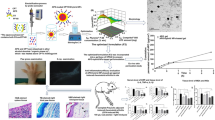
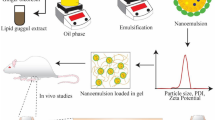
Abstract
Rheumatoid arthritis restricts the physical ability of patients and increases the disease burden; therefore, research has always been focused on evaluating better therapeutic options. The present research aimed to design Continentalic acid (CA)–loaded transfersomes (CA-TF) embedded in Carbopol gel containing permeation enhancer (PE) for the treatment of rheumatoid arthritis. CA-TF was developed via a modified thin film hydration method and incorporated into Carbopol 934 gel containing Eucalyptus oil (EO) as PE. The fabricated CA-TF showed particle size of < 140 nm with spherical geometry, optimal encapsulation efficiency (EE), and sustained drug release pattern. CA-TF-gel along with PE (CA-TF-PE-gel) showed better ex vivo skin penetration than plain CA gel and CA-TF-gel without PE. In vivo evaluation supported improved therapeutic outcomes of CA-TF-PE-gel in terms of behavioral findings, arthritic index, and histological findings whereas biochemical assays and pro-inflammatory cytokines (TNF-α and IL-1β) showed a significant decrease in their levels. Furthermore, immunohistochemistry assay for Nrf2 and HO-1 signaling pathways showed significant improvement in the expression of the Nrf2, and HO-1 proteins to depict improvement in arthritic condition in the animal model. CA-TF-PE-gel significantly delivered CA to the diseased target site via a topical route with promising therapeutic outcomes displayed in the CFA-induced arthritic model.
Graphical Abstract









Similar content being viewed by others
Data Availability
All the data generated and analyzed in the study is included in this article.
References
McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328–37.
ElSherbiny DA. Frequency and predictors of extra-articular manifestations in patients with rheumatoid arthritis. Egypt J Hosp Med. 2019;76(5):4062–7.
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72.
Chuang S-Y, Lin C-H, Huang T-H, Fang J-Y. Lipid-based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials. 2018;8(1):42.
Choy EHS, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344(12):907–16.
Boissier M-C, Semerano L, Challal S, Saidenberg-Kermanac’h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39(3):222–8.
Darwish SF, El-Bakly WM, Arafa HM, El-Demerdash E. Targeting TNF-α and NF-κB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS ONE. 2013;8(11): e79284.
Zwerina J, Redlich K, Schett G, Smolen JS. Pathogenesis of rheumatoid arthritis: targeting cytokines. Ann N Y Acad Sci. 2005;1051(1):716–29.
Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Álvaro-Gracia JM, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017;76(6):948–59.
Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72(6):863–9.
Bullock J, Rizvi SA, Saleh AM, Ahmed SS, Do DP, Ansari RA, et al. Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract. 2018;27(6):501–7.
Nikolova M, Slavchov R, Nikolova G. Nanotechnology in medicine. Drug discovery and evaluation: methods in clinical pharmacology. 2020:533–46.
Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: properties, mechanisms of skin interactions and medical applications. Int J Pharm. 2018;535(1–2):1–17.
Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–6.
Patidar A, Thakur DS, Kumar P, Verma J. A review on novel lipid based nanocarriers. Int J Pharm Pharm Sci. 2010;2(4):30–5.
Janakiraman K, Krishnaswami V, Sethuraman V, Rajendran V, Kandasamy R. Development of methotrexate-loaded cubosomes with improved skin permeation for the topical treatment of rheumatoid arthritis. Appl Nanosci. 2019;9(8):1781–96.
Duangjit S, Obata Y, Sano H, Onuki Y, Opanasopit P, Ngawhirunpat T, et al. Comparative study of novel ultradeformable liposomes: menthosomes, transfersomes and liposomes for enhancing skin permeation of meloxicam. Biol Pharm Bull. 2014;37(2):239–47.
Cevc G. Transfersomes, liposomes and other lipid suspensions on the skin: permeation enhancement, vesicle penetration, and transdermal drug delivery. Critical reviews™ in therapeutic drug carrier systems. 1996;13(3–4).
Ahad A, Al-Saleh AA, Al-Mohizea AM, Al-Jenoobi FI, Raish M, Yassin AEB, et al. Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate. Saudi Pharm J. 2017;25(7):1040–6.
El-Gizawy SA, Nouh A, Saber S, Kira AY. Deferoxamine-loaded transfersomes accelerates healing of pressure ulcers in streptozotocin-induced diabetic rats. J Drug Deliv Sci Technol. 2020;58: 101732.
Mahmood S, Chatterjee B, Mandal UK. Pharmacokinetic evaluation of the synergistic effect of raloxifene loaded transfersomes for transdermal delivery. J Drug Deliv Sci Technol. 2021;63: 102545.
Castangia I, Caddeo C, Manca ML, Casu L, Latorre AC, Díez-Sales O, et al. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohyd Polym. 2015;134:657–63.
Kang O-H, Chae H-S, Choi J-G, Oh Y-C, Lee Y-S, Kim J-H, et al. ent-pimara-8 (14), 15-dien-19-oic acid isolated from the roots of Aralia cordata inhibits induction of inflammatory mediators by blocking NF-κB activation and mitogen-activated protein kinase pathways. Eur J Pharmacol. 2008;601(1–3):179–85.
Hong R, Kim KS, Choi GM, Yeom M, Lee B, Lee S, et al. Continentalic acid rather than kaurenoic acid is responsible for the anti-arthritic activity of Manchurian Spikenard in vitro and in vivo. Int J Mol Sci. 2019;20(21):5488.
Hong R, Sur B, Yeom M, Lee B, Kim KS, Rodriguez JP, et al. Anti-inflammatory and anti-arthritic effects of the ethanolic extract of Aralia continentalis Kitag. in IL-1β-stimulated human fibroblast-like synoviocytes and rodent models of polyarthritis and nociception. Phytomedicine. 2018;38:45–56.
Rajan R, Vasudevan DT. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J Adv Pharm Technol Res. 2012;3(2):112.
Biruss B, Kählig H, Valenta C. Evaluation of an eucalyptus oil containing topical drug delivery system for selected steroid hormones. Int J Pharm. 2007;328(2):142–51.
El Zaafarany GM, Awad GA, Holayel SM, Mortada ND. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm. 2010;397(1–2):164–72.
Sana E, Zeeshan M, Ain QU, Khan AU, Hussain I, Khan S, et al. Topical delivery of curcumin-loaded transfersomes gel ameliorated rheumatoid arthritis by inhibiting NF-κβ pathway. Nanomedicine. 2021;16(10):819–37.
Mazhar D, Haq NU, Zeeshan M, Ain QU, Ali H, Khan S, et al. Preparation, characterization, and pharmacokinetic assessment of metformin HCl loaded transfersomes co-equipped with permeation enhancer to improve drug bioavailability via transdermal route. J Drug Deliv Sci Technol. 2023;84: 104448.
Ramkanth S, Anitha P, Gayathri R, Mohan S, Babu D. Formulation and design optimization of nano-transferosomes using pioglitazone and eprosartan mesylate for concomitant therapy against diabetes and hypertension. Eur J Pharm Sci. 2021;162: 105811.
Shuwaili AHA, Rasool BKA, Abdulrasool AA. Optimization of elastic transfersomes formulations for transdermal delivery of pentoxifylline. Eur J Pharm Biopharm. 2016;102:101–14.
Dudhipala N, Phasha Mohammed R, Adel Ali Youssef A, Banala N. Effect of lipid and edge activator concentration on development of aceclofenac-loaded transfersomes gel for transdermal application: in vitro and ex vivo skin permeation. Drug Dev Ind Pharm. 2020;46(8):1334–44.
Zeb A, Qureshi OS, Yu C-H, Akram M, Kim H-S, Kim M-S, et al. Enhanced anti-rheumatic activity of methotrexate-entrapped ultradeformable liposomal gel in adjuvant-induced arthritis rat model. Int J Pharm. 2017;525(1):92–100.
Qindeel M, Khan D, Ahmed N, Khan S, Asim. ur R. Surfactant-free, self-assembled nanomicelles-based transdermal hydrogel for safe and targeted delivery of methotrexate against rheumatoid arthritis. ACS Nano. 2020;14(4):4662–81.
Prabhu P, Shetty R, Koland M, Vijayanarayana K, Vijayalakshmi KK, Nairy MH, et al. Investigation of nano lipid vesicles of methotrexate for anti-rheumatoid activity. Int J Nanomed. 2012;7:177.
Song H, Wen J, Li H, Meng Y, Zhang Y, Zhang N, et al. Enhanced transdermal permeability and drug deposition of rheumatoid arthritis via sinomenine hydrochloride-loaded antioxidant surface transethosome. Int J Nanomed. 2019;14:3177.
Khan AM, Khan AU, Ali H, Islam SU, Seo EK, Khan S. Continentalic acid exhibited nephroprotective activity against the LPS and E coli-induced kidney injury through inhibition of the oxidative stress and inflammation. Int Immunopharmacol. 2020;80:106209.
Mahdi HJ, Khan NAK, Asmawi MZB, Mahmud R, Vikneswaran A, Murugaiyah L. In vivo anti-arthritic and anti-nociceptive effects of ethanol extract of Moringa oleifera leaves on complete Freund’s adjuvant (CFA)-induced arthritis in rats. Integr Med Res. 2018;7(1):85–94.
Jaleel GAA, Azab SS, El-Bakly WM, Hassan A. ’Methyl palmitate attenuates adjuvant induced arthritis in rats by decrease of CD68 synovial macrophages. Biomed Pharmacother. 2021;137: 111347.
Khan D, Qindeel M, Ahmed N, Khan AU, Khan S, Rehman AU. Development of novel pH-sensitive nanoparticle-based transdermal patch for management of rheumatoid arthritis. Nanomedicine. 2020;15(06):603–24.
Mossiat C, Laroche D, Prati C, Pozzo T, Demougeot C, Marie C. Association between arthritis score at the onset of the disease and long-term locomotor outcome in adjuvant-induced arthritis in rats. Arthritis Res Ther. 2015;17(1):1–12.
Dewangan AK, Varkey S, Mazumder S. Synthesis of curcumin loaded CMCAB nanoparticles for treatment of rheumatoid arthritis. In International conference on chemical, environmental and biological sciences (CEBS). CEBS Dubai; 2015.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta (BBA)-Gen Subj. 1979;582(1):67–78.
Singla S, Kumar NR, Kaur J. In vivo studies on the protective effect of propolis on doxorubicin-induced toxicity in liver of male rats. Toxicol Int. 2014;21(2):191.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Atiq A, Shal B, Naveed M, Khan A, Ali J, Zeeshan S, et al. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur J Pharmacol. 2019;843:292–306.
Surini S, Leonyza A, Suh CW. Formulation and in vitro penetration study of recombinant human epidermal growth factor-loaded transfersomal emulgel. Adv Pharm Bull. 2020;10(4):586.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57.
Ahad A, Al-Saleh AA, Al-Mohizea AM, Al-Jenoobi FI, Raish M, Yassin AEB, et al. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol® gel under Dermaroller® on rats with methyl prednisolone acetate-induced hypertension. Biomed Pharmacother. 2017;89:177–84.
Moawad FA, Ali AA, Salem HF. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: preparation, in vitro and in vivo performance. Drug Deliv. 2017;24(1):252–60.
Kaur N, Yadav K, Garg R, Saroha K, Yadav D. Formulation and in vitro characterization of ketoconazole span 80 based transfersomes gel, its comparison with liposomal gel and evaluation of antimicrobial activity. J Bionanosci. 2016;10(3):191–204.
Gomes RP, Bressan E, Silva TMD, Gevaerd MDS, Tonussi CR, Domenech SC. Standardization of an experimental model suitable for studies on the effect of exercise on arthritis. Einstein (São Paulo). 2013;11:76–82.
Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol. 2017;38(1):5–19.
Zhao J, Zhao M, Yu C, Zhang X, Liu J, Cheng X, et al. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int J Nanomed. 2017:6735–46.
Qureshi AA, Tan X, Reis JC, Badr MZ, Papasian CJ, Morrison DC, et al. Suppression of nitric oxide induction and pro-inflammatory cytokines by novel proteasome inhibitors in various experimental models. Lipids Health Dis. 2011;10(1):1–25.
Mititelu RR, Pădureanu R, Băcănoiu M, Pădureanu V, Docea AO, Calina D, et al. Inflammatory and oxidative stress markers—mirror tools in rheumatoid arthritis. Biomedicines. 2020;8(5):125.
García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxid Med Cell Longev. 2020. https://doi.org/10.1155/2020/2082145
Vomhof-DeKrey EE, Picklo MJ Sr. The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem. 2012;23(10):1201–6.
Funding
The Higher Education Commission of Pakistan provided financial support for this work through the NRPU grant (No. 9272/Federal/NRPU/R&D/HEC/2017).
Author information
Authors and Affiliations
Contributions
Muhammad Waseem Akram: methodology, experimental work, visualization, data curation, writing original draft; Danish Mazhar: assisted in preparation, data curation, data arrangement, manuscript editing and review; Iqra Afzal: data curation, manuscript editing, review; Ahmad Zeb: investigation, assisted in animal studies and visualization; Qurat Ul Ain: validation, characterization, and manuscript editing; Salman Khan: validation, data curation, in vivo data designing; Hussain Ali: conceptualization, project administration, supervision, data curation, manuscript editing. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Patent Declaration
An application with application no. 405/2022 and receipt No. 220345433 has been submitted for patent approval to IPO-Pakistan (Intellectual Property Organization of Pakistan).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akram, M.W., Mazhar, D., Afzal, I. et al. Design and Evaluation of Continentalic Acid Encapsulated Transfersomal Gel and Profiling of its Anti-arthritis Activity. AAPS PharmSciTech 24, 192 (2023). https://doi.org/10.1208/s12249-023-02648-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02648-y