Abstract
Obesity is associated with metabolic dysfunction, including a positive energy balance, increased insulin resistance, dyslipidemia, hypertension and inflammation. Bariatric surgery is an effective treatment of many of the metabolic defects associated with obesity. In this review, we outline the effect of bariatric surgery on energy balance, glucose homeostasis, blood pressure control and lipid metabolism. We also examine the effect of bariatric surgery on inflammation, and in particular renal inflammation, and detail the evidence for the mechanisms underlying these effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is increasingly prevalent, and is associated with metabolic changes that predispose to diabetes, cardiovascular disease and inflammation [1]. These changes include increased insulin resistance, increased endogenous glucose production, increased dyslipidemia, and changes in the regulation of energy balance [1, 2].
Bariatric surgery can effectively treat the condition of obesity associated metabolic dysfunction [3]. As well as improving metabolic parameters such as insulin sensitivity and secretion, there are changes to energy balance with improved appetite and energy expenditure [4–6]. This can translate to remission of type 2 diabetes in approximately 40 % of surgical recipients, but can also result in marked reductions in inflammation, with clinically important effects in conditions such as kidney disease [4, 7].
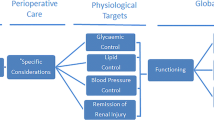
In this article, we review the effects of bariatric surgery on several key metabolic conditions associated with obesity. We will outline the major bariatric modalities, and then detail the changes that occur after bariatric surgery in each of the major processes mentioned above. Specifically, we will discuss the changes in glucose homeostasis, lipid metabolism, and energy regulation after bariatric surgery. Finally, we will examine the evidence for changes in inflammation, and discuss potential mechanisms associated with these changes.
Review
Major Bariatric Modalities
Several bariatric procedures are available. The most commonly performed procedures are Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB), and vertical sleeve gastrectomy (VSG) [8]. Biliopancreatic diversion, with or without duodenal switch (BPD and BPD-DS), is less commonly performed but is often considered in extremely obese individuals [9].
In RYGB, the stomach is divided into an upper gastric pouch, which is 15–30 milliliters (mL) in volume, and a lower gastric remnant. The jejunum is divided some 30 to 75 centimeters (cm) distal to the ligament of Treitz; this distal part is anastomosed to the gastric pouch. The excluded biliary limb is connected to the bowel 75 to 150 cm distal to the gastrojejunostomy.
In AGB, a band with an inner inflatable silastic balloon is placed around the proximal stomach just below the gastroesophageal junction. The band can be tightened through a subcutaneous access port by the injection or withdrawal of a saline solution.
In VSG, the stomach is transected vertically over a 34 or 36F bougie creating a gastric tube and leaving a pouch of 100–200 mL.
BPD involves a partial gastrectomy that results in a 400 mL gastric pouch [10]. The small bowel is divided 250 cm proximal to the ileocecal valve, and the alimentary limb is connected to the gastric pouch to create a Roux-en-Y gastroenterostomy. An anastomosis is performed between the excluded biliopancreatic limb and the alimentary limb 50–100 cm proximal to the ileocecal valve. In BPD-DS, a VSG is constructed and the division of the duodenum is performed immediately beyond the pylorus. The alimentary limb is connected to the duodenum, whereas the biliopancreatic limb is anastomosed to the ileum 75–100 cm proximal to the ileocecal valve [11].
Insulin Resistance, Insulin Secretion and Glycemia: Changes After Bariatric Surgery
Insulin resistance is associated with obesity and is a key factor in the development of obesity associated type 2 diabetes [12, 13, 14•, 15–18]. Insulin resistance can develop or be exacerbated in obesity via increased hepatic insulin resistance, or through the accumulation of adipose tissue [12, 13, 14•, 15–18]. Hepatic insulin resistance is very responsive to bariatric surgery, and can be ameliorated by RYGB in a non-diabetic population as measured by HOMA-IR [14•, 17].
This is also the case in those with diabetes, and the changes are seen within 7 days post-operatively [12, 13]. The improvement in HOMA-IR after bariatric surgery results in insulin sensitivity comparable to those with normal glucose tolerance, and improvements continue for up to 12 months post-operatively [14•, 15]. Similar results have been consistently shown in animal and human studies [12, 13, 14•, 15–18].
Peripheral insulin resistance does not immediately improve after RYGB to the same extent as hepatic insulin resistance [2, 15, 17, 18]. Peripheral glucose uptake as measured by euglycemic-hyperinsulinemic clamp is enhanced by RYGB in non-diabetic cohorts, although it can take up to 6 months for this to be measurable [18].
However, even if peripheral insulin resistance remain unchanged post-operatively almost 60 % of surgical recipients can still have near normal fasting blood glucose levels within a few weeks [15]. Pre-operative glucose tolerance has no affect on peripheral insulin sensitivity post-operatively [15]. This implies that the hepatic effects on insulin resistance, in concert with the other metabolic effects, are important in determining the impact of bariatric surgery on glucose homeostasis post-operatively.
The mechanism of the improvement in hepatic insulin resistance is not yet fully understood. Acute calorie restriction has been suggested as the main component of the improvement in insulin resistance after weight loss [19]. Low calorie diets, such as those that take effect immediately after bariatric surgery, can improve insulin sensitivity [19, 20]. This can occur within 1 week of initiating a very low calorie diet, with significant reductions in hepatic insulin resistance as measured by euglycemic-hyperinsulinemic clamp [19].
The improved hepatic insulin sensitivity following a reduction in food intake after bariatric surgery can be associated with significant improvements in glucose handling [19, 20, 29, 38]. The changes in glycemia can be seen as early as 4 weeks from the onset of low-calorie intake in this cohort [20–23]. The effects of reduced energy intake are limited to hepatic insulin resistance, and calorie restriction has a negligible effect on peripheral insulin resistance over an eight-week period [19].
There may be mechanisms other than calorie restriction responsible for the changes in insulin sensitivity seen after bariatric surgery. In a study of obese participants who maintained a very low calorie diet and subsequently underwent RYGB, insulin resistance as measured by insulin tolerance test and HOMA-IR reduced after calorie restriction but decreased further after RYGB [24]. Therefore, there are additive mechanisms associated with RYGB to improve insulin sensitivity above and beyond that of calorie restriction alone.
Weight loss can partially explain this with respect to hepatic insulin resistance, and may be the complete answer with respect to peripheral insulin resistance.
With weight loss insulin resistance does improve and this is maintained long term [2, 25]. A study measuring insulin resistance in a euglycemic-hyperinsulinemic clamp, and correcting for body weight and basal energy expenditure, could not detect a difference in hepatic insulin resistance beyond that associated with weight loss [2]. Other data find comparable improvements in HOMA-IR after AGB and RYGB that can be correlated with weight loss [5, 26]. Peripheral insulin resistance is not particularly responsive to bariatric surgery in the short term, but any improvements seen are associated with a greater degree of weight loss [18, 19, 27].
Some of the improvements in weight loss can be attributed to GLP-1 and other satiety gut hormones [4, 5, 28]. Incretin hormones may also affect insulin metabolism, therefore improving insulin sensitivity [27–31]. Bariatric procedures vary in how they enhance the incretin response. There is a greater improvement in RYGB as compared to AGB [27, 31]. However, similar improvements in peripheral insulin sensitivity can be achieved with AGB, a procedure that itself is not associated with altered entero-hormonal dynamics, thus implying that weight loss is the most important mediator of peripheral insulin sensitivity after bariatric surgery rather than improvements in incretin secretion [3–5].
Incretin hormones, and particularly glucagon-like-peptide-1 (GLP-1), have been implicated in the improvements in insulin secretion after bariatric surgery [29, 30]. A very low calorie diet can induce improvements in first-phase insulin secretion [19]. This can result in the better insulin secretion in obese cohorts with diabetes, to a level comparable to BMI matched control groups [19].
While this may contribute, RYGB results in increased insulin secretion after eating when compared to patients who had similar calorie restriction for 2 weeks in non-diabetic matched groups with equivalent weight loss [18]. An associated exaggerated increase in prandial GLP-1 secretion is associated with this improved insulin secretion [12, 14•, 17, 18]. Therefore, the reduced calorie intake may additively improve insulin dynamics post-operatively, and in combination with the enhanced incretin response, can result in improvements in insulin secretion after bariatric surgery.
Beta-cell function may be partially restored, and this can also be attributed to enhanced incretin secretion [28, 29]. Beta-cell gluco-sensitivity remains impaired in diabetic cohort after RYGB when compared to non-diabetic cohorts [28]. However, overall bariatric surgery improves beta-cell function, restores incretin dynamics and improves insulin secretion [12, 14•, 17, 18, 28, 29].
There are intracellular mechanisms involving regulators of insulin action that are augmented by GLP-1 analogues [32]. These data suggest that GLP-1 may act at a cellular level, to improve receptor sensitivity to insulin action [32]. There may also be effects on gene expression, to up regulate PPARy, thereby countering the down regulation of these genes in mouse models [33]. However, these studies are only now emerging and further work is needed to determine the effect and cellular mechanism of GLP-1 on insulin resistance.
GLP-1 also reduces lipotoxicity and glucotoxicity, which could indirectly improve insulin sensitivity [29, 30]. While this could potentially positively affect HOMA-IR calculations, it is not clear of the magnitude by which GLP-1 can influence clinically relevant hepatic insulin resistance [12, 14•].
Other gut hormones such as glucose-dependent insulinotropic polypeptide (GIP) may contribute to maintenance of intact adipocytes, and RYGB may reduce GIP secretion through bypassing the duodenum [34, 35]. These findings remain controversial.
There is an interplay between calorie restriction, weight loss, gut hormone secretion and potentially other factors in the remediation of hepatic insulin resistance. For now, the evidence base on gut hormones does not allow us to draw any definitive conclusions about their role in the improvements in insulin sensitivity. There is no doubt, however, that bariatric surgery does improve hepatic insulin resistance and that this is at least partially mediated by calorie restriction in the short-term, and facilitated by weight loss in the medium to long-term. The effect on peripheral resistance in the short term is minimal, and is associated with weight loss in the long term rather than any other mechanism.
However, the combined effect on insulin sensitivity and insulin secretion translates to greater improvements in glycemic control in type 2 diabetes with bariatric surgery when compared to medical therapy [36•, 37•]. Diabetes remission occurs in approximately 40 % of RYGB recipients, and up to 95 % of BPD recipients over 2 years [36•, 37•].
The positive effects on glucose metabolism are also beneficial in non-diabetic cohorts, with improved glucose handing after RYGB and VSG [22, 38]. These beneficial changes are seen within weeks of the procedure, and are maintained for at least 12 months [22, 38]. This can translate to long-term reductions in diabetes incidence in obese cohorts [25].
Lipid Metabolism
Bariatric surgery is associated with a number of changes in lipid metabolism associated with reduced cardiovascular risk. RYGB is associated with subtle reduction in low-density lipoprotein (LDL) and triglycerides, and dramatic increases in high-density lipoprotein (HDL) [16, 39, 40]. BPD also reduces plasma free fatty acids and triglycerides in patients with a BMI > 35 kg/m2 [30, 41].
As well as these changes on serum lipids, adipokines are increased following bariatric surgery and the increase correlates with weight loss, less insulin resistance and a lower risk of developing diabetes mellitus [42–44]. The increase in adipokines can also be associated with remission of diabetes [45].
The mechanisms of these positive changes in lipid and adipokine metabolism may be related to the reduction in fat mass [39, 42–44]. However, some of the hormonal effects of bariatric surgery may also contribute. GLP-1 has been associated with increased fat oxidization during meals, and improvements in lipid metabolism, with a noted reduction in intracellular lipolysis [46, 47].
Appetite and Energy Expenditure
Bariatric surgery is associated with improved satiety and reduced appetite through central and humoral mechanisms [4–6]. This effect is regulated through a complex neuroendocrine network that is still being defined [6, 48]. RYGB can increase the secretion of the satiety promoting gut hormones peptide YY (PYY) and GLP-1 [49, 50]. The neural pathways regulating energy intake and energy expenditure include the arcuate nucleus, which has receptors to which peripherally and centrally administered PYY can bind [48, 51]. In patients after RYGB and AGB, antagonism of PYY and GLP-1 results in increased appetite [49]. Bile acids may also participate in this system by augmenting post-prandial gut hormone secretion [52•]. In RYGB, bile acid secretion is increased and this is associated with greater gut hormone levels, thus reducing appetite [52•].
Therefore, the enhanced gut hormone response has a central effect on appetite after surgery [5, 48, 51]. However, there are also effects on entero-neural systems involving the vagus nerve and its derivatives [48, 53, 54]. The contribution of GLP-1 mediated vagal responses in regulating appetite is not fully understood [53, 54]. Selective vagotomy can impair glycemic control, and have some effect on appetite [54]. However, vagotomized rats do eat less than controls and the reasons for this are not yet known [54]. Studies comparing central and intraperitoneal administration of GLP-1 in vagotomized rats suggest that there is significant contribution to satiety from both central and vagal incretin stimulation [48].
Further studies are needed to establish how the entero-endocrine system interacts with the central nervous system to affect satiety, but further research may yield breakthroughs that change the way we understand obesity and appetite. For now, we can be sure that bariatric surgery generally reduces appetite, at least in the medium term, and that the gut hormone effects, augmented by enhanced bile secretion, drive this change in appetite through central and vagal mechanisms.
RYGB in rats increases resting energy expenditure compared to sham operated and sham operated body weight matched rats [55–57]. The increased energy expenditure is not related to physical activity or thermogenesis, and central mechanisms have been implicated [6, 56, 57]. In contrast weight loss after RYGB in humans most likely reduces basal energy expenditure in diabetic and non-diabetic patients [2, 58]. However, this is in the context of reduced calorie intake and weight loss. Patients usually have a greater resting energy expenditure pre-intervention, and therefore the resting energy expenditure may be greater than expected [40, 59, 60].
This change in energy expenditure has been related to weight loss, although the data are conflicting [40, 56, 60]. The more important determinant may be the individual’s metabolic rate before surgery [40]. Those with greater metabolic rates pre-operatively may adapt to a reduced level that, when acting in the context of reduced energy intake, remains at a level that can contribute to weight loss [40, 60].
The mechanisms underpinning this change in energy expenditure are not well elucidated. Gut hormones may be implicated [61, 62, 63•]. Oxyntomodulin, which mediates some of its action through the GLP-1 receptor, increases energy expenditure while reducing energy intake in overweight and obese humans [64]. However, oxyntomodulin also acts on the glucagon receptor and its response to bariatric surgery is not clear. Preferential oxidation of fat rather than carbohydrate after RYGB may also play a role in the changes in energy expenditure [65].
Inflammation: Bariatric Surgery and Kidney Disease
Obesity is recognized as a state of chronic low grade inflammation [66]. Inflammatory markers such as C-reactive protein (CRP) and pro-inflammatory cytokines are reduced by bariatric surgery [66–68]. There is an increase in anti-inflammatory cytokine and chemokine levels [66–71]. This can be implicated in the improvements in a number of end-organ complications of obesity, including nephropathy [7].
These data are based on cohort studies, and the evidence from randomized studies still need confirmation [66]. It remains controversial to use cytokines like tumour necrosis factor alpha (TNFα) or interleukins in monitoring the changes in inflammation after bariatric surgery [66–70]. The difficulty arises when the tissue specific relationship between cytokines and chemokines is seen to improve after surgery, as the basis for the improvements in these measurements can be multi-factorial, leading to distortion of the data [69–72]. To best determine the effect of bariatric surgery on the inflammatory process within an organ system, the studies need to be focused on a particular disease process with pre-defined outcome measures.
When research in obesity and bariatric surgery is directed at specific organ system, more valid conclusions can often be found. Bariatric surgery may be associated with improvements in renal function, but the limitations of serum creatinine in patients losing lean body mass needs to be taken into account [7, 73]. Histological appearances in obesity associated kidney disease improve after bariatric surgery, and in some cases, bariatric surgery has resulted in the recovery of renal function in dialysis-dependent patients [74].
However, the mechanisms for these improvements are not fully understood. Bariatric surgery improves blood pressure and this has been implicated in the improvements in estimated glomerular filtration rates (eGFR) [75, 76]. While improvements in body weight, blood pressure and glucose homeostasis are likely to contribute, the role of inflammation is increasingly recognized to be key to the process of kidney disease in obese cohorts, with and without diabetes [7, 77, 78].
Improvements in renal inflammation following bariatric surgery may be associated with improvements in renal function [7, 78, 79]. The amelioration in inflammation has been best described in RYGB, VSG and AGB, with data finding comparable effects in these three modalities [7, 79].
There are particular cytokines that can be correlated with the development of renal disease [80]. Monocyte-chemoattractant protein-1 (MCP-1), also known as CC-chemokine ligand 2 (CCL2), is one that is strongly associated with renal disease in obesity, and with diabetic nephropathy in particular [81]. MCP-1 contributes to macrophage accumulation and renal inflammation [82–84]. Blockade of the MCP-1 pathway delays the development of renal disease in diabetic animal models, and is associated with improved cell function in human in-vitro studies [82–84].
Bariatric surgery is associated with reductions in MCP-1 and is correlated to improved renal function [7, 79, 85]. MCP-1 is also associated with the weight loss post-operatively [7, 79]. Other data seem to hint at other mechanisms acting in addition to weight loss to reduce inflammation. After RYGB, there was a post-operative reduction in MCP-1 that can result in levels lower than that of lean controls, inferring that the relationship between MCP-1 improvements and weight or fat mass, may not be as simple as it seems [45].
Gut hormones are increasingly being associated with reduction of inflammation in obese cohorts losing weight [86•, 87]. In kidney disease, this occurs, at least in part, through T-cell mediated regulation of macrophage aggregation and inflammation [86•, 87, 88]. The reduction in inflammation correlates with improved histological appearances [87]. This gut hormone effect on inflammation can be independent of changes in glycemia [87, 89, 90].
In other diseases, GLP-1 mediated improvements in inflammation have resulted in demonstrable clinical benefits [86•]. The effect of bariatric surgery augmented gut hormone responses or GLP-1 analogues in psoriasis have shown particularly impressive results [86•, 91, 92]. This effect has been shown to act through the T-cell population, and therefore could act to regulate macrophage activity, which is a major immunological component of renal inflammation and disease progression in diabetic kidney disease [86•, 91, 93].
Therefore, bariatric surgery may improve outcomes in renal disease, and this effect can be achieved through improvements in blood pressure, glycemia, dyslipidemia and inflammation [7, 73]. Inflammation is a key component in this mechanism. It seems likely that the enhanced gut hormone response following bariatric surgery improves gut hormone regulation of the inflammatory response. However, this remains to be proven.
It should be remembered that renal impairment has also been associated with increased operative mortality in surgical subjects in observational data [94]. Therefore, more studies are needed to fully define the safety and benefits of bariatric surgery in this group, although the evidence to date suggests than those with stage 3 renal impairment or better may benefit [72, 73, 75].
Conclusion
Bariatric surgery can facilitate positive metabolic changes that improve insulin resistance, insulin secretion, blood pressure, dyslipidemia and inflammation. These changes are driven mainly by reduced appetite, weight loss and improvements in energy balance. The enhanced gut hormone responses post-operatively in procedures such as RYGB also contribute to these beneficial effects. These changes can aid diabetes remission or prevention, and can improve outcomes in disease mediated by inflammation including obesity associated nephropathy and diabetic kidney disease. As we continue to define and understand these disease processes through our ongoing research, we may find greater use for bariatric surgery and incretin-based therapy in treating and preventing diabetes and renal disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Salazar MR, Carbajal HA, Espeche WG, et al. Relationships among insulin resistance, obesity, diagnosis of the metabolic syndrome and cardio-metabolic risk. Diabetes Vasc Dis Res. 2011;8:109–16.
Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093–102.
Meijer RI, van Wagensveld BA, Siegert CE, et al. Bariatric surgery as a novel treatment for type 2 diabetes mellitus: a systematic review. Arch Surg. 2011;146:744–50.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56 e5.
le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14.
Rao RS. Bariatric surgery and the central nervous system. Obes Surg. 2012;22:967–78.
Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2012 Apr 10.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–11.
Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Med Clin N Am. 2011;95:1009–30.
Scopinaro N, Gianetta E, Pandolfo N, et al. Bilio-pancreatic bypass. Proposal and preliminary experimental study of a new type of operation for the functional surgical treatment of obesity. Minerva Chir. 1976;31:560–6.
Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267–82.
Umeda LM, Silva EA, Carneiro G, et al. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21:896–901.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–81.
• Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–31. This important study demonstates the changes in glucose metabolism and hepatic insulin resistance in diabetic and non-diabetic matched cohorts after RYGB. This well designed study shows that the improvements in glucose handling and insulin sensitivity occur within days, and raises interesting considerations for the use of bariatric surgery as metabolic surgery in non-diabetic individuals.
Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–42.
Garcia-Marirrodriga I, Amaya-Romero C, Ruiz-Diaz GP, et al. Evolution of lipid profiles after bariatric surgery. Obes Surg. 2012;22:609–16.
Promintzer-Schifferl M, Prager G, Anderwald C, et al. Effects of gastric bypass surgery on insulin resistance and insulin secretion in nondiabetic obese patients. Obesity (Silver Spring). 2011;19:1420–6.
Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23.
Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–14.
Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care. 1991;14:802–23.
Ash S, Reeves MM, Yeo S, et al. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with type II diabetes: a randomised trial. Int J Obes Relat Metab Disord. 2003;27:797–802.
Ramon JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–22.
Heilbronn LK, Noakes M, Clifton PM. The effect of high- and low-glycemic index energy restricted diets on plasma lipid and glucose profiles in type 2 diabetic subjects with varying glycemic control. J Am Coll Nutr. 2002;21:120–7.
Foo J, Krebs J, Hayes MT, Bell D, Macartney-Coxson D, Croft T, et al. Studies in insulin resistance following very low calorie diet and/or gastric bypass surgery. Obes Surg. 2011;21:1914–20.
Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA. 2012;308:1132–41.
Lee WJ, Lee YC, Ser KH, et al. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–25.
Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–71.
Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:E1372–9.
Anderwald CH, Tura A, Promintzer-Schifferl M, et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes Care. 2012 Aug 24. [Epub ahead of print].
Cummings BP, Strader AD, Stanhope KL, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–46, 46 e1.
Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond). 2010;34:462–71.
Yang M, Zhang L, Wang C, et al. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One. 2012;7:e48392.
Li L, Miao Z, Liu R, Yang M, Liu H, Yang G. Liraglutide prevents hypoadiponectinemia-induced insulin resistance and alterations of gene expression involved in glucose and lipid metabolism. Mol Med. 2011;17:1168–78.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42.
• Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85. This article is of major importance as it outlines the clinical effects of bariatric surgery in diabetic cohorts, and places bariatric surgery among the treatment options for glycaemic control in a selected population.
• Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. Similarly, this study confirms the superiority of bariatric surgery over intenstive medical therapy in obesity associated type 2 diabetes mellitus.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740–8.
Asztalos BF, Swarbrick MM, Schaefer EJ, et al. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J Lipid Res. 2010;51:2405–12.
Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–16.
Benedetti G, Mingrone G, Marcoccia S, et al. Body composition and energy expenditure after weight loss following bariatric surgery. J Am Coll Nutr. 2000;19:270–4.
Kopp HP, Krzyzanowska K, Mohlig M, et al. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond). 2005;29:766–71.
Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8.
Woelnerhanssen B, Peterli R, Steinert RE, et al. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy—a prospective randomized trial. Surg Obes Relat Dis. 2011;7:561–8.
Hirsch FF, Pareja JC, Geloneze SR, Chaim E, et al. Comparison of metabolic effects of surgical-induced massive weight loss in patients with long-term remission versus non-remission of type 2 diabetes. Obes Surg. 2012;22:910–7.
Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, et al. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:846–52.
Sancho V, Trigo MV, Martin-Duce A, et al. Effect of GLP-1 on D-glucose transport, lipolysis and lipogenesis in adipocytes of obese subjects. Int J Mol Med. 2006;17:1133–7.
Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–12.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5.
Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond). 2009;33:786–95.
Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4.
• Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–9. This paper introduces the role of bile acids in weight change and improvements in glucose homeostasis, and is an area that is increasingly recognised as playing an important part in the positive metabolic changes after surgery.
Ruttimann EB, Arnold M, Hillebrand JJ, et al. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–81.
Hayes MR, Kanoski SE, De Jonghe BC, et al. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1479–85.
Furnes MW, Tommeras K, Arum CJ, et al. Gastric bypass surgery causes body weight loss without reducing food intake in rats. Obes Surg. 2008;18:415–22.
Liu X, Lagoy A, Discenza I, et al. Metabolic and neuroendocrine responses to Roux-en-Y gastric bypass. I: energy balance, metabolic changes, and fat loss. J Clin Endocrinol Metab. 2012;97:E1440–50.
Bueter M, Lowenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–53.
Olbers T, Bjorkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–22.
Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg. 2006;16:1602–8.
Flancbaum L, Choban PS, Bradley LR, Burge JC. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery. 1997;122:943–9.
Pannacciulli N, Bunt JC, Koska J, et al. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am J Clin Nutr. 2006;84:556–60.
Shalev A, Holst JJ, Keller U. Effects of glucagon-like peptide 1 (7-36 amide) on whole-body protein metabolism in healthy man. Eur J Clin Investig. 1997;27:10–6.
• Lee YS, Park MS, Choung JS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55:2456–68. In this study, the potential immunological effects of GLP-1 are explored, with interesting results in terms on inflammatory regulation and macrophage immunophenotype, albeit in a transfected mouse model (i.e. not a moue model given exogenous GLP-1).
Wynne K, Park AJ, Small CJ, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond). 2006;30:1729–36.
Flint A, Raben A, Rehfeld JF, et al. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obes Relat Metab Disord. 2000;24:288–98.
Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res. 2012;61:789–807.
Illan-Gomez F, Gonzalvez-Ortega M, Orea-Soler I, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–5.
Catalan V, Gomez-Ambrosi J, Ramirez B, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–74.
Pardina E, Ferrer R, Baena-Fustegueras JA, et al. Only C-reactive protein, but not TNF-alpha or IL6, reflects the improvement in inflammation after bariatric surgery. Obes Surg. 2012;22:131–9.
Miller GD, Nicklas BJ, Fernandez A. Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2011;7:618–24.
Zhang H, Wang Y, Zhang J, et al. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31:2063–9.
Dalmas E, Rouault C, Abdennour M, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. 2011;94:450–8.
Schuster DP, Teodorescu M, Mikami D, et al. Effect of bariatric surgery on normal and abnormal renal function. Surg Obes Relat Dis. 2011;7:459–64.
Huan Y, Tomaszewski JE, Cohen DL. Resolution of nephrotic syndrome after successful bariatric surgery in patient with biopsy-proven FSGS. Clin Nephrol. 2009;71:69–73.
Navaneethan SD, Yehnert H. Bariatric surgery and progression of chronic kidney disease. Surg Obes Relat Dis. 2009;5:662–5.
Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17:S213–7.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
Miras AD, Chuah LL, Lascaratos G, et al. Bariatric surgery does not exacerbate and may be beneficial for the microvascular complications of type 2 diabetes. Diabetes Care. 2012;35:e81.
Bueter M, Dubb SS, Gill A, et al. Renal cytokines improve early after bariatric surgery. Br J Surg. 2010;97:1838–44.
Chow FY, Nikolic-Paterson DJ, Ma FY, et al. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–80.
Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–701.
Kanamori H, Matsubara T, Mima A, et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360:772–7.
Tarabra E, Giunti S, Barutta F, et al. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes. 2009;58:2109–18.
Darisipudi MN, Kulkarni OP, Sayyed SG, et al. Dual blockade of the homeostatic chemokine CXCL12 and the proinflammatory chemokine CCL2 has additive protective effects on diabetic kidney disease. Am J Pathol. 2011;179:116–24.
Monte SV, Caruana JA, Ghanim H, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. 2012;151:587–93.
• Hogan AE, Tobin AM, Ahern T, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54:2745–54. This elegant paper demonstrates very clearly the direct effect of GLP-1 on T-cells, and thereby inflammation, with direct implications for the clinical outcomes of psoriasis.
Kodera R, Shikata K, Kataoka HU, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965–78.
Chow FY, Nikolic-Paterson DJ, Ozols E, et al. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80.
Cummings BP, Stanhope KL, Graham JL, et al. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes. 2010;59:2653–61.
Blandino-Rosano M, Perez-Arana G, et al. Anti-proliferative effect of pro-inflammatory cytokines in cultured beta cells is associated with extracellular signal-regulated kinase 1/2 pathway inhibition: protective role of glucagon-like peptide -1. J Mol Endocrinol. 2008;41:35–44.
Ahern T, Tobin AM, Corrigan M, et al. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2012 Jun 13. [Epub ahead of print].
Hossler EW, Wood GC, Still CD, et al. The effect of weight loss surgery on the severity of psoriasis. Br J Dermatol. 2012 Aug 9. [Epub ahead of print].
Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–40.
Nguyen NT, Masoomi H, Laugenour K, et al. Predictive factors of mortality in bariatric surgery: data from the Nationwide Inpatient Sample. Surgery. 2011;150(2):347–51.
Disclosure
Conflict of interest: K.J. Neff: none; C.W. le Roux: is employed by the University College Dublin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neff, K.J., le Roux, C.W. Metabolic Effects of Bariatric Surgery: A Focus on Inflammation and Diabetic Kidney Disease. Curr Obes Rep 2, 120–127 (2013). https://doi.org/10.1007/s13679-013-0050-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-013-0050-2