release of a hydrophilic drug from coaxially electrospun polycaprolactone nanofibers. Int J Pharm 505:133–138. https://doi.org/10.1016/J.IJPHARM.2016.03.032
Talebi N, Lopes D, Lopes J et al (2023) Natural polymeric nanofibers in transdermal drug delivery. Appl Mater Today 30:101726. https://doi.org/10.1016/j.apmt.2022.101726
TenBroek EM, Yunker L, Nies MF, Bendele AM (2016) Randomized controlled studies on the efficacy of antiarthritic agents in inhibiting cartilage degeneration and pain associated with progression of osteoarthritis in the rat. Arthritis Res Ther 18:24. https://doi.org/10.1186/s13075-016-0921-5
Uprit S, Kumar Sahu R, Roy A, Pare A (2013) Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J 21:379–385. https://doi.org/10.1016/j.jsps.2012.11.005
Wsoo MA, Shahir S, Mohd Bohari SP et al (2020) A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: a new perspective. Carbohydr Res 491:107978. https://doi.org/10.1016/j.carres.2020.107978
Wu X, Long L, Liu J et al (2017) Gambogic acid suppresses inflammation in rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol Med Rep 16:7112–7118. https://doi.org/10.3892/mmr.2017.7459
Xu X, Qin Z, Zhang C et al (2023) TRIM29 promotes podocyte pyroptosis in diabetic nephropathy through the NF-kB/NLRP3 inflammasome pathway. Cell Biol Int 47:1126–1135. https://doi.org/10.1002/cbin.12006
Yang L, Xia H (2021) TRIM Proteins in Inflammation: from expression to emerging regulatory mechanisms. Inflammation 44:811–820. https://doi.org/10.1007/s10753-020-01394-8
Yasser M, El Naggar EE, Elfar N et al (2024) Formulation, optimization and evaluation of ocular gel containing nebivolol Hcl-loaded ultradeformable spanlastics nanovesicles: in vitro and in vivo studies. Int J Pharm X. https://doi.org/10.1016/J.IJPX.2023.100228
Zhai K-F, Duan H, Luo L et al (2017) Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology. https://doi.org/10.1007/s10787-017-0385-5
Zhang F, Cheng T, Zhang S-X (2023) Mechanistic target of rapamycin (mTOR): a potential new therapeutic target for rheumatoid arthritis. Arthritis Res Ther 25:187. https://doi.org/10.1186/s13075-023-03181-w
Acknowledgements
None
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding.
Author information
Authors and Affiliations
Contributions
The authors conceptualized, designed, carried out, and supervised the experiment, analyzed and interpreted the data, participated in writing the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
All authors (S.F. ELhabal, M.A. El‑Nabarawi, S. Hassanin, F.E. Hassan, S.S. Abbas, S.M. Gebril, and R. Albash) declare that they have no conflict of interest.
Ethical approval
The animal studies were performed after receiving approval from the Research Ethics Committee-Faculty of Pharmacy, Cairo University (REC-FOPCU) serial number (code: PI 2942), valid from 28/3/2022. Animal models are among the most crucial in vivo models concerning basic parameters like drug efficiency, safety, and toxicological studies, as these pre-clinical data are essential before translating into humans. The use and handling of animals in our study complied with ARRIVE guidelines for animal experiments.
Consent to participate
All the authors approved the participation.
Consent for publication
All the authors approved the publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
ELhabal, S.F., El-Nabarawi, M.A., Hassanin, S.O. et al. Transdermal fluocinolone acetonide loaded decorated hyalurosomes cellulose acetate/polycaprolactone nanofibers mitigated Freund’s adjuvant-induced rheumatoid arthritis in rats. J. Pharm. Investig. (2024). https://doi.org/10.1007/s40005-024-00693-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40005-024-00693-8