Abstract
Purpose of Review
Existing dietary and lifestyle interventions and recommendations, to improve the risk factors of obesity and type 2 diabetes with the target to mitigate this double global epidemic, have produced inconsistent results due to interpersonal variabilities in response to these conventional approaches, and inaccuracies in dietary assessment methods. Precision nutrition, an emerging strategy, tailors an individual’s key characteristics such as diet, phenotype, genotype, metabolic biomarkers, and gut microbiome for personalized dietary recommendations to optimize dietary response and health. Precision nutrition is suggested to be an alternative and potentially more effective strategy to improve dietary intake and prevention of obesity and chronic diseases. The purpose of this narrative review is to synthesize the current research and examine the state of the science regarding the effect of precision nutrition in improving the risk factors of obesity and type 2 diabetes.
Recent Findings
The results of the research review indicate to a large extent significant evidence supporting the effectiveness of precision nutrition in improving the risk factors of obesity and type 2 diabetes. Deeper insights and further rigorous research into the diet-phenotype-genotype and interactions of other components of precision nutrition may enable this innovative approach to be adapted in health care and public health to the special needs of individuals.
Summary
Precision nutrition provides the strategy to make individualized dietary recommendations by integrating genetic, phenotypic, nutritional, lifestyle, medical, social, and other pertinent characteristics about individuals, as a means to address the challenges of generalized dietary recommendations. The evidence presented in this review shows that precision nutrition markedly improves risk factors of obesity and type 2 diabetes, particularly behavior change.
Similar content being viewed by others
Introduction
Obesity and diabetes have emerged as enormous public health problems not only in the USA but also globally. Diabetes is a significant global challenge to the health and well-being of individuals and societies [1]. With a continued global increase in diabetes, the current prevalence of 537 million adults living with diabetes is projected to rise to 643 million by 2030 [1]. In the USA, an estimated 37.3 million people have diabetes, of which 90–95% of cases, including children, adolescents, and young adults are attributed to type 2 diabetes [2,3,4]. Diabetes data and trends for 2019 available at the Centers for Disease Control and Prevention indicated that diabetes is the sixth leading cause of death, and number one cause of kidney failure and lower limb amputation [3, 4]. Obesity is the strongest risk factor for the development of type 2 diabetes [5,6,7]. Thus, the burden of type 2 diabetes is increasing in parallel to increasing cases of obesity [8]. Clinical data show that of the people diagnosed with type 2 diabetes, about 80–90% are highly likely to be diagnosed as obese [9,10,11,12]. The associated medical expenses of obesity and type 2 diabetes are steep. Obesity costs the US health care system nearly $173 billion a year [13, 14], while the total estimated economic burden of type 2 diabetes was $327 billion in medical costs and lost productivity [15].
Both obesity and type 2 diabetes have related multifactorial etiology, making them highly complex diseases and investment in their effective prevention and management has become necessary to tackle this global epidemic. While obesity and type 2 diabetes have traditionally been studied to be diseases of energy imbalance, other risk factors such as high body weight and fat, dyslipidemia, high blood glucose, and insulin resistance are also involved in the etiology [16,17,18]. Unhealthy diet characterized by foods high in fat, sugars, and calories, but low in plant-based sources, and lack of physical activity are now considered top risk factors for the development and progression of obesity and type 2 diabetes [19]. Thus, improving dietary intake and physical activity is a global priority [20].
Dietary recommendations and public health campaigns for tackling risk factors of obesity and type 2 diabetes have focused on using population averages, have been based on generalized advice, or have been poorly adhered to [21,22,23,24]. Moreover, there have been great challenges with the validity, consistency, and reproducibility of dietary assessments [25]. Because obesity and type 2 diabetes are heterogeneous diseases from the pathophysiological, genetic, and clinical perspectives, and there is dramatic inter-individual variability in response to any therapeutic diet or physical activity regime, there is a need to shift to or complement the population perspective with patient-centric interventions [26,27,28]. These variabilities are attributed to differences in genetics, biomarkers of metabolic pathways, gut microbiome, environmental, physiological, behavioral, social, and economic factors. Given the substantial burden of obesity and its related comorbidities, research and practice efforts should adopt a holistic approach for sustainable solutions in preventing and treating the obesity and type 2 diabetes epidemic [9].
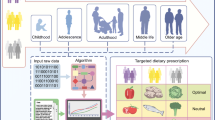
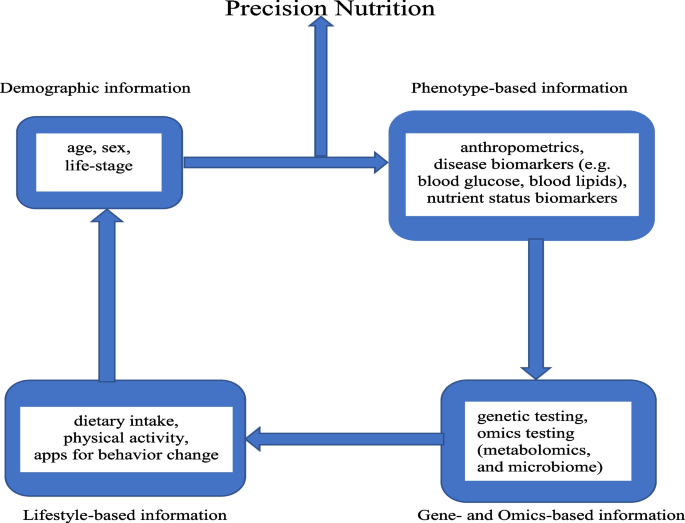
Precision nutrition (or personalized nutrition) has emerged as a new area of lifestyle intervention that allows dietary recommendations to be tailored at the individual level through integration of demographic information, lifestyle-based information (e.g., dietary intake, and physical activity), phenotype-based information (e.g., anthropometrics, and standard clinical biomarkers of disease risk), and gene- and omics-based information (e.g., genetic testing of single nucleotide polymorphisms, and gut microbiome) (Fig. 1) [29, 30]. The current use of nutrigenetics, metabolomics, and metagenomics in precision nutrition enables the holistic interrogation of dietary and lifestyle factors to objectively assess risk factors of obesity and type 2 diabetes. The identification of various genes and polymorphisms has been determined as the basis for the interpersonal variability in metabolic response to specific diets [31,32,33]. Metabolomics investigates, among other things, the effect of food-derived biomarkers metabotypes variation among individuals in metabolizing the same diets in health and disease states for customized dietary interventions through metabolic patterns [34]. The identification of metabolites of food intake to serve as target of nutrition intervention makes metabolomics have potential to improve the accuracy of dietary assessment [35]. Metagenomics is vital in precision nutrition because it can be used to comprehensively analyze the diet-microbiome interaction to identify various metabotypes that characterize metabolic risk and tailor dietary intervention approaches for improved health [36].
Components of the precision nutrition approach. The individual characteristics of demographic, phenotype, lifestyle, genetic, and omics information are incorporated into the precision nutrition intervention to address the interpersonal variabilities in response to general nutrition intervention and recommendations to improve the risk factors of obesity and type 2 diabetes
It is suggested that precision nutrition interventions could result in greater weight loss and blood glucose control than non-personalized strategies [37, 38]. In personalizing nutritional advice, there is evidence that people are more motivated to make appropriate behavioral changes [39, 40]. The interest in precision nutrition has not only significantly increased in the scientific community [41], but is already becoming more accessible to consumers, largely through self-administered test-kits coupled with diet plans and subscription programs [41,42,43]. Thus, precision nutrition has been identified as the individualized solution to prevent and manage obesity and type 2 diabetes in lieu of the population-based dietary interventions, whose effectiveness in reducing the risks of these conditions using the “one-way diet” approach for all individuals is questionable [44].
The purpose of this review is to examine the current state of the science regarding precision nutrition in improving the risk factors of obesity and type 2 diabetes with emphasis on studies that included more than one component of precision nutrition and not only genetic testing to provide individualized/personalized dietary advice. While progress has been made on the quantity of research focused on precision nutrition, reviews discussing particularly behavior change and changes in nutrient/diet quality and physical activity as part of a comprehensive analysis of the utility of precision nutrition intervention and its outcomes are lacking.
Nutrigenetics
Nutrigenetics is considered the foundation of precision nutrition (Table 1) [45, 46]. Genetic variation in the form of single nucleotide polymorphisms (SNPs) is considered to account for the heterogeneity in individual dietary response and risk for obesity and type 2 diabetes [47, 48]. Nutrigenetic research has investigated the interactions between SNPs influencing body composition, insulin signaling, and dietary factors in relation to adiposity and glucose homeostasis in obesity and type 2 diabetes. In an observational study, a genetic risk score-diet interaction used to provide precision nutrition based on 16 SNPs related to obesity or lipid metabolism demonstrated its value in obesity prediction. Specifically, in individuals carrying > 7 risk alleles, there was higher body mass index (BMI), body fat mass, waist circumference, and waist-to-hip ratio more than the individuals with ≤ 7 risk alleles [49]. Additionally, there was a significant interaction between genetic risk score and the macronutrient intake used in personalized intervention. Similarly, a systematic review and meta-analyses and two observational studies reported genetic interactions with specific macronutrients, that is, carbohydrate [50], fat [51], and protein intakes, respectively [52]. SNPs in the apolipoprotein A1 and C3 (APOA1 and APOC3) genes and cluster of differentiation 36 (CD36) gene led to increased risk of metabolic syndrome in subjects with Western dietary pattern and dyslipidemia in individuals who consumed high amounts of fat, respectively. Two randomized controlled trials (RCT) showed that personalized prescription of energy-restricted diets (low-fat and moderately high-protein) based on 95 different genetic variants related to energy homeostasis, phenotypic, and environmental factors was associated with differential adiposity outcomes, with waist circumference and total body fat loss particularly among obese subjects who carried the Peroxisome Proliferator Activator Receptor Gamma Coactivator 1 (PPARGC1A Gly482Gly) genotype [53••, 54]. In an observational prospective cohort design from the RCT, Prevención con Dieta Mediterránea (PREDIMED), the investigators concluded that genetic predisposition to type 2 diabetes associated with the Transcription Factor 7-Like 2 Gene [TCF7L2 gene (rs790314 TT)] homozygosity could be counteracted through precision nutrition interventions with the Mediterranean diet [55]. While precision nutrition effectively addresses the genetic variability in nutrient metabolism, and other physiological processes among individuals, it was found in a parallel-group, pragmatic, RCT that providing nutrigenetic information and advice for management could help reduce body fat percentage up to 6 months, and reductions in body fat were similar to the standard weight loss intervention after 12 months. The clinical implications of this study are that the genetic-based precision nutrition approach should be considered for use for clinical cases which require short- to long-term body fat loss, particularly for individuals needing that to undergo surgery or transplant [56]. The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) RCT was conducted to determine the impact of precision nutrition on fasting glucose, fasting insulin, hemoglobin A1C (HbA1C), insulin resistance, and β cell function. The precision nutrition diet varied in macronutrient composition and was investigated with type 2 diabetes genetic risk scores on these parameters of glucose metabolism. At 2 years of intervention, low-protein diet responses significantly interacted with lower genetic risk score and greater decreases in fasting insulin, HbA1C, insulin resistance, and a lesser increase in β cell function, compared to those with a higher genetic risk score [57]. A post hoc analysis of the POUNDS LOST RCT showed that in response to high-fat diets, participants with the highest genetic risk score showed increased fasting glucose, insulin resistance, and decreased insulin sensitivity at 6-month follow-up than those with low-fat diets [58]. The influence of genetic factors and nutrient-gene interactions in precision nutrition applications has been indicated by twin studies. In the Personalized Responses to Dietary Composition Trial (PREDICT) RCT [59••], a large inter-individual variability in postprandial blood glucose and insulin responses was observed following the same meals among 1002 twins and unrelated healthy adults in the UK. Genetic variants had modest impact on predictions of glucose, triglycerides, and C-peptide. These results were independently validated among 100 US adults. In addition, a machine learning algorithm predicted these variabilities to precision nutrition. An observational retrospective pre/post comparison of digital twin-enabled precision nutrition therapy was used to examine diabetes reversal [60••]. The authors reported diabetes reversal (that is, achieving HbA1C < 6.5% at least 3 months after stopping antidiabetic medications) during 90 days of precision nutrition therapy at varying rates of subgroups of obese and non-obese type 2 diabetes patients. Baseline data showed that only 9.5% of patients were in reversal stage 4 or better; however, over the first 90 days, 82.1% achieved advanced stages of reversal with improved clinical outcomes and fewer pharmacotherapy. Furthermore, a retrospective study reported that there was a decrease in HbA1C, body weight, fasting blood glucose, and insulin resistance at 90-day follow-up assessment [61]. In contrast, a prospective RCT [62] that randomized overweight or obese individuals to receive a nutrigenetic-based precision nutrition diet or standard balanced diet reported no difference in weight loss between the two groups. However, the results highlight the need for larger macronutrient differences between groups and adherence to the recommended intervention diet plan. Further research should be conducted to provide new data and make the use of genetic-based precision nutrition management in the clinical setting more effective [62]. Studies on diet-gene interactions among non-Caucasians are limited. In a prospective cohort study of Hispanics of Caribbean origin who were genotyped for the Perilipin SNP [PLIN 11482G > A (rs894160)] to determine whether dietary macronutrients modulated the associations of the SNP with obesity (measured as BMI, waist and hip circumference), the investigators found that the minor allele was protective against obesity for subjects who consumed higher complex carbohydrate, whereas among those with lower complex carbohydrate intake, the minor allele was linked with increased risk of obesity [63].
Metabolomics
Metabolomics, an emerging technology which encompasses comprehensive analysis of metabolites, holds promise to inform precision nutrition recommendations (Table 1) [64]. The various metabolites produced from metabolism of dietary factors have been used to characterize metabolic phenotypes or biomarkers that can be used for individual stratification. This metabolic specificity enables precision nutrition to resolve metabolic derangements that underlie obesity and type 2 diabetes [34]. Additionally, metabotyping which stratifies individuals with metabolic similarity into metabotype subgroups using their metabolic and phenotype patterns could be used for population stratification to customize dietary interventions [65]. Earlier studies that paved the way for the use of metabolomics in precision nutrition showed that dietary intake patterns were revealed in metabolomic profiles [66], and were associated with biomarkers such as high levels of lipid metabolites, amino acids, and ferritin that mediated red meat consumption and risk of type 2 diabetes [67]. Recently, a study analyzed blood metabolites using metabolomics among normoglycemic healthy adults to predict the risk of developing type 2 diabetes. A web-based platform interventional study was used to deliver precision nutrition intervention based on the blood metabolites health risk score to lower the blood metabolites to normal levels for 40 participants. A follow-up assessment of the blood metabolites showed significant reductions in the health risks associated with the development of type 2 diabetes, insulin resistance, and related comorbidities [68•]. A replication of the study through observational longitudinal analysis in a larger cohort of 1000 US adults demonstrated similar positive results with the precision nutrition intervention given based on biomarkers measured through metabolomics [69]. Bouwman et al. [70] in a double-blind placebo-controlled cross-over design used a health space model to visualize the effect of personalized nutrition intervention on metabolic stress profile including inflammatory and oxidative processes associated with obesity and type 2 diabetes. After following the recommendations for 5 weeks, the 145 metabolites and 79 proteins measured prior and before treatment were able to distinguish modulation of metabolic stress and specific oxidative and inflammatory response to treatment. Fiamoncini et al. [71] in an experimental design identified 2 metabotype clusters and tested their responses to a personalized nutrition intervention over a 12-week weight loss program. The researchers reported that only the study participants with higher disease-linked metabotype demonstrated improvements in glucose and insulin levels when fed a low caloric diet. They concluded that through the application of metabolomics in precision nutrition advice, a responsive and non-responsive metabotype was revealed. In the DIRECT (Dietary Intervention Randomized Controlled Trial) trial, personalized weight-loss diets decreased circulating amino acid metabolites that were associated with risk of type 2 diabetes, and improved insulin resistance. In addition, the reduction in the level of circulating amino acid metabolites which is indicative of an increase in insulin sensitivity was independent of weight loss [72]. Walford and colleagues performed plasma metabolite profiling to elucidate new pathways of type 2 diabetes incidence and the role of personalized nutrition interventions in a nested case–control design [73]. Dietary and lifestyle modifications based on the metabolites effectively raised betaine concentration from baseline to 2-year follow-up, which predicted lower risk of type 2 diabetes. Interestingly, a 10-week RCT that allocated 100 overweight and obese adults to a personalized diet and control diet based on their metabolomic and genetic information did not show significant difference between groups in fat mass; however, the individual diets produced significant improvements in insulin resistance and lipid profile, which was not significantly different between groups. The soundness of various precision nutrition approaches is required to translate such findings into clinical relevance [74•].
Metagenomics
Metagenomics is the comprehensive study of host microbial and their genetic material (Table 1) [75]. The role of the gut microbiota in obesity and type 2 diabetes has been underscored, and this has been an area of immense research [76]. It is believed that the metabolism of dietary compounds into other metabolites by the gut microbiota, which is associated with disease risk, mediates the impact of the gut microbiota on human health [77,78,79]. For example, the metabolism of dietary fibers and resistant starches into bacterial metabolites of short-chain fatty acids such as acetate, propionate, and butyrate presents a mechanism that modulates the pathways involved in obesity, insulin resistance, and type 2 diabetes [80]. Studies show that the diet-gut microbiota interactions vary in composition and functionality among individuals [81], and this appears to be a determinant to integrate metagenomics into precision nutrition [36]. Pioneering work by Zeevi et al. [82] in an observational study and blinded randomized controlled dietary intervention showed that postprandial glucose responses have high interpersonal variability even when individuals consumed identical standardized diets. The authors further used a machine learning algorithm that integrated dietary habits, blood parameters, anthropometrics, physical activity, and gut microbiota features for precision nutrition recommendations in the 800 person cohort. The precision nutrition recommendations accurately predicted personalized postprandial glucose response to the recommendations and resulted in significantly lower glucose levels and consistent alterations in gut microbiome. In modifying and extending the model created by Zeevi and colleagues, two cohort studies that evaluated the utility of such precision nutrition approaches to predict postprandial glucose responses found that across the cohort of non-diabetic adults that were examined, a personalized model was more predictive than current models of carbohydrate content [83, 84]. Similarly, Kovatcheva-Datchary et al. [85] in a cross-over study demonstrated that among 39 healthy Swedes, improved postprandial glucose metabolism was in those with statistically significant higher ratio of Prevotella/Bacteroides spp., following an intervention of 3-day consumption of barley kernel bread diet. Another RCT demonstrated through metagenomic analysis and a dietary weight loss intervention that compared to individuals with a low bacterial ratio, subjects with a high Prevotella/Bacteroides genera ratio lost more weight and body fat in response to high-fiber diets [86]. In a sub-study of a larger RCT, researchers examined whether the baseline composition and diversity of gut microbiota was associated with weight loss in a sample of 49 participants. Findings from the study showed that baseline gut microbiota composition was not associated with weight loss; however, there were substantial changes in gut microbiota in response to each diet, 3 months after initiating the intervention. The changes were attributed specifically to the healthy low-carbohydrate diet used in the intervention, although the changes were attenuated after 12 months [87]. Another important step in the use of metagenomics in precision nutrition was the work conducted by Vangay et al. [88] in an observational study that provided valuable insight into differences in population groups that requires racial considerations and sociocultural influences when employing precision nutrition approaches. In this study, Karen and Hmong natives residing in Thailand and the USA as well as European Americans born in the USA were assessed for the impact of migration to the USA on the gut microbiota in development of metabolic diseases such as obesity. After metagenomic DNA sequencing, the investigators found that US immigration rapidly depleted gut microbiota diversity and function and was replaced by US-associated strains and functions, and was exacerbated by obesity. These results were confirmed in a prospective cohort study that used similar metagenomic approaches of 16S and deep shotgun DNA sequencing among 144 Chinese individuals in Shanghai. A long-term healthy diet intervention was associated with greater diversity of Tenericutes, Firmicutes, and Actinobacteria, with or without adjustment for BMI [89]. Data from an RCT of an integrative model using gut microbiota and genetic information to personalize weight loss prescription among 190 Spanish overweight and obese participants suggested that the mixed models’ microbiota scores facilitated the selection of the optimal diet in 84% of men and 72% of women for weight loss [90••].
Behavioral (Dietary Patterns, and Physical Activity) Aspects of Precision Nutrition
Healthy behaviors (e.g., consuming a healthy diet and engaging in regular physical activity) are associated with the incidence of morbidity and mortality of chronic diseases including obesity and type 2 diabetes [91]. Behavior change components that may be beneficial to improve adoption of healthier options are goal setting, social interactions, and customized messages [92, 93]. Diet and physical activity behaviors are the strongest risk factors for obesity and type 2 diabetes prevention and outcomes [94]. Given this crucial role of behavior in preventing and treating chronic diseases, it is important to assess behavior change in dietary patterns and physical activity for improvement. The 2019 global burden of disease study reported that among the 3 largest increases in risk exposure for disability-adjusted life years (DALYs) lost across the world, 2 were high BMI and high fasting plasma glucose, and 6 of the top 10 causes of DALYs are due to poor health behaviors, including unhealthy dietary patterns and low physical activity levels [95]. Diet quality which represents the nutritional adequacy of a diet with varied nutrient composition, measured by how closely dietary patterns are within core nutrient-dense food groups, is a higher priority than the quantity of dietary intake [96,97,98,99]. In a systematic review of prospective cohort studies, a strong association was found between poor diet quality and greater weight gain, irrespective of gender [100]. In addition, higher diet quality is demonstrated in several studies to be associated with chronic disease risk, cause-specific mortality, and all-cause mortality [101,102,103]. Diet quality in the USA remains far from optimal and for all Americans, the average diet quality measured by the Healthy Eating Index (HEI) score is 58, which is far from the maximum of 100 points [104]. The top dietary risk factors in the USA are diets low in fruits, vegetables, whole grains, nuts, and legumes, and high in refined grains, red or processed meats, sodium, saturated and trans fats, and sugar-sweetened beverages [21, 105,106,107]. The transition from heavy labor to sedentary livelihoods, increased screen time, decrease in school physical education, and improved transportation has been implicated in the decline in physical activity levels [18, 107]. Studies show that moderate to vigorous-intensity physical activity such as walking or running is necessary for optimal health. A systematic review and meta-analysis of prospective cohort studies [108] reported that individuals who engaged in the minimum recommended amount of physical activity had potentially significant benefits to reduce the risk for type 2 diabetes by 26%, compared with inactive individuals. Thus, improvement in diet and physical activity signifies a huge potential for obesity and type 2 diabetes reduction either directly or indirectly through improvements in weight gain and blood glucose levels. It has been suggested that conventional dietary advice does not have as big of an impact on improving dietary health as expected [109, 110].
Precision nutrition interventions have demonstrated encouraging changes in dietary behaviors (Table 1). Precision nutrition studies that reported on behavior changes observed as healthy dietary patterns found that optimizing dietary patterns through individualized care improves management of obesity and type 2 diabetes [111,112,113]. For example, a randomized controlled trial that provided personalized nutrition advice using individualized information on diet and lifestyle, phenotype and/or genotype, produced larger, more appropriate, and sustained changes in dietary behavior to healthier diet as food groups compared to a conventional approach. Study participants in the precision nutrition group consumed less red meat, salt, and saturated fat, increased folate intake, and had higher HEI scores [114]. In line with these results, another RCT [115] that considered application of a dietary pattern technique instead of individual food items in isolation has reported that the use of precision nutrition enhanced dietary behavior changes associated with higher Mediterranean-style diet scores. The Mediterranean diet, characterized by high intakes of fruit and vegetables and low intakes of sugar-sweetened beverages and snacks, has been consistently linked with a beneficial effect on health, including obesity and type 2 diabetes [116,117,118]. Thus, it is strongly suggested that changing dietary intakes so as to align more appropriately with the Mediterranean diet would yield extensive public health benefit [119]. Through post hoc analyses, findings of the study further supported the importance of personalized nutritional advice which, when done with increased frequency, promoted sustained changes in dietary behavior and larger improvements in overall diet quality [120]. The changes in behavior of dietary patterns through the implementation of precision nutrition recommendations have also been associated with reduced intake of calories, carbohydrates, sugar, total fat, and saturated fat which correlated with significant weight loss, reduced waist circumference, and increased high density lipoprotein (HDL), decreased total cholesterol and low density lipoprotein (LDL) with improved glucose levels through observational studies, single-arm, multi-phase, open-label exploratory trial, and retrospective analysis of an RCT [121, 122••, 123, 124]. A pretest–posttest pilot study that organized a personalized dietary advice in a real-life setting found that dietary quality measured by the Dutch Healthy Diet Index was significantly improved compared with baseline. In addition, this research revealed that personalized dietary advice resulted in positive effects in self-perceived health in motivated pre-metabolic syndrome adults. Because the study was performed in the real-life setting (do-it-yourself), it highlighted the potential of at-home health behavior improvement through dietary changes [125]. The EatWellUK is another RCT that attests to the advancement of precision nutrition research beyond the USA. The authors of this research reported that an automated precision nutrition advice via a mobile web app was effective to elicit beneficial dietary change, improve diet quality, and increase engagement in healthy dietary behaviors in UK adults, relative to general population-based dietary guidelines [126••]. Similarly, other precision nutrition interventions found behavior change in dietary intake which favored healthier choices and increase in diet quality irrespective of the setting and/or platform used for delivery of the intervention, as well as measure used to assess diet quality score [127, 128••]. Short-term dietary behavior changes are usually very short lived, thus long-term compliance to dietary behavior change should not be compromised because it is crucial in maintaining body weight and blood glucose levels [129]. Generally, long-term dietary changes are difficult when it comes to consistency; however with the application of precision nutrition, there is a potential to optimize dietary behavior change by motivating greater adherence and change in dietary intake for the long-term for improved weight and glucose management [130,131,132]. The nutrigenomics overweight/obesity and weight management (NOW) trial was an RCT that shed more light on long-term dietary behavior change and adherence. More specifically, the investigators described that the use of precision nutrition increased motivation to long-term reduction in total fat intake, and long-term adherence to total fat and saturated fat advice [133].
Evidence shows that fixed step goals that are not personalized can discourage individuals, leading to unchanged behavior or even reduced physical activity levels [134,135,136]. There are findings, however, that show that the effect of precision nutrition to promote behavior change in physical inactivity and improve physical activity levels is not as consistent as observed for behavior changes in dietary patterns and diet quality. The findings of an RCT that included 1279 participants in 7 European countries to determine the effects of personalized advice on physical activity showed that while self-report-based physical activity levels increased to a greater extent with more personalized nutrition advice, there was no difference between the effect of personalized advice to promote changes in physical activity levels and conventional guidelines when physical activity was objectively measured. The authors concluded that it is vital to measure physical activity objectively in any physical activity intervention study [137]. Studies that analyzed objective measurement of physical activity levels in personalized advice support this theory as they found association between personalized and adaptive goal-setting intervention and steady daily steps, but not with constant steps in the control group, thus promoting behavior change in physical activity [138]. These data are in contrast with the results of an RCT that reported no changes in physical activity behavior after a precision nutrition intervention using objectively measured physical activity [139]. Nevertheless, an observational study found that precision nutrition significantly increased strength exercise frequency which was attributed to direct motivation of their personal genetic testing results to make behavior changes [140]. However, genetic results were not consistently associated with physical activity changes. Together these studies provide important insights into the precision nutrition effects on physical activity behavior changes, which highlights the need for further research.
Conclusion
The current review provides evidence that although the application of precision nutrition is emerging, it is to a large extent associated with obesity and type 2 diabetes and may be effective approach in improving the risks factors including dietary patterns, physical activity, body weight and fat, blood lipids, blood glucose, and insulin resistance. This advancement has been enabled through the use of cutting-edge omics technologies which provide genetic, biomarkers, and microbiome insights into variabilities in individual metabolic pathways in response to dietary intakes that may impact health. It is worth noting as presented in this review that the evidence for precision nutrition is stronger for behavior change than for actual hard endpoints but maintaining the behavior changes in the long term is important for the hard endpoints to change, and this is challenging. The choosing of genetic and phenotypic parameters as a rational basis for individual-level, precision nutrition advice is a key factor that motivates people to make appropriate behavioral changes. However, individual health aspirations, food preferences, and barriers/facilitators to behavior change need to be considered and integrated more using a biopsychosocial model in developing precision nutrition approaches to maintain long-term behavior change and promote sustainability for better health outcomes [141]. In addition, there are still methodological challenges in the design and application of precision nutrition in clinical settings and scale up to the population level in addressing obesity and type 2 diabetes. While sensitivity and specificity issues of the omics technologies exist, some studies do not incorporate all the sources of individual variability in their assessment, and others do not have relevant behavior change techniques, are of short duration in their intervention, low diet quality, and of small sample sizes to observe an effect. More rigorous and well-executed RCTs are required to reinforce the evidence base for precision nutrition to be widely and effectively used in clinical setting and the public health domain. Moreover, increasing the reliability and reducing the cost of cutting-edge omics technologies and new frontiers in machine learning will undoubtedly pave the way for comprehensive and integrated framework of big data to combine multi-omics approaches with lifestyle and behavioral, phenotype, sociocultural, and demographic factors. This will help apprise the optimal design of precision nutrition interventions in clinical settings, and improve population diets at scale in improving the risk factors of obesity and type 2 diabetes. The vast majority of present knowledge and research on precision nutrition has been derived from developed countries [142]. It is crucial to conduct original research in other populations with different dietary habits, disease susceptibility, genetic makeup, socioeconomic characteristics, and health-related lifestyles. Extending precision nutrition research and application by examining and understanding a wider array of multi-race population health, technological and digital landscape, and political will are needed to ensure that there is equity prior to implementation of such approaches.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
International Diabetes Federation. Diabetes around the world in 2021. 2021. https://diabetesatlas.org/. Accessed 5 Apr 2022.
Centers for Disease Control and Prevention. National diabetes statistics report. 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 9 Jul 2022.
Centers for Disease Control and Prevention. Type 2 diabetes. 2021. https://www.cdc.gov/diabetes/basics/type2.html. Accessed 5 Apr 2022.
American Diabetes Association. Statistics about diabetes. 2022. https://www.diabetes.org/about-us/statistics/about-diabetes. Accessed 9 Jul 2022.
Centers for Disease Control and Prevention. Prevalence of overweight and obesity among adults with diagnosed diabetes–United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep. 2004;19:1066–8.
American Diabetes Association. Extra Weight, Extra Risk. 2022. https://diabetes.org/healthy-living/weight-loss/extra-weight-extra-risk. Accessed 5 Aug 2022.
Koren D, Taveras EM. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metab. 2018;84:67–75.
Cameron NA, Petito LC, McCabe M, Allen NB, O'Brien MJ, Carnethon MR, N. et al. Quantifying the sex-race/ethnicity-specific burden of obesity on incident diabetes mellitus in the United States, 2001 to 2016: MESA and NHANES. J Am Heart Assoc. 2021;10:e018799–e018810.
Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118:1723–35.
American Society for Metabolic and Bariatric Surgery. Type 2 diabetes and metabolic surgery. 2018. https://asmbs.org/resources/type-2-diabetes-and-metabolic-surgery-fact-sheet. Accessed 5 Apr 2022.
Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44–58.
Ganz ML, Wintfeld N, Li Q, Alas V, Langer J, Hammer M. The association of body mass index with the risk of type 2 diabetes: a case–control study nested in an electronic health records system in the United States. Diabetol Metab Syndr. 2014;6:50–8.
Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS ONE. 2021;16:e0247307–11.
Cawley J, Biener A, Meyerhoefer C, Ding Y, Zvenyach T, Smolarz BG, et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27:354–66.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–28.
Yoo S. Dynamic energy balance and obesity prevention. J Obes Metab Syndr. 2018;27:203–12.
Gassasse Z, Smith D, Finer S, Gallo V. Association between urbanisation and type 2 diabetes: an ecological study. BMJ Glob Health. 2017;2:e000473–7.
Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;1:3–21.
Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;10:954–64.
Interagency Committee on Human Nutrition Research. National Nutrition Research Roadmap 2016‒2021: advancing nutrition research to improve and sustain health. 2016. Interagency Committee on Human Nutrition Research, Washington (DC). 2019. https://www.nal.usda.gov/sites/default/files/page-files/2016-03-30-%20ICHNR%20NNRR.pdf. Accessed 7 Apr 2022.
U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. 2020. https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. Accessed 20 Apr 2022.
Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–42.
Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832–8.
Banfield EC, Liu Y, Davis JS, Chang S, Frazier-Wood AC. Poor adherence to US dietary guidelines for children and adolescents in the national health and nutrition examination survey population. J Acad Nutr Diet. 2016;116(1):21–7.
Trepanowski JF, Ioannidis JPA. Perspective: limiting dependence on nonrandomized studies and improving randomized trials in human nutrition research: why and how. Adv Nutr. 2018;9:367–77.
Florez JC. Precision medicine in diabetes: is it time? Diabetes Care. 2016;39:1085–8.
Scheen AJ. Precision medicine: the future in diabetes care? Diabetes Res Clin Pract. 2016;117:12–21.
Reddy SS. Evolving to personalized medicine for type 2 diabetes. Endocrinol Metab Clin North Am. 2016;45:1011–20.
Bush CL, Blumberg JB, El-Sohemy A, Minich DM, Ordovás JM, Reed DG, et al. Toward the definition of personalized nutrition: a proposal by the American Nutrition Association. J Am Coll Nutr. 2020;39:5–15.
Ordovas JM, Ferguson LR, Tai ES, Mathers JC. Personalised nutrition and health. BMJ. 2018;361:2173–83.
Rudkowska I, Pérusse L, Bellis C, Blangero J, Després J-P, Bouchard C, et al. Interaction between common genetic variants and total fat intake on low-density lipoprotein peak particle diameter: a genome-wide association study. Lifestyle Genomics. 2015;8:44–53.
Tremblay BL, Cormier H, Rudkowska I, Lemieux S, Couture P, Vohl M-C. Association between polymorphisms in phospholipase A 2 genes and the plasma triglyceride response to an n-3 PUFA supplementation: a clinical trial. Lipids Health Dis. 2015;14:12.
Ferguson LR. Genome-wide association studies and diet. World Rev Nutr Diet. 2010;101:8–14.
Tebani A, Bekri S. Paving the way to precision nutrition through metabolomics. Front Nutr. 2019;6:41–7.
Brouwer-Brolsma EM, Brennan L, Drevon CA, van Kranen H, Manach C, Dragsted LO, et al. Combining traditional dietary assessment methods with novel metabolomics techniques: present efforts by the Food Biomarker Alliance. Proc Nutr Soc. 2017;76(4):619–27.
European Joint Programming Initiative, “A Healthy Diet for a Healthy Life.” JPI-HDHL INTIMIC. 2020. https://www.healthydietforhealthylife.eu/index.php/ec-partnerships/hdhl-intimic. Accessed 5 Aug 2022.
Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. 2020;69: 110549.
Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser M, Rigdon J, Loannidis JPA, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–9.
Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, O'Donovan CB, et al. Food4Me Study. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46(2):578–8.
de Roos B. Personalised nutrition: ready for practice? Proc Nutr Soc. 2013;72:48–52.
Garwood G. U.S.-backed study draws renewed focus on precision nutrition. The Food Institute. 2022. https://foodinstitute.com/focus/u-s-backed-study-draws-renewed-focus-on-precisionnutrition/. Accessed 5 Aug 2022.
Poínhos R, Oliveira BMPM, van der Lans IA, Fischer ARH, Berezowska A, Rankin A, et al. Providing personalised nutrition: consumers’ trust and preferences regarding sources of information, service providers and regulators, and communication channels. Public Health Genom. 2017;20:218–28.
Stewart-Knox BJ, Poínhos R, Fischer ARH, Chaudhrey M, Rankin A, Davison J, et al. Sex and age differences in attitudes and intention to adopt personalised nutrition in a UK sample. Z Gesundh Wiss. 2021;14:1–7.
Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;11:1–10.
Livingstone KM, Brayner B, Celis-Morales C, Ward J, Mathers JC, Bowe SJ. Dietary patterns, genetic risk, and incidence of obesity: application of reduced rank regression in 11,735 adults from the UK Biobank study. Prev Med. 2022;158: 107035.
Marcum JA. Nutrigenetics/nutrigenomics, personalized nutrition, and precision healthcare. Curr Nutr Rep. 2020;9:338–45.
Zhang C, Qi L, Hunter DJ, Meigs JB, Manson JE, Dam RMD, et al. Variant of transcription factor #7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes. 2006;55(9):2645–48.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Goni L, Cuervo M, Milagro FI, Martínez JA. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr. 2015;10:445–51.
Martinez JA, Navas-Carretero S, Saris WH, Astrup A. Personalized weight loss strategies — the role of macronutrient distribution. Nat Rev Endocrinol. 2014;10:749–60.
Celis-Morales CA, Lyall DM, Gray SR, Steell L, Anderson J, Iliodromiti S, et al. Dietary fat and total energy intake modifies the association of genetic profile risk score on obesity: evidence from 48,170 UK Biobank participants. Int J Obes. 2017;41:1761–8.
Merritt DC, Jamnik J, El-Sohemy A. FTO genotype, dietary protein intake, and body weight in a multiethnic population of young adults: a cross-sectional study. Genes Nutr. 2018;13:4–11.
•• Ramos-Lopez O, Cuervo M, Goni L, Milagro FI, Riezu-Boj JI, Martinez JA. Modeling of an integrative prototype based on genetic, phenotypic, and environmental information for personalized prescription of energy-restricted diets in overweight/obese subjects. Am J Clin Nutr. 2020;1:459–70. This article examined an integrative genetic, phenotype, and environmental information for personalized prescription of energy-restricted diets and the effect on BMI in overweight/obese subjects. The use of the model revealed how personalized dietary advice based on genetic, phenotypic, and environmental profile had impact on management of excessive body weight.
Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Cuervo M, Goni L, Martinez JA. Models integrating genetic and lifestyle interactions on two adiposity phenotypes for personalized prescription of energy-restricted diets with different macronutrient distribution. Front Genet. 2019;30:686–93.
Corella D, Coltell O, Sorlí JV, Estruch R, Quiles L, Martínez-González MÁ, et al. Polymorphism of the transcription factor 7-like 2 gene (TCF7L2) interacts with obesity on type-2 diabetes in the PREDIMED study emphasizing the heterogeneity of genetic variants in type-2 diabetes risk prediction: time for obesity-specific genetic risk scores. Nutrients. 2016;8(12):793–8.
Horne JR, Gilliland JA, O’Connor CP, Seabrook JA, Madill J. Change in weight, BMI, and body composition in a population-based intervention versus genetic-based intervention: the NOW trial. Obesity. 2020;28:1419–27.
Huang T, Ley SH, Zheng Y, Wang T, Bray GA, Sacks FM, et al. Genetic susceptibility to diabetes and long-term improvement of insulin resistance and β cell function during weight loss: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Am J Clin Nutr. 2016;104:198–204.
Wang T, Huang T, Zheng Y, Rood J, Bray GA, Sacks FM, et al. Genetic variation of fasting glucose and changes in glycemia in response to 2-year weight-loss diet intervention: the POUNDS LOST trial. Int J Obes (Lond). 2016;40:1164–9.
• Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26:964–73. This large-scale high-resolution study investigated postprandial metabolic responses in identical meal intake using person-specific factors such as gut microbiome and genetics, and machine learning model. Gut microbiome had greater influence for postprandial lipemia, and the model predicted both triglyceride and glycemic response.
•• Shamanna P, Joshi S, Shah L, Dharmalingam M, Saboo B, Mohammed J, et al. Type 2 diabetes reversal with digital twin technology-enabled precision nutrition and staging of reversal: a retrospective cohort study. Clin Diabetes Endocrinol. 2021;7:21–27. This retrospective cohort study assessed type 2 diabetes reversal and measure changes in reversal stages before and after 90 days of digital twin-enabled precision nutrition therapy. It revealed how precision nutrition had effect in reversing high levels of HbA1C, weight, BMI without diabetes medication.
Shamanna P, Saboo B, Damodharan S, Mohammed J, Mohamed M, Poon T, et al. Reducing HbA1c in type 2 diabetes using digital twin technology-enabled precision nutrition: a retrospective analysis. Diabetes Ther. 2020;11:2703–14.
Frankwich KA, Egnatios J, Kenyon ML, Rutledge TR, Liao PS, Gupta S, et al. Differences in weight loss between persons on standard balanced vs nutrigenetic diets in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1625–32.
Smith CE, Tucker KL, Yiannakouris N, Garcia-Bailo B, Mattei J, Lai CQ, et al. Perilipin polymorphism interacts with dietary carbohydrates to modulate anthropometric traits in Hispanics of Caribbean origin. J Nutr. 2008;138(10):1852–8.
O’Donovan CB, Walsh MC, Gibney MJ, Gibney ER, Brennan L. Can metabotyping help deliver the promise of personalised nutrition? Proc Nutr Soc. 2015;75:106–14.
Allam-Ndoul B, Guénard F, Garneau V, Cormier H, Barbier O, Pérusse L, et al. Association between metabolite profiles, metabolic syndrome and obesity status. Nutrients. 2016;8:324–8.
O’Sullivan A, Gibney MJ, Brennan L. Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am J Clin Nutr. 2010;93:314–21.
Wittenbecher C, Mühlenbruch K, Kröger J, Jacobs S, Kuxhaus O, Floegel A, et al. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am J Clin Nutr. 2015;101:1241–50.
• Anwar MA, Barrera-Machuca AA, Calderon N, Wang W, Tausan D, Vayali T, et al. Value-based healthcare delivery through metabolomics-based personalized health platform. Healthc Manage Forum. 2020;33(3):126–34. This article discusses the use of a metabolomics-based personalized lifestyle recommendations in type 2 diabetes, insulin resistance, and associated comorbidities. Results indicated reductions in health risks associated with type 2 diabetes and associated diseases.
Westerman K, Reaver A, Roy C, Ploch M, Sharoni E, Nogal B, et al. Longitudinal analysis of biomarker data from a personalized nutrition platform in healthy subjects. Sci Rep. 2018;8:14685–710.
Bouwman J, Vogels JT, Wopereis S, Rubingh CM, Bijlsma S, Ommen B. Visualization and identification of health space, based on personalized molecular phenotype and treatment response to relevant underlying biological processes. BMC Med Genomics. 2012;5:1–6.
Fiamoncini J, Rundle M, Gibbons H, Thomas EL, Geillinger-Kästle K, Bunzel D, et al. Plasma metabolome analysis identifies distinct human metabotypes in the postprandial state with different susceptibility to weight loss-mediated metabolic improvements. FASEB J. 2018;32(10):5447–58.
Zheng Y, Ceglarek U, Huang T, Li L, Rood J, Ryan DH, et. al.: Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr. 2016;103:505–11.
Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE; Diabetes Prevention Program Research Group. Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes. 2016;65(5):1424–33.
• Aldubayan MA, Pigsborg K, Gormsen SMO, Serra F, Palou M, Galmés S, et al. A double-blinded, randomized, parallel intervention to evaluate biomarker-based nutrition plans for weight loss: the PREVENTOMICS study. Clin Nutr. 2022;41(8):1834–44. This double-blinded randomized intervention examined biomarker-based personalized diet and control group diet on fat mass. There was no change in fat mass, and while the individual diets produced significant improvements in insulin resistance and lipid profile, there was no significant differences between groups. This article is an example of studies that did not show an effect of precision nutrition approach as compared to general healthy diet on weight loss, and warrants further research on the topic.
Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341–55.
Harsch IA, Konturek PC. The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: new insights into “old” diseases. Med Sci (Basel). 2018.17;6(2):32.
Wilson K, Situ C. Systematic review on effects of diet on gut microbiota in relation to metabolic syndromes. J Clin Nutr Metab. 2017;1(2).
Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019. 22;11(12):2862.
Dahl WJ, Mendoza DR, Lambert JM. Diet, nutrients and the microbiome. Prog Mol Biol Transl Sci. 2020;171:237–63.
Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23(3):1105.
Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64.
Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–94.
Mendes-Soares H, Raveh-Sadka T, Azulay S, Edens K, Ben-Shlomo Y, Cohen Y, et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw Open. 2019;2: e188102.
Mendes-Soares H, Raveh-Sadka T, Azulay S, Ben-Shlomo Y, Cohen Y, Ofek T, S, et al. Model of personalized postprandial glycemic response to food developed for an Israeli cohort predicts responses in Midwestern American individuals. Am J Clin Nutr. 2019;1:63–75.
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22(6):971–82.
Hjorth MF, Roager HM, Larsen TM, Poulsen SK, Licht TR, Bahl MI, et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes (Lond). 2018;42(3):580–3.
Fragiadakis GK, Wastyk HC, Robinson JL, Sonnenburg ED, Sonnenburg JL, Gardner CD. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am J Clin Nutr. 2020;111(6):1127–36.
Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, et al. US Immigration Westernizes the Human Gut Microbiome. Cell. 2018;175(4):962–72.
Yu D, Nguyen SM, Yang Y, Xu W, Cai H, Wu J, et al. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am J Clin Nutr. 2021;113(3):684–94.
•• Cuevas-Sierra A, Milagro FI, Guruceaga E, Cuervo M, Goni L, García-Granero M, et al. A weight-loss model based on baseline microbiota and genetic scores for selection of dietary treatments in overweight and obese population. Clin Nutr. 2022;41(8):1712–1723. This article determined the use of an integrative model using gut microbiota and genetic information to personalize weight loss prescription. The use of the model facilitated the selection of diet in 84% of men and 72% of women for weight loss.
Bacon SL, Lavoie K, Ninot G, Czajkowski SM, Freedland KE, Michie S, et al. An international perspective on improving the quality and potential of behavioral clinical trials. Curr Cardiovasc Risk Rep. 2013;9:1–6.
Shilts M, Horowitz M, Townsend M. Goal setting as a strategy for dietary and physical activity behavior change: a review of the literature. Am J Health Promot. 2004;19:81–93.
Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215.
Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, et al. Trends in dietary quality among adults in the united states, 1999 through 2010. JAMA Intern Med. 2014;174:1587–95.
GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;17:1223–49.
Collins CE, Morgan PJ, Jones P, Fletcher K, Martin J, Aguiar EJ, et al. A 12-week commercial web-based weight-loss program for overweight and obese adults: randomized controlled trial comparing basic versus enhanced features. J Med Internet Res. 2012;14:e57–62.
Hutchesson MJ, Collins CE, Morgan PJ, Watson JF, Guest M, Callister R. Changes to dietary intake during a 12-week commercial web-based weight loss program: a randomized controlled trial. Eur J Clin Nutr. 2013;68:64–70.
Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care. 2016;39:833–46.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Collins CE, Young AF, Hodge A. Diet quality is associated with higher nutrient intake and self-rated health in mid-aged women. J Am Coll Nutr. 2008;27:146–57.
Collins CE, Morgan PJ, Jones P, Fletcher K, Martin J, Aguiar EJ, et al. Evaluation of a commercial web-based weight loss and weight loss maintenance program in overweight and obese adults: a randomized controlled trial. BMC Public Health. 2010;10:669–76.
O’Brien KM, Hutchesson MJ, Jensen M, Morgan P, Callister R, Collins CE. Participants in an online weight loss program can improve diet quality during weight loss: a randomized controlled trial. Nutr J. 2014;9:82–6.
Collins CE, Boggess MM, Watson JF, Guest M, Duncanson K, Pezdirc K, et al. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin Nutr. 2014;33:906–14.
United States Department of Agriculture. Food and Nutrition Service. Healthy Eating Index. 2022. https://www.fns.usda.gov/hei-scores-americans. Accessed 6 Aug 2022.
The US, Burden of Disease Collaborators. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–72.
Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007.
World Health Organization. Physical Activity. 2020. https://www.who.int/news-room/fact-sheets/detail/physical-activity#:~:text=Levels%20of%20physical%20activity%20globally&text=Globally%2C%2028%25%20of%20adults%20aged,intensity%20physical%20activity%20per%20week. Accessed 5 Apr 2022.
Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:2527–45.
Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010;6:CD007275-CD007280.
Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285:1172–7.
Celis-Morales C, Lara J, Mathers JC. Personalising nutritional guidance for more effective behaviour change. Proc Nutr Soc. 2015;74:130–218.
Hietaranta-Luoma HL, Tahvonen R, Iso-Touru T, Puolijoki H, Hopia A. An intervention study of individual, apoE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161–74.
Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. PLoS ONE. 2014;9:e112665–21126.
Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, O’Donovan CB, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578–88.
Livingstone KM, Celis-Morales C, Navas-Carretero S, San-Cristobal R, Macready AL, Fallaize R, et al. Effect of an Internet-based, personalized nutrition randomized trial on dietary changes associated with the Mediterranean diet: the Food4Me Study. Am J Clin Nutr. 2016;104:288–97.
Rodríguez-Rejón AI, Castro-Quezada I, Ruano-Rodríguez C, Ruiz-López MD, Sánchez-Villegas A, Toledo E, et al. Effect of a Mediterranean diet intervention on dietary glycemic load and dietary glycemic index: the PREDIMED study. J Nutr Metab. 2014;2014:985373–83.
Pérez-Martínez P, García-Ríos A, Delgado-Lista J, Pérez-Jiménez F, López-Miranda J. Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Curr Pharm Des. 2011;17:769–77.
Beunza JJ, Toledo E, Hu FB, Bes-Rastrollo M, Serrano-Martínez M, Sánchez-Villegas A, et al. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2010;92:1484–93.
Hu EA, Toledo E, Diez-Espino J, Estruch R, Corella D, Salas-Salvado J, et al. Lifestyles and risk factors associated with adherence to the Mediterranean diet: a baseline assessment of the PREDIMED trial. PLoS ONE. 2013;8: e60166.
Celis-Morales C, Livingstone KM, Petermann-Rocha F, Navas-Carretero S, San-Cristobal R, O’Donovan CB, et al. Frequent nutritional feedback, personalized advice, and behavioral changes: findings from the European Food4Me Internet-based RCT. Am J Prev Med. 2019;57:209–19.
Héroux M, Watt M, McGuire KA, Berardi JM. A personalized, multi-platform nutrition, exercise, and lifestyle coaching program: a pilot in women. Internet Interv. 2016;28:16–22.
•• de Hoogh IM, Winters BL, Nieman KM, Bijlsma S, Krone T, van den Broek TJ, et al. A novel personalized systems nutrition program improves dietary patterns, lifestyle behaviors and health-related outcomes: results from the habit study. Nutrients. 2021;22:1763–70. This single-arm exploratory study evaluated the impact of personalized systems nutrition program on lifestyle behavior and health outcomes. Results showed that the personalized nutrition recommendation improves lifestyle habits and reduces body weight, BMI and other health-related outcomes.
Ritz C, Astrup A, Larsen TM, Hjorth MF. Weight loss at your fingertips: personalized nutrition with fasting glucose and insulin using a novel statistical approach. Eur J Clin Nutr. 2019;73:1529–35.
Hjorth MF, Astrup A, Zohar Y, Urban LE, Sayer RD, Patterson BW, et al. Personalized nutrition: pretreatment glucose metabolism determines individual long-term weight loss responsiveness in individuals with obesity on low-carbohydrate versus low-fat diet. Int J Obes (Lond). 2019;43:2037–44.
van der Haar S, Hoevenaars FPM, van den Brink WJ, van den Broek T, Timmer M, Boorsma A, et al. Exploring the potential of personalized dietary advice for health improvement in motivated individuals with premetabolic syndrome: pretest-posttest study. JMIR Form Res. 2021;24: e25043.
•• Zenun Franco R, Fallaize R, Weech M, Hwang F, Lovegrove JA. Effectiveness of web-based personalized nutrition advice for adults using the eNutri web app: evidence from the EatWellUK randomized controlled trial. J Med Internet Res. 2022;25:e29088. This randomized controlled trial investigated the effectiveness of an automated precision nutrition advice for improving diet quality compared to general dietary guidelines. The precision nutrition recommendation was found to improve short-term diet quality and increased healthy eating behaviors.
Marmash D, Ha K, Sakaki JR, Hair R, Morales E, Duffy VB, et al. A feasibility and pilot study of a personalized nutrition intervention in mobile food pantry users in Northeastern Connecticut. Nutrients. 2021;25:2939–45.
•• Rollo ME, Haslam RL, Collins CE. Impact on dietary intake of two levels of technology-assisted personalized nutrition: a randomized trial. nutrients. 2020;29: 3334–3339. This study of a randomized controlled trial compared the impact of low personalized nutrition feedback and high personalized nutrition feedback on dietary intake. The results allowed for differences between the two groups to emerge.
Horne J, Madill J, O’Connor C, Shelley J, Gilliland J. A systematic review of genetic testing and lifestyle behaviour change: are we using high-quality genetic interventions and considering behaviour change theory? Lifestyle Genom. 2018;11:49–63.
Nielsen DE, Shih S, El-Sohemy A. Perceptions of genetic testing for personalized nutrition: a randomized trial of DNA-based dietary advice. J Nutrigenet Nutrigenomics. 2014;7:94–104.
Horne J, Madill J, Gilliland J. Incorporating the ‘Theory of Planned Behavior’ into personalized healthcare behavior change research: a call to action. Per Med. 2017;14:521–9.
Drabsch T, Holzapfel C. A scientific perspective of personalised gene-based dietary recommendations for weight management. Nutrients. 2019;11:617–24.
Horne J, Gilliland J, O’Connor C, Seabrook J, Madill J. Enhanced long-term dietary change and adherence in a nutrigenomics-guided lifestyle intervention compared to a population-based (GLB/DPP) lifestyle intervention for weight management: results from the NOW randomised controlled trial. BMJ Nutr Prev Health. 2020;21:49–59.
Adams MA, Hurley JC, Todd M, Bhuiyan N, Jarrett CL, Tucker WJ, et al. Adaptive goal setting and financial incentives: a 2 × 2 factorial randomized controlled trial to increase adults’ physical activity. BMC Public Health. 2017;29:286–92.
Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA. 2016;20:1161–71.
Joseph R, Keller C, Adams M, Ainsworth B. Print versus a culturally-relevant Facebook and text message delivered intervention to promote physical activity in African American women: a randomized pilot trial. BMC Womens Health. 2015;27:30–6.
Marsaux CF, Celis-Morales C, Fallaize R, Macready AL, Kolossa S, Woolhead C, et al. Effects of a web-based personalized intervention on physical activity in European adults: a randomized controlled trial. J Med Internet Res. 2015;14:e231–7.
Zhou M, Fukuoka Y, Mintz Y, Goldberg K, Kaminsky P, Flowers E, et al. Evaluating machine learning-based automated personalized daily step goals delivered through a mobile phone app: randomized controlled trial. JMIR Mhealth Uhealth. 2018;25:e28–36.
Godino JG, van Sluijs EM, Marteau TM, Sutton S, Sharp SJ, Griffin SJ. Lifestyle advice combined with personalized estimates of genetic or phenotypic risk of type 2 diabetes, and objectively measured physical activity: a randomized controlled trial. PLoS Med. 2016;29:e1002185–91.
Nielsen DE, Carere DA, Wang C, Roberts JS, Green RC, PGen Study Group. Diet and exercise changes following direct-to-consumer personal genomic testing. BMC Med Genomics. 2017;2:24–30.
National Academies of Sciences, Engineering, and Medicine. Challenges and Opportunities for Precision and Personalized Nutrition: Proceedings of a Workshop—in Brief. 1st ed. Washington, DC: The National Academies Press. 2021.
Sight and Life. Precision nutrition for low- and middle-income countries: hype or hope? Special Report. 2022. https://sightandlife.org/10.52439/PRCJ5543.pdf. Accessed 27 Feb 2023.
Acknowledgements
The author would like to thank the Cooperative Agriculture Research Center of the College of Agriculture and Human Sciences at Prairie View A&M University for supporting Open Access publication of this work.
Funding
Open Access publication support was received from the Cooperative Agriculture Research Center of the College of Agriculture and Human Sciences at Prairie View A&M University.
Author information
Authors and Affiliations
Contributions
JA conceptualized the review, conducted literature searches, and wrote the paper. The sole author had responsibility for all parts of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antwi, J. Precision Nutrition to Improve Risk Factors of Obesity and Type 2 Diabetes. Curr Nutr Rep 12, 679–694 (2023). https://doi.org/10.1007/s13668-023-00491-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-023-00491-y