Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by systemic synovitis and bone destruction. Proinflammatory cytokines activate pathways of immune-mediated inflammation, which aggravates RA. The mechanistic target of rapamycin (mTOR) signaling pathway associated with RA connects immune and metabolic signals, which regulates immune cell proliferation and differentiation, macrophage polarization and migration, antigen presentation, and synovial cell activation. Therefore, therapy strategies targeting mTOR have become an important direction of current RA treatment research. In the current review, we summarize the biological functions of mTOR, its regulatory effects on inflammation, and the curative effects of mTOR inhibitors in RA, thus providing references for the development of RA therapeutic targets and new drugs.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by erosive arthritis and a pathological basis of synovitis, which causes morning stiffness, joint pain, and impairment of movement functions [1]. Extraarticular symptoms of severe active RA generally occur in the cardiovascular, respiratory, blood, and urinary system and may cause other comorbidities [2]. The global incidence of RA is approximately 1%, and two thirds of the cases are women [3]. At the joint inflammatory site, abnormal modulation of mTOR signaling results in continuous feedback between stromal cells and infiltrating immune cells, leading to persistent inflammation and tissue damage, driving the pathological tissue refactoring and eventually causing organ dysfunction [4]. Recent studies reported that activation of the mechanistic target of rapamycin (mTOR) affects the abundance and functioning of immune and stromal cells and may thus be an essential pathway in the pathogenesis of RA. In this review, we describe the structure and processes of the mTOR pathway and highlight its role in the pathogenesis of RA to provide a reference for the development of novel clinical treatment avenues.
The biology of mTOR
In 1975, Sehgal et al. [5] discovered an antifungal macrolide antibiotic produced by the bacterium Streptomyces hygroscopicus, which was termed rapamycin. Rapamycin binds to the 12-kD intracellular protein FKBP12, thus forming a high-affinity complex with the protein FK506 [6]. The FKBP12-rapamycin complex then fuses with the mTOR protein, which was first isolated from mammalian tissue [7]. mTOR is a highly conserved serine/threonine protein kinase that regulates cell proliferation, differentiation, and angiogenesis [8].
mTOR complexes
mTOR forms two functionally distinct complexes with proteins: mTOR complex (mTORC)1 and mTORC2 [9]. mTORC1 comprises mTOR, regulatory-associated protein of TOR (raptor), mammalian lethal with sec-13 protein 8 (mlst8), DEP domain–containing mTOR interacting protein (deptor), and proline-rich Akt substrate of 40kD (pras40) [8, 10]. mTORC2 is composed of mTOR, rapamycin-insensitive companion of mTOR (rictor), stress-activated protein kinase interacting protein 1 (msin1), mlst8, deptor, and protor-1/2 [11,12,13]. mTOR bears several conserved domains, including the N terminus HEAT repeats, FAT domain, FRB domain, Kinase domain, and the C-terminal FATC domains [14] (Fig. 1).
The mTORC1 and mTORC2 complex. The FKBP12-rapamycin complex binds to the FK506-binding protein 12 and inhibits mTORC1 by blocking its association with the FRB domain. This leads to steric hindrance and prevents the activation of mTORC1. On the other hand, mTORC2 is resistant to rapamycin due to the presence of rictor and msin1, which block the binding of the FKBP12-rapamycin complex to the FRB domain of mTORC2. In the mTORC1 complex, raptor plays a crucial role in promoting the phosphorylation of various downstream targets. mlst8 stabilizes the mTORC1 complex, whereas deptor negatively regulates its activity. pras40 also interacts with mTORC1. In the mTORC2 complex, rictor is an essential component that helps stabilize the complex while msin1 contributes to its regulation. This figure provides insight into the structural components and interactions within mTORC1 and mTORC2 complexes, highlighting their sensitivity or insensitivity to the FKBP12-rapamycin complex and the respective roles of key proteins. raptor, regulatory-associated protein of TOR; mlst8, mammalian lethal with sec-13 protein 8; deptor, DEP domain–containing mTOR interacting protein; pras40, proline-rich Akt substrate of 40kD; rictor, rapamycin-insensitive companion of mTOR; msin1, stress-activated protein kinase interacting protein 1
In more detail, the FKBP12-rapamycin complex binds to the FRB domain of mTORC1 to produce steric hindrance, which interferes with interactions between the substrate and the active site of mTORC1 [15, 16]. Furthermore, mTORC1 promotes cell growth by controlling glycolysis, upregulating mRNA transcription, and synthesizing and breaking down proteins and lipids [17]. In addition, mTORC1 regulates catabolic processes including autophagy [18]. Unlike mTORC1, in mTORC2, rictor and msin1 block the binding of the FKBP12-rapamycin complex to the FRB domain of mTORC2, thus revealing the potential mechanism underlying the insensitivity of mTORC2 to rapamycin (Fig. 1) [19, 20]. mTORC2 regulates cell survival, migration, metabolism, and cytoskeleton arrangement through molecular networks [21].
Upstream regulators of mTOR
Several ligands activate mTOR signaling, including growth factors, amino acids, antigens, and cytokines [22]. The pathways of mTORC1 activation have been extensively researched; however, mTORC2 regulation is currently less understood. Compared to mTORC1, mTORC2 is an insensitive signaling factor [8].
The activity of mTORC1 is primarily mediated by phosphatidylinositol-3-OH kinase/RAC-α serine/threonine-protein kinase/tuberous sclerosis complex (PI3K/AKT/TSC) and liver kinase B1/the mitogen-activated protein kinase (LKB1/AMPK) axis. Growth factor receptor, cytokines, and Toll-like receptor (TLR) ligands regulate mTORC1 activity by the PI3K/AKT/TSC signaling pathway. They phosphorylate PI3K to activate downstream effector AKT, then restrain the TSC1/TSC2 complex [23]. Ras homolog enriched in the brain (Rheb) primarily occurs in a GTP-bound activated state [14, 24]. Inactivation of the TSC complex restrains the hydrolysis of Rheb-GTP, preventing it from conversion into Rheb-GDP. Finally, Rheb-GTP directly activates mTORC1 [25]. Through continuously improving understanding of the pathway, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was found to neutralize the activity of PI3K by dephosphorylating the downstream products of PI3K, which include ephosphatidylinositol-3,4-bisphosphate (PIP2) and phosphatidylinositol -3,4,5-triphosphate (PIP3) [14, 26]. The most remarkable characteristic of mTORC1 is its activating ability on the surface of the lysosomal membrane [27]. Growth factors activate Rheb, and amino acids recruit mTORC1, both of which are indispensable for the activation of mTORC1. AMPK regulates glucose metabolism and perceives changes in ATP levels [28, 29]. Energy stress activates LKB1-dependent AMPK, which phosphorylates downstream TSC2, then promotes the formation of the TSC1/TSC2 complex, and further inhibits the activity of mTORC1 [30]. In addition, AMPK directly phosphorylates raptor and suppresses mTORC1 signaling [31] (Fig. 2A).
A The upstream pathway of mTORC1 and mTORC2. Growth factor, cytokines, or TLR signaling activates PI3K, which in turn activates AKT. The AMPK, LKB1, and IRS are also involved in regulating mTORC1 and mTORC2. TLR, Toll-like receptor; PI3K, phosphatidylinositol-3-OH kinase; AKT, RAC-α serine/threonine-protein kinase; TSC, tuberous sclerosis complex; Rheb, Ras homolog enriched in the brain; PTEN, phosphatase and tensin homolog deleted on chromosome 10; PIP2, ephosphatidylinositol-3,4-bisphosphate; PIP3, phosphatidylinositol-3,4,5-triphosphate; AMPK, the mitogen-activated protein kinase; LKB1, liver kinase B1, IRS, insulin receptor substrate. B The downstream pathway of mTORC1. mTORC1 activates S6K1 and eIF4E to promote protein synthesis and cell growth. mTORC1 also inhibits 4E-BP1 to initiate translation. Additionally, mTORC1 influences PDCD4, SKAR, ATF4, MTHFD2, CAD, HIF1α, ULK1, and TFEB to regulate glucose metabolism, lipid synthesis, and autophagy. S6K1, S6 kinase 1; eIF4E, eukaryotic translation initiation factor 4E; eIF4B, eukaryotic translation initiation factor 4B; 4E-BP1, the eIF4E binding protein 1; PDCD4, programmed cell death protein 4; SKAR, S6K1 Aly/REF-like target; ATF4, activating transcription factor 4; MTHFD2, methylenetetrahydrofolate dehydrogenase 2; CAD, carbamoyl-phosphate synthetase; HIF1α, hypoxia-inducible factor-1α; ULK1, unc-51-like kinase1; TFEB, transcription factor EB. C The downstream pathway of mTORC2. mTORC2 activates SGK1 and PKCα, which play roles in cell survival and cytoskeletal organization. SGK1, serum and glucocorticoid inducible kinase 1; PKCα, protein kinase C-α
The activity of mTORC2 is mainly regulated through the PI3K/AKT axis. In detail, PI3K induces PtdIns(3,4,5)P(3) to combine with the pleckstrin homology domain of msin1, blocking inhibition of the mTOR kinase domain by msin1, thereby activating mTORC2 [32, 33]. PI3K also promotes the combination of the ribosome and mTORC2, and the ribosome is necessary for inducing mTORC2 kinase activity [34]. Of note, partial activation of Akt boosts the activation of mTORC2, which phosphorylates and ultimately activates Akt, resulting in a positive feedback loop [35] (Fig. 2A).
Downstream targets of mTOR
mTORC1 maintains metabolic homeostasis by regulating biological synthesis and catabolic processes. Activated mTORC1 phosphorylates its downstream targets S6 kinase 1 (S6K1) and the eIF4E binding protein 1 (4E-BP1) to regulate protein synthesis [36]. Phosphorylated 4E-BP1 releases cap-binding protein eukaryotic translation initiation factor 4E (eIF4E), which counteracts inhibition of protein synthesis by enabling eIF4E to form the eIF4F complex and to participate in cap-dependent translation [37, 38]. S6K1 regulates eukaryotic translation initiation factor 4B (eIF4B), programmed cell death protein 4 (PDCD4) [39], and S6K1 Aly/REF-like target (SKAR) [40] to participate in protein synthesis. eIF4B is a positive regulator of eIF4F complex, and PDCD4 is a negative regulator of eIF4A [41, 42]. The mTORC1 phosphorylates lipin 1 to increase the activity of SREBP1 or activates SREBP1 through an S6K1-dependent mechanism, thus participating in lipid synthesis [43, 44]. In addition, mTORC1 promotes nucleotide synthesis by stimulating the mTHF cycle, which increases ATF4 levels to upregulate the expression of methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) [45]. Moreover, S6K1 phosphorylates carbamoyl-phosphate synthetase (CAD) to activate the de novo pyrimidine synthesis pathway [46]. Furthermore, mTORC1 increases the expression of transcription factor HIF1α to promote glucose metabolism [44]. SREBP1 is also involved in the regulation of the pentose phosphate pathway. Taken together, mTORC1 regulates various metabolic pathways to coordinate anabolism (Fig. 2B).
mTORC1 negatively regulates catabolic processes such as autophagy to promote cell growth [17, 47]. Under nutrient sufficiency, activated mTORC1 phosphorylates and suppresses unc-51-like kinase1 (ULK1) [48]. Transcription factor EB (TFEB) regulates the expression of autophagy and lysosomal genes, which is also phosphorylated and inhibited by mTORC1 to regulate autophagy indirectly [49] (Fig. 2B).
Primary downstream targets of mTORC2 include AKT [50], serum and glucocorticoid inducible kinase 1 (SGK1) [51], and protein kinase C-α (PKCα) [11] (Fig. 2C). PKC-α regulates the actin cytoskeleton to adjust cell shape [52]. Activated SGK1 controls ion transport and cell survival [51]. Additionally, Akt phosphorylates and inhibits transcription factor FoxO1/3a to promote metabolic homeostasis [53].
The role of mTOR in the pathogenesis of RA
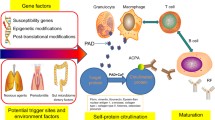
The pathogenesis of RA reflects the complex interactions between various cell groups in the synovium, mediated by direct contact between cells and various types of secreted or exfoliated molecules. mTOR signaling controls the recruitment and activation of innate, acquired immune cells and fibroblast-like synoviocytes (FLSs) during RA, resulting in the production of numerous chemokines, pro-inflammatory cytokines, and cathepsin to degrade extracellular matrix and cartilage, further contributing to the early characteristics of synovitis [3, 54] (Fig. 3).
Inflammation in RA-affected joints. The pathogenesis of RA reflects the complex interactions between various cell groups in the synovium, mediated by direct contact between cells and various types of secreted or exfoliated molecules. mTOR signaling controls the recruitment and activation of innate, acquired immune cells and FLSs during RA, thus producing numerous chemokines, pro-inflammatory cytokines, and cathepsin to degrade the extracellular matrix and cartilage, further contributing to the early characteristics of synovitis
mTOR regulates proliferation and differentiation of T lymphocytes
T lymphocytes play crucial roles in the progression of RA. Effector T cells (Teffs) such as Th1, Th2, and Th17 cells stimulate monocytes or macrophages to synthesize pro-inflammatory cytokines, leading to destructive changes in RA-affected joints [55]. By contrast, regulatory T cells (Tregs) promote the secretion of inhibitory cytokines to inhibit the proliferation and activation of autoreactive T cells and maintain immune homeostasis [56]. Thus, a disturbed Teffs/Tregs balance determines the progression of RA [57]. mTOR signaling is essential for the proliferation and activation of T cell lineages, and mTORC1 and mTORC2 make distinct respective contributions [58, 59].
mTORC1 regulates HIF1α to promote the change from oxidative phosphorylation to glycolysis, thereby supporting the differentiation of T lymphocytes [60]. Increased glycolysis promotes T cells to differentiate into Th1, Th2, and Th17 cells [61]. By contrast, deprivation of glycolysis promotes the development of Tregs [61]. Glycolysis activates mTORC1 to block the expression of FOXP3 and affects the function of Tregs [62]. Leucine or serine deficiency can inhibit the activity of mTORC1 and restrict the differentiation of T cells into Teffs [63]. Indoleamine 2,3-dioxygenase negatively regulates mTORC1 signal transduction via PTEN, preventing proliferation of Teffs and promoting induction of Tregs [62, 64]. De novo fatty acid synthesis inhibits differentiation of Th17 cells and stimulates expansion of Tregs [65]. By contrast, mTORC2 regulates the activity of RhoA to accelerate Th2 cell differentiation [66]. Consequently, mTORC1-deficient T cells cannot differentiate into Th1 or Th17 cells, and mTORC2-deficient T cells lack the ability of Th2 differentiation [66, 67]. Further mTORC1 and mTORC2 negatively regulate the differentiation and functioning of Tregs [68].
mTOR regulates the proliferation and activation of B lymphocytes
B cells participate in the pathogenesis of RA by producing autoantibodies, proinflammatory cytokines, and chemokines, acting as efficient antigen-presenting cells and regulating T cell activation and differentiation [69, 70]. mTOR signaling pathway regulates immune function and homeostasis of peripheral B cell in RA [71].
Inhibition of the mTOR pathway in B cells restrains the growth of mitogen-dependent embryonic cells and suppresses cell proliferation by blocking cell cycle progression in the G1 phase, which markedly inhibits the differentiation and secretory functions of B cells. mTOR acts as an essential factor in B cell lineage differentiation; for example, mTOR negatively regulates the development of memory B cells [72]. Activated mTORC1 combines with Syk and induces EZH2 expression to inhibit BACH2 transcription, resulting in increased expression of Blimp-1 and XBP1, which are essential for differentiation and antibody secretion of plasma B cells [73,74,75]. mTORC1 signaling is critical for the early development of B cells and primary or secondary immune responses to T-dependent antigens. However, excessive activation of mTORC1 is pernicious to B cell maturation, especially with respect to marginal zone B cells [76, 77].
mTORC2 signaling regulates PI3K signaling in early B cells to upregulate IL-7R signaling and Rag gene expression, and pathway disruption results in interrupted B cell development [76]. Moreover, mTORC2 regulates the expression of NF-κB target genes, which affects cell survival downstream, mediated by BCR and BAFF-R signaling, and also influences antibody production in rictor-deficient mice [78]. BAFF-R plays a role in the activation of PI3K signaling pathways, as well as classical and alternative NF-κB signaling networks. The alternative NF-κB signal pathway involves phosphorylation of IKK1 by NF-κB -induced kinase (NIK), and IKK1 phosphorylates mTOR, leading to the transduction of downstream signaling. Moreover, BAFF-R is also involved in the induction of PI3K signaling, which enhances the activity of mTORC1 [79]. In addition, IL-27 has been identified as the critical driver of mTOR activation in B cells during RA. The mTOR inhibitor effectively restores IL-27-induced excessive activation, proliferation, and secretion of B cells and the abnormal ratio of regulatory B cells to plasma cells in vivo and in vitro [71]. Therefore, the mTOR pathway plays a crucial role in B cell immune dysfunctions during RA.
mTOR regulates macrophage activation, polarization, and migration
Macrophages are the first line of defense against pathogen invasion and the principal response to infection [80]. The normal synovial membrane is divided into the lining and synovial sub-lining layers. The lining layer includes macrophage-like synoviocytes (MLS) and FLSs. The sub-lining comprises synovial macrophages (SMs), a network of vascular capillaries, and fibrous and adipose tissue [81]. The occurrence of abundant activated macrophages in the synovia is an early marker of RA. M1 secretes pro-inflammatory cytokines (mainly IL-1, IL-6, and TNF), which lead to osteoclast (OC) activation and joint erosion, and M2 produces anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGF-β) to promote angiogenesis and tissue remodeling [82, 83]. M1 macrophages show high expression of MHCII and costimulatory molecules such as CD80 and CD88, which are the basis of effective T cell antigen presentation. M1 macrophages in RA synovium also secrete multiple chemokines to promote the recruitment of macrophages or neutrophils in arthritic sites, which corresponds to clinical severity of RA [84]. Taken together, during RA, macrophages produce proinflammatory cytokines, chemokines, and free radicals to activate immune and non-immune cells, leading to joint inflammation and destruction.
The essential roles of the mTOR pathway in macrophage biology have been partially elucidated over the past few years. Macrophage function depends on activation and polarization into subtypes with distinct effector functions. Macrophage polarization is regulated by various factors, including cytokines, growth factors, and environmental stimuli. Therefore, the mTOR pathway changes environmental and metabolic factors to influence the polarization of macrophages [85]. Inhibition of mTORC1 enhances the polarization of M1 macrophages, whereas TSC1 deletion increases the activity of mTORC1 and reduces mTORC2 activity to promote the polarization of M1 and decrease M2 macrophage polarization [86, 87]. In addition, differences of AKT subtypes control macrophage polarization [88]. AKT1 deletion promotes M1 macrophages, and AKT2 ablation produces M2 macrophages. Consistent with this, AMPK deletion decreases the activation of AKT and promotes M1 macrophage polarization [89]. With regard to metabolism functions, the activation of pyruvate dehydrogenase kinase 1 (PDK1) promotes M1 macrophage polarization, and aerobic glycolysis stimulator MYC polarizes M2 macrophages [90]. Overall, macrophage polarization is controlled by the mTOR pathway; however, the role of individual pathway members requires further research. mTORC1 inhibits the migration of macrophages and reduces the migratory activity of immune cells, and activation of mTORC1 induces the translation conversion of transcription factors to reduce the expression of chemokines CCL2, CCL3, and CCL4 [91]. In addition, it has been confirmed that the arthritogenicity of RA induced by Syntenin-1 relies on the remodeling of macrophages activated by mTOR and their capacity to cross-regulate Th1 cells through induction of IL-12 and IL-18. This interaction closely associated with mTOR's regulation of T cell and macrophage differentiation, and holds significant implications for guiding future research in this field [92].
mTOR regulates dendritic cell (DC) differentiation, maturation, and function
DCs are efficient antigen-presenting cells that initiate the initial immune response. During infection or under stressful conditions, mature DCs present antigens to naive T cells, resulting in the differentiation of effector CD4+T cells, production of B cell antibodies, and activation of macrophages [93]. DCs also contribute significantly to maintaining immune homeostasis and tolerance [94]. DCs secrete chemokines to attract macrophages, neutrophils, and T cells to the synovium during RA, which potentiates subsequent immune responses [95]. The specific mechanisms of various pro-inflammatory immune cells are described in detail above. DCs are directly or indirectly involved in the development of RA, and interactions between DCs and Tregs also interfere with RA development. Tregs express cytotoxic T-lymphocyte-associated protein-4, lymphocyte-activating gene-3, and neuropilin-1 to suppress DC functions [96].
The suppressive effects of mTOR inhibition on DCs have thoroughly investigated. Inhibition of mTOR reduces the expression of antigen uptake receptors and costimulatory molecules in DCs and restrains receptor-mediated phagocytosis [97]. Furthermore, inactivation of mTOR was suggested to inhibit CD86 expression induced by TLR ligands (such as LPS) or CD40-specific during DCs differentiation [98]. mTOR inhibitors attenuate the HIF-1α pathway to suppress hypoxia-induced inflammation and affect the differentiation of immature DCs [99]. mTOR is induced by Flt3 ligand (Flt3L) and is necessary for the Flt3L-driven development of DCs [100], and mTOR inhibits IL-1β production to impede DC maturation and the ability to stimulate effector T cell responses. In contrast, DCs under mTOR inactivation conditions favor differentiation of FOXP3+ Tregs [98]. In conclusion, mTOR exerts multiple effects on DC differentiation and functioning, which interferes with antigen uptake and presentation, and it affects cytokine production and chemokine receptor expression to modulate immune responses.
mTOR regulates the proliferation of RA FLSs
Abnormal proliferation, as well as high invasion and migration of FLSs in joints, are the main characteristics of RA [101]. Inflammation of RA-affected joints commences with FLS proliferation and invasion of immune cells into the synovium. FLSs then elicit overproduction of chemokines, cytokines, and matrix degradation molecules, thereby promoting immune cell infiltration and cartilage degradation [102]. Additionally, activated FLSs migrate to the synovial sub-lining layer to promote angiogenesis and exacerbate synovial hyperplasia and bone destruction [103]. In RA synovial cells, the mTOR signaling pathway is abnormally activated to participate in the regulation of RA FLS invasion [104]. Aberrant activation of PI3K/Akt/mTOR leads to high expression of anti-apoptosis genes, reduced autophagy, and continuous proliferation of FLSs [105, 106]. Inactivating mTOR prevents FLSs from recombining the actin cytoskeleton and eliciting bone destruction [104].
mTOR regulates differentiation and formation of OCs
OCs, differentiated giant multinucleated cells derived from the monocyte/macrophage lineage, decompose the bone matrix by producing various enzymes and acids [107]. OCs are hyperfunctioning during RA, resulting in bone destruction. mTOR controls the autophagy pathway to participate in OC differentiation and formation [108]. Inhibition of autophagy blocks the differentiation of RA mouse macrophages into OCs, decreases bone erosion, and reduces OC abundances. Conditional deletion of mTOR (raptor) in OCs results in decreased OC differentiation and activity. The increased expression of structurally active S6K1 rescues damage of OCs differentiation under Raptor-deficiency [109]. The AMPK/mTOR/p70S6K signaling pathway induces autophagy to inhibit OC differentiation, thus regulating bone mineral density and improving bone mass [110]. Furthermore, inhibition of the AMPK/mTOR/ULK1 pathway reduces autophagy in OCs exposed to high levels of glucose [111], and activation of the PI3K/AKT/mTOR axis suppresses autophagy of OCs treated with hydrogen sulfide [108]. Additionally, pharmacological inhibition of mTOR induces OC apoptosis and inhibits OC activity and differentiation [112, 113]. In conclusion, elucidating the mTOR pathway provides essential insights into the molecular mechanism of regulating OCs.
mTOR-targeted therapy
As described above, mTOR regulates many cellular processes and participates in the development of RA. Blocking this pathway through mTOR inhibitors is beneficial for RA treatment through changes in the immune and metabolic environment.
Rapamycin
Rapamycin is a potent mTOR inhibitor that controls antigen-induced T-cell expansion, antibody production, and cellular proliferation [114]. This compound was originally approved by regulatory authorities in 1999, with the purpose to prevent kidney allograft rejection. Further research revealed that mTOR is generally activated in neoplasms and controls cancer cell metabolism by regulating key metabolic enzymes; thus, it has been targeted for cancer treatment [115]. Moreover, rapamycin inhibits the proliferation of endothelial cells and prevents the deterioration of disease by intra-arterial drug-eluting stents [116]. In short, rapamycin has been successfully used for numerous medical applications, such as anti-inflammation, anti-immune rejection, anti-tumor, and anti-endothelial proliferation treatments, and it plays a key role in the treatment of many diseases.
Rapamycin significantly inhibits the activity of arthritis and improves immune function in RA mice [117, 118]. Rapamycin was also successfully used in clinical trials for RA treatment [119,120,121]. For instance, Wen et al. [120] found that patients receiving rapamycin therapy achieved profound clinical improvement through increased circulating Tregs during RA. In addition, rapamycin reduces the secretion of inflammatory cytokines such as IL-6, TNF, and IL-1β, relieving the symptoms of RA [122]. Moreover, rapamycin also decreases the necessity for conventional disease-modifying antirheumatic drugs in controlling RA activities [120]. Taken together, rapamycin induces autoimmune tolerance to reduce joint inflammation and is expected to be a new option for treating RA.
Rapalogues
Semi-synthetic rapamycin analogs are collectively referred to as rapalogues, such as everolimus (also known as RAD001), temsirolimus (CCI779), and ridaforolimus [123,124,125]. Compared with rapamycin, rapalogues are designed for higher water solubility and oral administration [126]. Everolimus inhibits the proliferation of synovial cells and the activity of OCs, which affects bone erosion during RA [112, 127]. Everolimus has the advantages of a simple administration route (oral), low cost, and low risk of infection [128]. A multicenter, randomized, double-blind trial investigating the safety and efficacy of everolimus reported that everolimus plus methotrexate showed better clinical efficacy and reduced adverse reactions [129]. Temsirolimus neutralizes the stimulation of LAT1 by IL-17 and reduces leucine uptake and fibroblast migration to prevent further erosion of the cartilage and bone [130].
Second-generation mTOR inhibitors
Dual PI3K and mTOR inhibitor NVP-BEZ23527 decreases mTOR and Akt phosphorylation to accelerate the apoptosis of bone cells. Furthermore, the dual effects of BEZ235 on PI3K/Akt and mTOR signaling pathways inhibit the activation of fibroblasts and eliminate the defect of rapamycin in p-Akt feedback activation of Ser473 after treatment with TGF-β [131]. Clinical trials with other dual mTOR/PI3K inhibitors, such as GSK2126458, PI103, SF1126, and GSK2126458, for the treatment of rheumatic diseases are underway [132].
N-acetylcysteine (NAC)
NAC is an antioxidant and anti-inflammatory agent whose functions are to promote glutathione biosynthesis and scavenge free radicals [133]. Glutathione modulates T-cell differentiation by regulating mitochondrial transmembrane potential (Δψm) and mTOR [134]. During RA, oxidative stress stimulates OC formation and activation to promote bone resorption. NAC reduces IL-17-induced activation of the mTOR/JNK/NF-κB (nuclear factor κB) pathway to regulate the expression of RANKL in synovial fibroblasts and osteoblasts, preventing inflammation and bone destruction during RA [135]. NAC supplementation improves clinical indicators of RA [136]. Additionally, continued exposure of T cells at sites of inflammation elicits high levels of reactive oxygen species (ROS), which results decreased intracellular levels of GSH, dysregulation of redox balance, impaired signal transduction of the TCR/CD3 complex, and ultimately to synovial T cell hyporesponsiveness during RA [137]. Accumulation of ROS induces oxidative damage and chondrocyte senescence, thereby accelerating the degeneration of the cartilage matrix, and antioxidant NAC reverses this process [138]. NAC effectively scavenges free-oxygen radicals, decelerates the process of cartilage degradation, reduces synovitis, and relieves pain [139]. Furthermore, NAC prevented chondrocyte apoptosis and cartilage destruction in an experimentally induced rat model of RA [140]. However, long-term oral NAC administration is associated with higher risk of RA [141], and potential clinical application of NAC requires further study.
Metformin (Met)
Met is the cornerstone of diabetes treatment, and during treatment of rheumatic diseases, it activates AMPK and inhibits mTORC1 [142,143,144]. Met-mediated AMPK activation and inhibition of mTOR activity regulate autophagy flux, inhibit NF-κB signal transduction and production of inflammatory cytokines, and reduce inflammation during experimentally induced arthritis [145, 146]. Met effectively inhibits the proliferation of RA-FLS by inducing G2/M cell cycle phase arrest, thus upregulating and downregulating phosphorylation of p70S6K and 4E-BP1 [147]. Furthermore, Met exerts an immunomodulatory effect of collagen induced arthritis by inhibiting Th17 cell differentiation and upregulating Treg differentiation [148]. Met inhibits differentiation of B cells into plasma cells and formation of spontaneous germinal center through the AMPK/mTOR/STAT3 signal pathway and thus reduces autoantibodies production and inflammation [149]. A retrospective cohort study found that long-term use of Met is related to reduced risk of developing RA [150].
Statins
Statins are lipid-lowering drugs that prevent cholesterol synthesis by inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase, commonly used in treating hypercholesterolemia and cardiovascular-related diseases [151]. Statins are also used as immunomodulators to block the adhesion of antigen-presenting cells to T cells, thus preventing the proliferation and function of T cells. Statins influence the activation of GTPases, such as Rho-GTPases, thereby regulating the transduction of PI3K/Akt/mTOR and ERK signaling pathways. The effects of statins on Tregs in RA patients in vivo and in vitro confirm that Tregs participate in the immunomodulatory impact of statins on RA [152].
Conclusion
During the pathogenesis of RA, the mTOR pathway is activated by multiple antigens, resulting in the recruitment and differentiation of immune cells and the activation of synovial and osteoclastic cells. Current studies suggest that targeting the mTOR pathway holds promise as a treatment for RA. However, indiscriminate suppression of mTOR to prevent RA may have unintended consequences. Long-term immunosuppressive therapy can compromise immune defense and surveillance, thereby increasing the risk of infections and tumors. Therefore, it is crucial to clarify the precise role of mTOR pathway in different stages of RA and its interaction with other signaling pathways and develop targeted therapies that do not disrupt essential physiological processes. Future investigations should prioritize understanding the potential side effects of immunotherapy in order to ensure the safety and efficacy of mTOR-based therapy for RA.
Availability of data and materials
Not applicable.
Abbreviations
- RA:
-
Rheumatoid arthritis
- mTOR:
-
Mechanistic target of rapamycin
- raptor:
-
Regulatory-associated protein of TOR
- mlst8:
-
Mammalian lethal with sec-13 protein 8
- deptor:
-
DEP domain–containing mTOR interacting protein
- pras40:
-
Proline-rich Akt substrate of 40kD
- rictor:
-
Rapamycin-insensitive companion of mTOR
- msin1:
-
Stress-activated protein kinase interacting protein 1
- PI3K:
-
Phosphatidylinositol-3-OH kinase
- AKT:
-
RAC-α serine/threonine-protein kinase
- TSC:
-
Threonine-protein kinase/tuberous sclerosis complex
- LKB1:
-
Liver kinase B1
- AMPK:
-
Mitogen-activated protein kinase
- TLR:
-
Toll-like receptor
- Rheb:
-
Ras homolog enriched in the brain
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome 10
- PIP2:
-
Ephosphatidylinositol-3,4-bisphosphate
- PIP3:
-
Phosphatidylinositol-3,4,5-triphosphate
- S6K1:
-
S6 kinase 1
- eIF4E:
-
Eukaryotic translation initiation factor 4E
- eIF4B:
-
Eukaryotic translation initiation factor 4B
- 4E-BP1:
-
EIF4E binding protein 1
- PDCD4:
-
Programmed cell death protein 4
- SKAR:
-
S6K1 Aly/REF-like target
- ATF4:
-
Activating transcription factor 4
- MTHFD2:
-
Methylenetetrahydrofolate dehydrogenase 2
- CAD:
-
Carbamoyl-phosphate synthetase
- HIF1α:
-
Hypoxia-inducible factor-1α
- ULK1:
-
Unc-51-like kinase1
- TFEB:
-
Transcription factor EB
- SGK1:
-
Serum and glucocorticoid inducible kinase 1
- PKCα:
-
Protein kinase C-α
- FLSs:
-
Fibroblast-like synoviocytes
- Teffs:
-
Effector T cells
- Tregs:
-
Regulatory T cells
- MLS:
-
Macrophage-like synoviocytes
- SMs:
-
Synovial macrophages
- OC:
-
Osteoclast
- TGF-β:
-
Factor-β
- DC:
-
Dendritic cell
- Flt3L:
-
Flt3 ligand
- NAC:
-
N-acetylcysteine
- NF-κB:
-
Nuclear factor κB
- ROS:
-
Reactive oxygen species
- Met:
-
Metformin
References
McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–37. https://doi.org/10.1016/S0140-6736(17)31472-1.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. https://doi.org/10.1016/S0140-6736(10)60826-4.
Liu S, Ma H, Zhang H, Deng C, Xin P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin Immunol. 2021;230:108793. https://doi.org/10.1016/j.clim.2021.108793.
Suto T, Karonitsch T. The immunobiology of mTOR in autoimmunity. J Autoimmun. 2020;110:102373. https://doi.org/10.1016/j.jaut.2019.102373.
Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo). 1975;28:721–6. https://doi.org/10.7164/antibiotics.28.721.
Aghdasi B, et al. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc Natl Acad Sci U S A. 2001;98:2425–30. https://doi.org/10.1073/pnas.041614198.
Sabers CJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–22. https://doi.org/10.1074/jbc.270.2.815.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. https://doi.org/10.1016/j.cell.2012.03.017.
Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12:169–82. https://doi.org/10.1038/nrrheum.2015.172.
Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. https://doi.org/10.1016/s0092-8674(02)00808-5.
Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. https://doi.org/10.1038/ncb1183.
Pearce LR, et al. Identification of protor as a novel rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–22. https://doi.org/10.1042/BJ20070540.
Jacinto E, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. https://doi.org/10.1016/j.cell.2006.08.033.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. https://doi.org/10.1101/gad.1212704.
Yang H, et al. 4.4 A resolution cryo-EM structure of human mTOR complex 1. Protein Cell. 2016;7:878–87. https://doi.org/10.1007/s13238-016-0346-6.
Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–74. https://doi.org/10.1016/j.molcel.2010.05.017.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–76. https://doi.org/10.1016/j.cell.2017.02.004.
Zhao H, Zhang X, Wang M, Lin Y, Zhou S. Stigmasterol simultaneously induces apoptosis and protective autophagy by inhibiting Akt/mTOR pathway in gastric cancer cells. Front Oncol. 2021;11:629008. https://doi.org/10.3389/fonc.2021.629008.
Chen X, et al. Cryo-EM structure of human mTOR complex 2. Cell Res. 2018;28:518–28. https://doi.org/10.1038/s41422-018-0029-3.
Aylett CH, et al. Architecture of human mTOR complex 1. Science. 2016;351:48–52. https://doi.org/10.1126/science.aaa3870.
Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–92. https://doi.org/10.1038/nrneurol.2016.81.
Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. https://doi.org/10.1038/nature04869.
Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. https://doi.org/10.1038/ncb839.
Im E, et al. Rheb is in a high activation state and inhibits B-Raf kinase in mammalian cells. Oncogene. 2002;21:6356–65. https://doi.org/10.1038/sj.onc.1205792.
Zhang Y, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–81. https://doi.org/10.1038/ncb999.
Haddadi N, et al. PTEN/PTENP1: “Regulating the regulator of RTK-dependent PI3K/Akt signalling”, new targets for cancer therapy. Mol Cancer. 2018;17:37. https://doi.org/10.1186/s12943-018-0803-3.
Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–9. https://doi.org/10.1038/nrm3522.
Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75. https://doi.org/10.1038/nrc2676.
Kemp BE, et al. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–5. https://doi.org/10.1016/s0968-0004(98)01340-1.
Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–8. https://doi.org/10.1016/j.tibs.2011.03.006.
Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. https://doi.org/10.1016/j.molcel.2008.03.003.
Liu P, et al. PtdIns(3,4,5)P3-dependent activation of the mTORC2 kinase complex. Cancer Discov. 2015;5:1194–209. https://doi.org/10.1158/2159-8290.CD-15-0460.
Hua H, et al. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12:71. https://doi.org/10.1186/s13045-019-0754-1.
Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–68. https://doi.org/10.1016/j.cell.2011.02.014.
Yang G, Murashige DS, Humphrey SJ, James DE. A positive feedback loop between Akt and mTORC2 via SIN1 phosphorylation. Cell Rep. 2015;12:937–43. https://doi.org/10.1016/j.celrep.2015.07.016.
Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279:169–97. https://doi.org/10.1007/978-3-642-18930-2_11.
Wang L, Rhodes CJ, Lawrence JC Jr. Activation of mammalian target of rapamycin (mTOR) by insulin is associated with stimulation of 4EBP1 binding to dimeric mTOR complex 1. J Biol Chem. 2006;281:24293–303. https://doi.org/10.1074/jbc.M603566200.
Gingras AC, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–37. https://doi.org/10.1101/gad.13.11.1422.
Dorrello NV, et al. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–71. https://doi.org/10.1126/science.1130276.
Richardson CJ, et al. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol. 2004;14:1540–9. https://doi.org/10.1016/j.cub.2004.08.061.
Methot N, Pickett G, Keene JD, Sonenberg N. In vitro RNA selection identifies RNA ligands that specifically bind to eukaryotic translation initiation factor 4B: the role of the RNA remotif. RNA. 1996;2:38–50.
Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–80. https://doi.org/10.1016/j.cell.2005.10.024.
Peterson TR, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–20. https://doi.org/10.1016/j.cell.2011.06.034.
Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83. https://doi.org/10.1016/j.molcel.2010.06.022.
Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–33. https://doi.org/10.1126/science.aad0489.
Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–8. https://doi.org/10.1126/science.1228792.
Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. https://doi.org/10.1146/annurev.nutr.27.061406.093749.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. https://doi.org/10.1038/ncb2152.
Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–14. https://doi.org/10.4161/auto.19653.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. https://doi.org/10.1126/science.1106148.
Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J. 2008;416:375–85. https://doi.org/10.1042/BJ20081668.
Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. https://doi.org/10.1016/j.cub.2004.06.054.
Guertin DA, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. https://doi.org/10.1016/j.devcel.2006.10.007.
Dai Q, Zhou D, Xu L, Song X. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des Devel Ther. 2018;12:4095–105. https://doi.org/10.2147/DDDT.S175763.
Gaffen SL. Biology of recently discovered cytokines: interleukin-17–a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res Ther. 2004;6:240–7. https://doi.org/10.1186/ar1444.
Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. https://doi.org/10.1146/annurev.immunol.25.022106.141623.
Kosmaczewska A, Swierkot J, Ciszak L, Wiland P. The role of Th1, Th17, and Treg cells in the pathogenesis of rheumatoid arthritis including anti-inflammatory action of Th1 cytokines. Postepy Hig Med Dosw (Online). 2011;65:397–403. https://doi.org/10.5604/17322693.948971.
Zeng H, Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol. 2013;25:347–55. https://doi.org/10.1016/j.coi.2013.05.002.
Xu X, Ye L, Araki K, Ahmed R. mTOR, linking metabolism and immunity. Semin Immunol. 2012;24:429–35. https://doi.org/10.1016/j.smim.2012.12.005.
Guma M, Tiziani S, Firestein GS. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat Rev Rheumatol. 2016;12:269–81. https://doi.org/10.1038/nrrheum.2016.1.
Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–76. https://doi.org/10.1084/jem.20110278.
Gerriets VA, et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol. 2016;17:1459–66. https://doi.org/10.1038/ni.3577.
Sundrud MS, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–8. https://doi.org/10.1126/science.1172638.
Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–60. https://doi.org/10.1084/jem.20151159.
Berod L, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–33. https://doi.org/10.1038/nm.3704.
Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. https://doi.org/10.1038/ni.2005.
Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–53. https://doi.org/10.1016/j.immuni.2010.06.002.
Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. https://doi.org/10.1016/j.immuni.2009.04.014.
Dorner T, Lipsky PE. B cells: depletion or functional modulation in rheumatic diseases. Curr Opin Rheumatol. 2014;26:228–36. https://doi.org/10.1097/BOR.0000000000000000.
Franks SE, Getahun A, Hogarth PM, Cambier JC. Targeting B cells in treatment of autoimmunity. Curr Opin Immunol. 2016;43:39–45. https://doi.org/10.1016/j.coi.2016.09.003.
Tang Y, et al. Altered peripheral B lymphocyte homeostasis and functions mediated by IL-27 via activating the mammalian target of rapamycin signaling pathway in patients with rheumatoid arthritis. Clin Exp Immunol. 2021;206:354–65. https://doi.org/10.1111/cei.13663.
Inoue T, et al. Exit from germinal center to become quiescent memory B cells depends on metabolic reprograming and provision of a survival signal. J Exp Med. 2021;218:e20200866. https://doi.org/10.1084/jem.20200866.
Nutt SL, Fairfax KA, Kallies A. BLIMP1 guides the fate of effector B and T cells. Nat Rev Immunol. 2007;7:923–7. https://doi.org/10.1038/nri2204.
Gonzalez-Garcia I, Ocana E, Jimenez-Gomez G, Campos-Caro A, Brieva JA. Immunization-induced perturbation of human blood plasma cell pool: progressive maturation, IL-6 responsiveness, and high PRDI-BF1/BLIMP1 expression are critical distinctions between antigen-specific and nonspecific plasma cells. J Immunol. 2006;176:4042–50. https://doi.org/10.4049/jimmunol.176.7.4042.
Shaffer AL, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. https://doi.org/10.1016/j.immuni.2004.06.010.
Iwata TN, Ramirez-Komo JA, Park H, Iritani BM. Control of B lymphocyte development and functions by the mTOR signaling pathways. Cytokine Growth Factor Rev. 2017;35:47–62. https://doi.org/10.1016/j.cytogfr.2017.04.005.
Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur J Immunol. 2007;37:2923–36. https://doi.org/10.1002/eji.200737281.
Lee K, et al. Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood. 2013;122:2369–79. https://doi.org/10.1182/blood-2013-01-477505.
McAllister E, Jellusova J. BAFF signaling in B cell metabolism. Curr Opin Immunol. 2021;71:69–74. https://doi.org/10.1016/j.coi.2021.05.011.
Liu W, et al. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front Immunol. 2018;9:2228. https://doi.org/10.3389/fimmu.2018.02228.
Veale DJ, Orr C, Fearon U. Cellular and molecular perspectives in rheumatoid arthritis. Semin Immunopathol. 2017;39:343–54. https://doi.org/10.1007/s00281-017-0633-1.
Tardito S, et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18: 102397. https://doi.org/10.1016/j.autrev.2019.102397.
Siouti E, Andreakos E. The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol. 2019;165:152–69. https://doi.org/10.1016/j.bcp.2019.03.029.
Rohde G, et al. CXC chemokines and antimicrobial peptides in rhinovirus-induced experimental asthma exacerbations. Clin Exp Allergy. 2014;44:930–9. https://doi.org/10.1111/cea.12313.
Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. https://doi.org/10.1038/nri3901.
Mercalli A, et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology. 2013;140:179–90. https://doi.org/10.1111/imm.12126.
Zhu L, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5:4696. https://doi.org/10.1038/ncomms5696.
Arranz A, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–22. https://doi.org/10.1073/pnas.1119038109.
Galic S, et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–15. https://doi.org/10.1172/JCI58577.
Tan Z, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol. 2015;194:6082–9. https://doi.org/10.4049/jimmunol.1402469.
Perino A, et al. TGR5 reduces macrophage migration through mTOR-induced C/EBPbeta differential translation. J Clin Invest. 2014;124:5424–36. https://doi.org/10.1172/JCI76289.
Meyer, Anja. et al. Syntenin-1-mediated arthritogenicity is advanced by reprogramming RA metabolic macrophages and Th1 cells. Ann Rheum Dis. 2023;2022:223284. https://doi.org/10.1136/ard-2022-223284.
Wehr P, Purvis H, Law SC, Thomas R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin Exp Immunol. 2019;196:12–27. https://doi.org/10.1111/cei.13256.
Khan S, Greenberg JD, Bhardwaj N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:566–71. https://doi.org/10.1038/nrrheum.2009.185.
Moret FM, et al. Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res Ther. 2013;15:R155. https://doi.org/10.1186/ar4338.
Hu XX, Wu YJ, Zhang J, Wei W. T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. Int Immunopharmacol. 2019;70:428–34. https://doi.org/10.1016/j.intimp.2019.03.008.
Monti P, et al. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation. 2003;75:137–45. https://doi.org/10.1097/00007890-200301150-00025.
Turnquist HR, et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–31. https://doi.org/10.4049/jimmunol.178.11.7018.
Rama I, et al. Hypoxia stimulus: an adaptive immune response during dendritic cell maturation. Kidney Int. 2008;73:816–25. https://doi.org/10.1038/sj.ki.5002792.
Sathaliyawala T, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. https://doi.org/10.1016/j.immuni.2010.09.012.
Wang J, Zhao Q. Kaempferitrin inhibits proliferation, induces apoptosis, and ameliorates inflammation in human rheumatoid arthritis fibroblast-like synoviocytes. Phytother Res. 2019;33:1726–35. https://doi.org/10.1002/ptr.6364.
Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19:110. https://doi.org/10.1186/s13075-017-1303-3.
Ma JD, et al. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther. 2019;21:153. https://doi.org/10.1186/s13075-019-1935-6.
Laragione T, Gulko PS. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol Med. 2010;16:352–8. https://doi.org/10.2119/molmed.2010.00049.
Ito Y, Hart JR, Vogt PK. Isoform-specific activities of the regulatory subunits of phosphatidylinositol 3-kinases - potentially novel therapeutic targets. Expert Opin Ther Targets. 2018;22:869–77. https://doi.org/10.1080/14728222.2018.1522302.
Perlman H, et al. Bcl-2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. J Immunol. 2000;164:5227–35. https://doi.org/10.4049/jimmunol.164.10.5227.
Sato K. Th17 cells and rheumatoid arthritis–from the standpoint of osteoclast differentiation. Allergol Int. 2008;57:109–14. https://doi.org/10.2332/allergolint.R-07-158.
Wang S, et al. The role of autophagy and mitophagy in bone metabolic disorders. Int J Biol Sci. 2020;16:2675–91. https://doi.org/10.7150/ijbs.46627.
Dai Q, et al. Inactivation of regulatory-associated protein of mTOR (Raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J Biol Chem. 2017;292:196–204. https://doi.org/10.1074/jbc.M116.764761.
Tong X, et al. Suppression of AMP-activated protein kinase reverses osteoprotegerin-induced inhibition of osteoclast differentiation by reducing autophagy. Cell Prolif. 2020;53: e12714. https://doi.org/10.1111/cpr.12714.
Zhao H, et al. Antiosteoclastic bone resorption activity of osteoprotegerin via enhanced AKT/mTOR/ULK1-mediated autophagic pathway. J Cell Physiol. 2020;235:3002–12. https://doi.org/10.1002/jcp.29205.
Cejka D, et al. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:2294–302. https://doi.org/10.1002/art.27504.
Chen S, Jia L, Zhang S, Zheng Y, Zhou Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res Ther. 2018;9:185. https://doi.org/10.1186/s13287-018-0935-9.
Sehgal SN, Bansbach CC. Rapamycin: in vitro profile of a new immunosuppressive macrolide. Ann N Y Acad Sci. 1993;685:58–67. https://doi.org/10.1111/j.1749-6632.1993.tb35852.x.
Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–57. https://doi.org/10.1038/s41568-018-0074-8.
Furukawa Y, et al. Sirolimus-eluting stent for in-stent restenosis of left main coronary artery in Takayasu arteritis. Circ J. 2005;69:752–5. https://doi.org/10.1253/circj.69.752.
Drosos AA. Newer immunosuppressive drugs: their potential role in rheumatoid arthritis therapy. Drugs. 2002;62:891–907. https://doi.org/10.2165/00003495-200262060-00003.
Yocum DE. Cyclosporine, FK-506, rapamycin, and other immunomodulators. Rheum Dis Clin North Am. 1996;22:133–54. https://doi.org/10.1016/s0889-857x(05)70266-0.
Niu HQ, et al. Sirolimus selectively increases circulating Treg cell numbers and restores the Th17/Treg balance in rheumatoid arthritis patients with low disease activity or in DAS28 remission who previously received conventional disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol. 2020;38:58–66.
Wen HY, et al. Low-dose sirolimus immunoregulation therapy in patients with active rheumatoid arthritis: a 24-week follow-up of the randomized, open-label, parallel-controlled trial. J Immunol Res. 2019;2019:7684352. https://doi.org/10.1155/2019/7684352.
Foroncewicz B, Mucha K, Paczek L, Chmura A, Rowinski W. Efficacy of rapamycin in patient with juvenile rheumatoid arthritis. Transpl Int. 2005;18:366–8. https://doi.org/10.1111/j.1432-2277.2004.00070.x.
Shao P, Ma L, Ren Y, Liu H. Modulation of the immune response in rheumatoid arthritis with strategically released rapamycin. Mol Med Rep. 2017;16:5257–62. https://doi.org/10.3892/mmr.2017.7285.
Rini BI. Temsirolimus, an inhibitor of mammalian target of rapamycin. Clin Cancer Res. 2008;14:1286–90. https://doi.org/10.1158/1078-0432.CCR-07-4719.
Gabardi S, Baroletti SA. Everolimus: a proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology. Pharmacotherapy. 2010;30:1044–56. https://doi.org/10.1592/phco.30.10.1044.
Mita M, Sankhala K, Abdel-Karim I, Mita A, Giles F. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs. 2008;17:1947–54. https://doi.org/10.1517/13543780802556485.
Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–80. https://doi.org/10.1038/nrd3531.
Yoon KH. Proliferation signal inhibitors for the treatment of refractory autoimmune rheumatic diseases: a new therapeutic option. Ann N Y Acad Sci. 2009;1173:752–6. https://doi.org/10.1111/j.1749-6632.2009.04663.x.
Olsen NJ. Is there a role for everolimus in the treatment of RA? Nat Clin Pract Rheumatol. 2009;5:68–9. https://doi.org/10.1038/ncprheum0980.
Bruyn GA, et al. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomised, placebo-controlled, parallel-group, proof-of-concept study. Ann Rheum Dis. 2008;67:1090–5. https://doi.org/10.1136/ard.2007.078808.
Yu Z, Lin W, Rui Z, Jihong P. Fibroblast-like synoviocyte migration is enhanced by IL-17-mediated overexpression of L-type amino acid transporter 1 (LAT1) via the mTOR/4E-BP1 pathway. Amino Acids. 2018;50:331–40. https://doi.org/10.1007/s00726-017-2520-4.
Liang M, et al. Vertical inhibition of PI3K/Akt/mTOR signaling demonstrates in vitro and in vivo anti-fibrotic activity. J Dermatol Sci. 2014;76:104–11. https://doi.org/10.1016/j.jdermsci.2014.08.002.
Jung SM, Park KS, Kim KJ. Deep phenotyping of synovial molecular signatures by integrative systems analysis in rheumatoid arthritis. Rheumatology (Oxford). 2021;60:3420–31. https://doi.org/10.1093/rheumatology/keaa751.
Mokhtari V, Afsharian P, Shahhoseini M, Kalantar SM, Moini A. A review on various uses of N-acetyl cysteine. Cell J. 2017;19:11–7. https://doi.org/10.22074/cellj.2016.4872.
Fernandez DR, et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol. 2009;182:2063–73. https://doi.org/10.4049/jimmunol.0803600.
Kim HR, Kim KW, Kim BM, Lee KA, Lee SH. N-acetyl-l-cysteine controls osteoclastogenesis through regulating Th17 differentiation and RANKL production in rheumatoid arthritis. Korean J Intern Med. 2019;34:210–9. https://doi.org/10.3904/kjim.2016.329.
Jamali F, Ahmadzadeh A, Sahraei Z, Salamzadeh J. Study of the effects of N-acetylcysteine on inflammatory biomarkers and disease activity score in patients with rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2021;20:574–83. https://doi.org/10.18502/ijaai.v20i5.7407.
Maurice MM, et al. CD28 co-stimulation is intact and contributes to prolonged ex vivo survival of hyporesponsive synovial fluid T cells in rheumatoid arthritis. Eur J Immunol. 1998;28:1554–62. https://doi.org/10.1002/(SICI)1521-4141(199805)28:05%3c1554::AID-IMMU1554%3e3.0.CO;2-N.
Kang D, et al. Selenophosphate synthetase 1 deficiency exacerbates osteoarthritis by dysregulating redox homeostasis. Nat Commun. 2022;13:779. https://doi.org/10.1038/s41467-022-28385-7.
Ozcamdalli M, et al. Comparison of intra-articular injection of hyaluronic acid and N-acetyl cysteine in the treatment of knee osteoarthritis: a pilot study. Cartilage. 2017;8:384–90. https://doi.org/10.1177/1947603516675915.
Kaneko Y, et al. Oral administration of N-acetyl cysteine prevents osteoarthritis development and progression in a rat model. Sci Rep. 2019;9:18741. https://doi.org/10.1038/s41598-019-55297-2.
Yeh YT, Liang CC, Chang CL, Hsu CY, Li PC. Increased risk of knee osteoarthritis in patients using oral N-acetylcysteine: a nationwide cohort study. BMC Musculoskelet Disord. 2020;21:531. https://doi.org/10.1186/s12891-020-03562-1.
Miranda VC, Barroso-Sousa R, Glasberg J, Riechelmann RP. Exploring the role of metformin in anticancer treatments: a systematic review. Drugs Today (Barc). 2014;50:623–40. https://doi.org/10.1358/dot.2014.50.9.2229920.
Kim JW, Choe JY, Park SH. Metformin and its therapeutic applications in autoimmune inflammatory rheumatic disease. Korean J Intern Med. 2022;37:13–26. https://doi.org/10.3904/kjim.2021.363.
Salvatore T, et al. Metformin: a potential therapeutic tool for rheumatologists. Pharmaceuticals (Basel). 2020;13:234. https://doi.org/10.3390/ph13090234().
Yan H, Zhou HF, Hu Y, Pham CT. Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation. J Rheum Dis Treat. 2015;1:5. https://doi.org/10.23937/2469-5726/1510005.
Song P, et al. Therapeutic applications of type 2 diabetes mellitus drug metformin in patients with osteoarthritis. Pharmaceuticals (Basel). 2021;14:152. https://doi.org/10.3390/ph14020152.
Chen K, et al. Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed Pharmacother. 2019;115:108875. https://doi.org/10.1016/j.biopha.2019.108875.
Son HJ, et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014;2014:973986. https://doi.org/10.1155/2014/973986.
Lee SY, et al. Metformin suppresses systemic autoimmunity in Roquin(san/san) mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. J Immunol. 2017;198:2661–70. https://doi.org/10.4049/jimmunol.1403088.
Naffaa ME, et al. Adherence to metformin and the onset of rheumatoid arthritis: a population-based cohort study. Scand J Rheumatol. 2020;49:173–80. https://doi.org/10.1080/03009742.2019.1695928.
Stone NJ, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-45. https://doi.org/10.1161/01.cir.0000437738.63853.7a.
Tang TT, et al. Atorvastatin upregulates regulatory T cells and reduces clinical disease activity in patients with rheumatoid arthritis. J Lipid Res. 2011;52:1023–32. https://doi.org/10.1194/jlr.M010876.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82001740) and the National Social Science Fund of China (21BTQ050).
Author information
Authors and Affiliations
Contributions
Fen Zhang, Ting Cheng and Sheng-xiao Zhang both wrote the main manuscript text and all authors prepared figures 1-3. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that all authors consent to participate in the writing of manuscript, and the order of authors listed in the manuscript has been approved by all of us.
Consent for publication
All authors approved the final version to be published.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, F., Cheng, T. & Zhang, SX. Mechanistic target of rapamycin (mTOR): a potential new therapeutic target for rheumatoid arthritis. Arthritis Res Ther 25, 187 (2023). https://doi.org/10.1186/s13075-023-03181-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-023-03181-w