Abstract
The IGROVCDDP cisplatin-resistant ovarian cancer cell line is an unusual model, as it is also cross-resistant to paclitaxel. IGROVCDDP, therefore, models the resistance phenotype of serous ovarian cancer patients who have failed frontline platinum/taxane chemotherapy. IGROVCDDP has also undergone epithelial-mesenchymal transition (EMT). We aim to determine if alterations in EMT-related genes are related to or independent from the drug-resistance phenotypes. EMT gene and protein markers, invasion, motility and morphology were investigated in IGROVCDDP and its parent drug-sensitive cell line IGROV-1. ZEB1 was investigated by qPCR, Western blotting and siRNA knockdown. ZEB1 was also investigated in publicly available ovarian cancer gene-expression datasets. IGROVCDDP cells have decreased protein levels of epithelial marker E-cadherin (6.18-fold, p = 1.58e−04) and higher levels of mesenchymal markers vimentin (2.47-fold, p = 4.43e−03), N-cadherin (4.35-fold, p = 4.76e−03) and ZEB1 (3.43-fold, p = 0.04). IGROVCDDP have a spindle-like morphology consistent with EMT. Knockdown of ZEB1 in IGROVCDDP does not lead to cisplatin sensitivity but shows a reversal of EMT-gene signalling and an increase in cell circularity. High ZEB1 gene expression (HR = 1.31, n = 2051, p = 1.31e−05) is a marker of poor overall survival in high-grade serous ovarian-cancer patients. In contrast, ZEB1 is not predictive of overall survival in high-grade serous ovarian-cancer patients known to be treated with platinum chemotherapy. The increased expression of ZEB1 in IGROVCDDP appears to be independent of the drug-resistance phenotypes. ZEB1 has the potential to be used as biomarker of overall prognosis in ovarian-cancer patients but not of platinum/taxane chemoresistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of ovarian-cancer patients present with advanced disease as ovarian cancer has non-specific symptoms [1]. The 5-year survival of women who present with late-stage disease is 29–35% [2, 3]. Advanced ovarian cancer is treated by debulking surgery followed by 3-weekly platinum/taxane chemotherapy [3, 4]. Recurrence, which is associated with resistance to platinum/taxane chemotherapy, is currently incurable in 75% of ovarian-cancer patients [3].
The IGROVCDDP cisplatin-resistant ovarian-cancer cell line is an unusual model, as it is cross-resistant to paclitaxel [5]. IGROVCDDP, therefore, models the resistance phenotype of ovarian-cancer patients who have failed standard frontline platinum/taxane chemotherapy. The paclitaxel resistance in IGROVCDDP is mediated through overexpression of the ABC transporter P-glycoprotein (ABCB1). The IC50s for paclitaxel in the IGROV-1 parental and IGROVCDDP cells over a 6-day exposure are 0.99 ± 1.13 and 127.92 ± 64.76 µg/mL, respectively, a ~ 129-fold change [5]. The platinum resistance in IGROVCDDP cells is multifactorial and involves changes in drug accumulation, glutathione metabolism and DNA repair. The IC50s for cisplatin in the IGROV-1 and IGROVCDDP cells over a 6-day exposure are 42.0 ± 18.0 and 810 ± 345.01 ng/mL, respectively, a ~ 19-fold change [5].
Epithelial to mesenchymal transition (EMT) normally occurs during embryonic development [6]. In EMT, epithelial cells lose their cell–cell adhesion and acquire mesenchymal features such as motility, invasiveness and increased resistance to apoptosis [6, 7]. Cancer cells undergoing EMT can progress from a non-invasive to an invasive phenotype, there can also be a change in cellular morphology associated with this process [6, 8]. Ovarian cancer cell lines have been previously shown to have undergone EMT in association with the development of drug resistance [6, 7, 9,10,11]. However, the mechanisms of drug resistance are not typically investigated or referred to in detail in the same study. There is also evidence to suggest that EMT occurs in conjunction with clinical ovarian-cancer progression and metastasis [8, 12, 13].
IGROVCDDP has undergone EMT relative to IGROV-1 parental cells. It is the aim of this study to determine if the alterations in EMT-related genes in IGROVCDDP are also involved in the platinum/taxane drug-resistance phenotype. ZEB1 was identified as differentially expressed in IGROVCDDP by microarray, and has been previously associated with EMT in ovarian-cancer cells [11, 14]. It is important to determine if these changes are associated with or independent of the drug-resistance phenotype to choose appropriate biomarkers of chemoresistance for use in the clinic.
Results
IGROVCDDP cells have undergone EMT
IGROV-1 and cisplatin/paclitaxel IGROVCDDP cells were analysed by Affymetrix microarray. The IGROVCDDP cells have mRNA expression changes consistent with EMT relative to parental IGROV-1 cells (Table 1). The gene expression of epithelial marker E-cadherin was decreased and mesenchymal marker N-cadherin was increased (Table 1). IGROVCDDP also has lower protein levels of epithelial marker E-cadherin (6.18-fold, p = 1.58e−04, Fig. 1A) and higher levels of mesenchymal markers N-cadherin (4.35-fold, p = 4.76e-−3, Fig. 1B) and vimentin (2.47-fold, p = 4.43e−03, Fig. 1C) consistent with EMT. Treatment with 200 ng/mL cisplatin did not alter the expression of E-cadherin, N-cadherin or vimentin in the IGROV-1 or IGROVCDDP cell lines (Fig. 1A–C).
The EMT phenotype in IGROVCDDP cells. Western blots of A E-cadherin, B N-cadherin, C vimentin and D ZEB1. IGROV-1 (blue) and IGROVCDDP (red) with and without treatment with 200 ng/mL cisplatin for 72 h (pale blue, pink). Representative images of n = 4 biological repeats are shown. Abundance of protein in arbitrary units was normalised to β-actin for each sample and then each biological series was normalised to IGROV-1 control expression. * Indicates significant difference from IGROV-1 p < 0.05 Student’s t test. No significant difference was observed in IGROVCDDP between the control and the cisplatin-treated cells
The mRNA expression of several transcription factors associated with EMT regulation were also altered in IGROVCDDP cells. The mRNA expression of TWIST1 was significantly decreased, while ZEB1 and SIP1(ZEB2) gene expression were significantly increased (Table 1). The protein expression level of ZEB1 was also significantly increased in IGROVCDDP (3.43-fold, p = 0.04, Fig. 1D). Treatment with a low-dose of cisplatin for 72 h did not significantly alter the protein expression of ZEB1 in the IGROV-1 or IGROVCDDP cells (Fig. 1D).
The change in cell morphology of IGROVCDDP from IGROV-1 is also consistent with EMT (Fig. 2A and B). In parental IGROV-1 cells, the shape of marginal cells was rounded, showing little formation of pseudopodia. In contrast, the morphological changes observed in IGROVCDDP cells include increased loss of cell polarity causing a spindle-shaped morphology in some cells and increased formation of pseudopodia.
Cellular morphology and invasion. Morphology images of A IGROV-1 and B IGROVCDDP. Images were taken of 70% confluent cells with ×100 magnification. Invasion and motility in IGROV-1 (blue) and IGROVCDDP (red) cells at C) 24 h and D) 48 h. Average of n = 6 biological repeats is shown. * Indicates significant difference from IGROV-1 p < 0.05 Student’s t test
The IGROVCDDP cells are more invasive than IGROV-1 at 24 h (2.61-fold, p = 0.008, Fig. 2C). Comparison between invasion and motility shows that for both the IGROV-1 and IGROVCDDP cells there is an increase in motility over invasion suggesting the cells are restricted in invasion by their ability to break down the Matrigel extracellular matrix (Fig. 2C). The motility for IGROVCDDP tended to be higher than IGROV-1 but this was not significant (Fig. 2C). At 48 h the IGROV-1 cells tend to be more invasive than the IGROVCDDP cells, but the data were more variable and the difference was not significant (Fig. 2D).
ZEB1 knockdown in IGROVCDDP alters the gene expression of EMT markers and cellular morphology but not chemoresistance
We investigated the effect of siRNA knockdown of ZEB1 in IGROVCDDP. ZEB1 knockdown was examined by qPCR at 48 h and 5 days post-treatment with siRNAs. Cell viability was good post-siRNA transfection and there was no change in cell growth compared to the scramble control at 48 h or 5 days (Fig. 3A). ZEB1 gene expression tended to be reduced at 48 h post-siRNA transfection (61–84%) but this was not significant (Fig. 3B). ZEB1 gene expression was significantly knocked down at 5 days post-siRNA transfection for all 3 siRNAs (41–52%).
Cell growth and mRNA expression after ZEB1 siRNA knockdown in IGROVCDDP cells. A Cell growth in IGROVCDDP at 48 h and 5 days in response to siRNA knockdown. B ZEB1 gene expression in response to ZEB1 siRNA knockdown at 48 h and 5 days. C E-cadherin; D N-cadherin and vimentin gene expression in response to ZEB1 siRNA knockdown at 5 days. Scramble control (red) with three different ZEB1 siRNAs (shades of green). * Indicates a significant difference from IGROVCDDP scramble control p < 0.05 Student’s t test
The gene expression of epithelial marker E-cadherin and mesenchymal markers vimentin and N-cadherin were also examined in the 5-day ZEB1 siRNA knockdown samples by qPCR. e-Cadherin expression tended to increase in response to knockdown but this was not significant due to high variability (Fig. 3C). There was no change in vimentin in response to knockdown (Fig. 3D). In contrast, the expression of N-cadherin was significantly decreased to a non-detectable level in 2 out of the 3 ZEB1 siRNAs (Fig. 3D).
The protein expression of ZEB1 was decreased to 60–73% of the scramble control at 72-h post transfection (Fig. 4A). The knockdowns with ZEB1-1 and ZEB1-2 siRNA were significant knockdowns (p = 0.04 and 8.0e−03, respectively), ZEB1-3 approached significance (p = 0.08). The protein expression of mesenchymal markers vimentin and N-cadherin were examined in the ZEB1 knockdown samples by Western blot (Fig. 4B and C). There was no change in vimentin. ZEB1-2 tended to increase N-cadherin and a small but significant increase was observed in response to ZEB1-3 (1.21-fold, p = 0.004, Fig. 4C).
Protein expression after ZEB1 siRNA knockdown in IGROVCDDP. A ZEB1, B vimentin and C N-cadherin protein expression in response to ZEB1 siRNA knockdown at 3 days. IGROVCDDP scramble control (red) and three different ZEB1 siRNAs (shades of green). Representative images of n = 4 biological repeats are shown. Abundance of protein in arbitrary units was normalised to β-actin for each sample and then each biological series was normalised to IGROVCDDP scramble control expression. * Indicates significant difference from IGROVCDDP scramble control p < 0.05 Student’s t test
The IGROVCDDP cells treated with the siRNA scramble control retain their spindle-like morphology (Fig. 5A). In contrast, the cells treated with the ZEB1 siRNA have a more epithelial-like morphology with colonies of cells clumped more tightly together suggesting a reversal of the EMT phenotype (Fig. 5B). Morphology images were analysed using Image J [15]. Cell circularity was examined, a figure of 1 indicates a perfect circle. IGROVCDDP cells are less circular than IGROV-1 reflecting their spindle-like morphology (0.59 ± 0.11 vs 0.74 ± 0.11; p = 2.74e−09). The ZEB1 siRNA treatment returns the IGROVCDDP cells to a similar circularity as IGROV-1 (Fig. 5C). IGROVCDDP cells are also larger than IGROV-1 cells, the ZEB1 siRNA knockdown decreases cell size, but not quite to the size of IGROV-1 (Fig. 5D).
Cellular Morphology after ZEB1 siRNA knockdown in IGROVCDDP. Morphology images of A IGROVCDDP Scramble and B IGROVCDDP ZEB1 siRNA knockdown, images were taken at 5-days post transfection with ×100 magnification. Image J analysis of C Cell circularity and D) Cell Area was performed on images from n = 3 biological repeats capturing a minimum of 50 cells per image. IGROV-1 (blue), IGROVCDDP Scramble control (red) with three different ZEB1 siRNAs (shades of green). * Indicates significant difference from IGROV-1 p < 0.05 Student’s t test. # Indicates significant difference from IGROVCDDP scramble control p < 0.05 Student’s t test
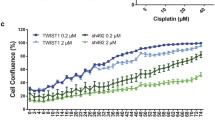
The response of IGROVCDDP to cisplatin (800 ng/mL, 2 µg/mL) or taxol (200 ng/mL, 2 µg/mL) treatment over 5 days was not significantly altered by ZEB1 siRNA knockdown (Fig. 6). Drug treatment induced a significant drop in cell viability but the ZEB1 siRNA treated cells responded in the same way as the scramble controls (Fig. 6).
Cytotoxicity after ZEB1 siRNA knockdown in IGROVCDDP. Response to treatment with 800 ng/mL and 2 µg/mL cisplatin as well as 200 ng/mL and 2 µg/mL taxol in ZEB1 siRNA knockdown cells. IGROVCDDP Scramble control (red) with three different ZEB1 siRNAs (shades of green). * Indicates a significant drop in cell viability compared to IGROVCDDP scramble treatment control p < 0.05 Student’s t test
ZEB1 is predictive of overall survival but not in ovarian-cancer patients treated with platinum.
ZEB1 was analysed in a meta-survival analysis of publicly available gene expression data from high-grade serous ovarian-cancer patients. Median expression of the gene in question was used to dichotomise the data and overall survival was chosen as the survival end point. A hazard ratio of greater than 1 indicates a negative effect on survival and a hazard ratio of less than one has a positive effect. The higher the hazard ratio the greater the effect the gene has on survival. ZEB1 was individually predictive of overall survival in ovarian cancer. (HR = 1.31, n = 2051, p = 1.13e−05, Fig. 6A). In contrast, ZEB1 was not predictive of overall survival when the dataset of ovarian-cancer patients was limited to those confirmed to be treated with platinum chemotherapy ((HR = 1.04, n = 622, p = 0.35, Fig. 6B). Similarly, ZEB1 was not predictive of overall survival in taxane treated patients (HR = 0.94, n = 516, p = 0.66) or in patients treated with both platinum and taxane (HR = 0.94, n = 515, p = 0.67). ZEB1 was also investigated with progression-free survival as an end point and was not found to be individually prognostic in high-grade serous ovarian-cancer patients (Fig. 7).
Prognostic role of ZEB1 gene expression in high-grade serous ovarian cancer. A ZEB1—high expression (blue) is associated with poor overall in ovarian cancer (HR = 1.31, n = 2051, p = 1.13e−05). B ZEB1—high expression (blue) is not prognostic of overall survival in platinum-treated ovarian-cancer patients
Discussion
We have demonstrated that the IGROVCDDP cisplatin/paclitaxel-resistant cell line has undergone EMT relative to parental IGROV-1 cells. The epithelial marker E-cadherin is decreased and mesenchymal markers N-cadherin is increased at both the gene and protein level (Table 1, Fig. 1A, B). Morphological changes (Fig. 2A and B) are also consistent with a shift to a mesenchymal phenotype. IGROVCDDP cells are, therefore, similar to other chemoresistant ovarian-cancer cell lines which have undergone EMT [6, 7, 9, 10]. E-cadherin is not expressed in normal ovarian surface epithelium. Early-stage epithelial ovarian-cancer is associated with a gain of epithelial features including E-cadherin expression; tumour progression is associated with a reacquisition of mesenchymal features [13]. IGROV-1 cells express E-cadherin. The loss of E-cadherin expression in IGROVCDDP and gaining of other mesenchymal markers suggests that IGROVCDDP cells model a progressed form of ovarian cancer, including drug resistance.
In the IGROVCDDP cells there was no change in the mRNA expression of transcription factors associated with EMT Snail and Slug (data not shown) and TWIST1 is decreased (Table 1). This is in contrast to other ovarian-cancer models of chemoresistance which usually show increases in Snail, Slug and TWIST in association with their EMT phenotypes [6, 7, 9, 10]. This suggests that ZEB1 is the primary transcription factor driving EMT in the IGROVCDDP cells.
Interestingly, low-level cisplatin treatment of IGROV-1 did not modulate the expression of EMT markers; E-cadherin expression tended to increase with cisplatin treatment rather than decrease (Fig. 1A), and there was no change in vimentin or N-cadherin expression (Fig. 1B, C) The IGROVCDDP cells were induced into EMT through repeated cisplatin treatment over many months of cell culture [16], they are stably drug resistant and are grown in the absence of cisplatin. The mRNA and protein changes associated with EMT are, therefore, maintained in IGROVCDDP in the absence of the EMT-inducer cisplatin. Short-term low-dose cisplatin (200 ng/mL), similar to that used in the original development of the IGROVCDDP resistant cell line, is not sufficient to induce an EMT-like state in IGROV-1 parental cells. The dose of 200 ng/mL cisplatin is clinically relevant [17, 18] and represents an IC25 in IGROV-1 and IC70 in IGROVCDDP. This dose has also been optimised to show the greatest difference between cell lines while still yielding enough cells in the sensitive IGROV-1 cell line.
ZEB1, platinum and taxane resistance and EMT
ZEB1 is a transcription factor that can regulate the suppression of E-cadherin and induce EMT [19]. The increase in ZEB1 protein expression (Fig. 1D) could, therefore, be one of the factors mediating EMT in IGROVCDDP. Knockdown of ZEB1 by siRNA does not impact chemoresistance or alter the growth rate of cells (Fig. 3A, E). In contrast, ZEB1 siRNA knockdown decreases the gene expression of mesenchymal marker N-cadherin (Fig. 3D) and causes a shift to epithelial-like morphology (Fig. 5). This suggests that ZEB1 is one of the drivers of the EMT phenotype in IGROVCDDP, but that ZEB1 is not involved in the mechanism of cisplatin or paclitaxel resistance in IGROVCDDP (Fig. 3E).
ZEB1 knockdown has been shown to reverse cisplatin resistance in SKOV3/CDDP ovarian carcinoma [20] and SGC7901/DDP gastric carcinoma cells [21]. The mechanisms of cisplatin resistance in SKOV3/CDDP has been linked to the expression of SLC3A2, a type 2 transmembrane cell surface molecule [20]. The mechanisms of cisplatin resistance of SGC7901/DDP include upregulation of the PI3K/Akt pathway and decreased apoptosis [22]. In contrast, the known mechanisms of platinum resistance in IGROVCDDP, include increased expression of glutathione-related genes, copper transporters and BRCA1 [5]. Therefore, ZEB1’s role in cisplatin resistance may be very mechanism specific. It is unclear if SGC7901/DDP or SKOV3/CDDP cells are cross-resistant to paclitaxel like IGROVCDDP cells. Paclitaxel has been studied in SGC7901/DPP cells but its relative resistance to parental SG7901 cells was not reported [23, 24]. Paclitaxel was not examined in the SKOV3/CDDP cells when they were developed [25].
ZEB1 gene knockdown has been shown to sensitise ovarian cell lines ES-2, SVOV3 and NOS3TR to paclitaxel [26]. The ES-2 and SKOV3 cell lines were relatively drug-sensitive models of intrinsic drug resistance in ovarian cancer, whereas the NOS3TR cells are an acquired model of resistance. It is unclear what mechanism of paclitaxel resistance is being altered in all three models [26]. Increased protein expression of ZEB1 was observed in two paclitaxel-resistant ovarian-cancer cell lines OV3R-PTX and SK3R-PTX [11]. ZEB1 was knocked down in these cell lines as part of a large study into the EMT mechanism but response to chemotherapy treatment was not investigated [11]. The known mechanisms of taxane resistance in IGROVCDDP, is increased expression of P-glycoprotein [5]. Therefore, ZEB1’s role in paclitaxel resistance may be very mechanism specific. It is unclear if NOS3TR, OV3R-PTX or SK3R-PTX cells are cross-resistant to cisplatin like IGROVCDDP cells as no studies could be found examining cisplatin resistance in these models.
A study on ZEB1 responsive genes in a panel of 38 lung-cancer cell lines found high levels of correlation with EMT-associated genes [27], but no correlation with genes that contribute to the mechanisms of resistance in IGROVCDDP such as P-glycoprotein, glutathione-related genes, copper transporters and BRCA1 [5]. This suggests that the transcription factor ZEB1 does not have a consistently strong influence on platinum and taxane drug-resistance pathways and is consistent with our findings that ZEB1 knockdown does not alter platinum and taxane cross resistance.
ZEB1 is predictive of overall survival in ovarian cancer, but not in platinum-treated patients.
The IGROV-1 cell line was obtained from a 47-year-old woman who had stage III ovarian cancer [28]. The histological profile was described as with multiple differentiations, primarily endometrioid with some serous clear cells and undifferentiated foci [28]. This histological profile would normally be suggestive of Type I ovarian cancer [29]. However, the BRCA1 and p53 mutations suggests that it is a Type II high-grade serous carcinoma of the SET (Solid, pseudo-endometrioid and transitional cell carcinoma-like morphology) subtype [29,30,31]. Our meta-survival analysis of publicly available ovarian cancer was performed on data from 2051 samples across 12 datasets [32,33,34,35,36,37,38,39,40,41,42,43]. Histological information was available on all of the datasets (Table 2). High-grade serous ovarian cancer was selected for in most studies, if it was not it was the dominant subtype. Therefore, this dataset was an appropriate clinical comparison for the IGROV-1 and IGROVCDDP cells.
High gene expression levels of ZEB1 are predictive of poor overall survival in high-grade serous ovarian-cancer patients (Fig. 4A). This is consistent with other studies which show that high protein levels of ZEB1 are associated with poor progression-free [44] and overall survival in ovarian cancer [26]. The Li et al. study also showed that ZEB1 was highly associated with FIGO stage, the more advanced the metastases the higher the ZEB1 protein expression [44]. In contrast, in this study we found that ZEB1 gene expression is not predictive of survival in high-grade serous ovarian-cancer patients confirmed to be treated with platinum chemotherapy (Fig. 6B). An analysis of chemotherapy treatment was not performed in Li et al.[44] or Sakata et al. [26]. This clinical finding is consistent with our data in IGROVCDDP. ZEB1 may be an important component of maintaining the more aggressive mesenchymal phenotype of IGROVCDDP but not drug resistance.
Ovarian cancer patients whose cancers grow while receiving platinum-based chemotherapy are considered very platinum resistant [45]. Whereas ovarian cancers that recur 6 or more months after platinum treatment are considered platinum sensitive and platinum-based chemotherapy can be given again [46]. There is uncertainly if ovarian cancer recurring within 6 months of platinum treatment is truly platinum resistant or are clinically aggressive independent of platinum resistance. Case studies have shown women who have responded well to platinum retreatment despite relapsing within 6 months of platinum treatment [45]. It is our hope that markers like ZEB1 could be useful in determining which ovarian cancers are likely to respond to platinum retreatment.
Conclusions
ZEB1 may be a useful as marker of progression, invasion and metastasis in ovarian cancer which is associated with poor overall survival. ZEB1 expression may occur concurrently with chemoresistance in ovarian cancer but is not a marker of the platinum/taxane cross resistance phenotype. ZEB1 may be useful as a marker for platinum retreatment in recurrent ovarian cancer.
Materials and methods
Cell culture and cytotoxicity assays
The human IGROV-1 ovarian-cancer cell line and its cisplatin-resistant variant IGROVCDDP were obtained from Prof. Jan Schellens, of the Netherlands Cancer Institute [16, 47]. IGROV-1 and IGROVCDDP cells were grown in antibiotic and chemotherapy-free RPMI (Sigma) with 10% FCS (Lonza). All cell lines were maintained in a humidified atmosphere with 5% CO2 at 37 °C. All cultures were tested routinely and were mycoplasma-free [48]. STR fingerprinting was used to confirm the identity of the cell lines. Cytotoxicity assays were performed as previously described [5]. Cisplatin was obtained from the St. James’ Hospital Pharmacy; Taxol was obtained from Sigma.
Affymetrix arrays
Cells (1.25 × 106 cells/10 cm dish) were plated and allowed to attach and grow for 3 days to reach 70–80% confluence. The cells were then trypsinised, washed in 10 mL PBS, centrifuged and the supernatant removed. The cell pellets were stored at − 80 °C prior to analysis. Total RNA was prepared using the RNeasy Mini Kit (Qiagen, UK). Affymetrix arrays were performed on biological triplicate samples of IGROV-1 and IGROVCDDP cells. A total of 400 ng of total RNA was reverse transcribed, fragmented and biotin labelled following recommended Affymetrix protocols. All samples run on the arrays had an RNA Integrity Number (RIN) > 9.5 (Bioanalyzer, Agilent), indicating that the RNA was of high quality. Samples were prepared according to the manufacturer’s instructions. Quality control metrics were carried out based on the Affymetrix quality control white paper [49]. Single stranded fragmented, biotin labelled DNA was hybridised to GeneChip® Human Gene 1.0 ST Arrays (Affymetrix). Hybridised arrays were scanned on an Affymetrix GeneChip® Scanner 3000 7G (Affymetrix).
Analysis and comparison of Affymetrix array data was performed using Bioconductor software libraries (www.bioconductor.org). The oligo package [50] was used to import data from CEL files and compute RNA expression values [50,51,52]. Differential expression analysis of the RNA expression values was performed using RankProd [53], a non-parametric statistical method for identifying significantly de-regulated genes based on the estimated percentage of false predictions. The RankProd method has been shown to perform well in cases where datasets had low numbers of samples or high levels of noise [54]. De-regulated genes were identified as those with a log-based fold-change in expression value of 1.0 or more, using a significance threshold p value of 0.05, adjusted for multiple testing. Gene annotation was provided by annaffy [55].
Invasion and motility assays
A modified version of the Boyden chamber invasion assay was performed [56]. A cell suspension of 1 × 105 cells/mL was incubated on Matrigel (1 mg/mL) pre-coated inserts at 37 °C for 24 h. The Matrigel and cells that had not migrated were removed. The inserts containing migrated cells, were stained with 0.25% crystal violet for 10 min, rinsed and dried. Invasive potential was assessed by counting the stained cells on the inserts. Motility was measured with a Boyden chamber as above but without the addition of Matrigel.
siRNA knockdown—reverse transfection
ZEB1 siRNAs (Table 3, Applied Biosystems) or a scramble control (Applied Biosystems #4611) were prepared to a final concentration of 30 nM in Opti-MEM Reduced-Serum Media (Life Technologies). The volume of 30 nM siRNA used was appropriate for the size of well (Table 4). An equal volume of Lipofectamine was added to the plate (Table 4) and the plate was gently rocked followed by a 15-min incubation to allow transfection complexes to form. A cell suspension of IGROVCDDP at a density of 2 × 104/mL was then added and plates were incubated at 37 °C with 5% to allow cells to attach overnight. The next day the media was changed an either replaced with drug-free complete RPMI or complete RPMI with cisplatin or taxol. Following 5 days of incubation cells in 96-well plates were analysed by cytotoxicity assay [5]; cells in 6-well plates were trypsinised, washed in 1 mL PBS, transferred to a sterile Eppendorf tube and frozen for RNA extraction or Western Blot Analysis at various time points.
qPCR
Frozen cell pellets from siRNA knockdown were re-suspended in 200 µL PBS and total RNA was extracted using the Roche High Pure RNA Isolation Kit (Roche) according to the manufacturer’s instructions. The purified RNA was then quantified using a Nanodrop 2000 UV–Vis Spectrophotometer (Thermo Scientific). A High Capacity Reverse Transcriptase Kit (Applied Biosystems) was used to convert RNA to cDNA according to the manufacturer’s instructions. 25-100 ng of total RNA was converted to cDNA depending on the yield of RNA in each experiment.
TaqMan™ Gene Expression Assays (Applied Biosystems) were used to assay the gene expression of ZEB1, VIM, CDH1 (E-cadherin) and CDH2 (N-cadherin). CDKNIB was used as the endogenous control. TaqMan™ Gene Expression Mastermix was used for all assays according to the manufacturer’s instructions. A TaqMan™ PreAmp Master Mix Kit was used on the ZEB1 siRNA knockdown cDNA samples to detect CDH1, CDH2 and for consistency CDKNIB. QPCR was performed using the Roche Light Cycler 96 RT-PCR and analysed using Roche LightCycler 96 software. Relative gene expression was determined using the comparative CT method (2−ΔΔCT) [57].
Western blots
Cells (1.25 × 106 cells/10 cm dish) were plated in 10 mL media and allowed to attach overnight. The next day either 2 mL of fresh media or media containing cisplatin was added to give a final concentration of 200 ng/mL. The plates were then incubated for 72 h. The cells were then trypsinised, washed in 10 mL PBS, centrifuged and the supernatant removed. The cell pellets were stored at − 20 °C prior to analysis. Western blots were performed as previously described [5]. Densitometry on a minimum of n = 3 biological replicates was performed using Quantity One software (Biorad), using local-background correction. Abundance of protein in arbitrary units was normalised to β-actin for each sample and then each biological series was normalised to IGROV-1 control, IGROV-1 scramble or IGROVCDDP scramble depending on the experiment. Antibodies used for Western blots are described in Table 5.
Morphology analysis
Images of IGROV-1, IGROVCDDP and ZEB1 siRNA treated IGROVCDDP cells were analysed using Image J [15]. Phase contrast images were captured at 100× magnification and saved as a tif file. A minimum of 50 cells per image were manually outlined and then analysed for cell size and circularity. Biological triplicate experiments were analysed for the ZEB1 siRNA knockdown.
Statistical analysis of cell line data
All experiments in cell lines were performed at minimum in biological triplicate, and statistical analysis was carried out using Minitab. A two-sample t test was used to determine statistical significance with a p value cut-off of < 0.05.
Meta-survival analysis of publicly-available ovarian-cancer datasets
Meta-survival analysis of publicly-available ovarian-cancer datasets was performed as described by [58]. Briefly, gene-expression data sets were downloaded from the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) in the form of raw data files. Ovarian cancer datasets with survival information and at least 50 patients were included. In total, 2051 samples across 12 datasets incorporating 6 different array platforms were used [32,33,34,35,36,37,38,39, 59]. This resulted in gene-expression data for a total of 20,017 Entrez gene IDs across 2051 samples. We combined detailed clinical data with this gene expression data for each of the 12 datasets. Overall survival or progression-free survival from the clinical data were chosen as the survival endpoints. Median expression was used to dichotomise the data, allowing stratification into high and low groups within each of the 12 individual datasets. Once a sample was assigned to a particular group, the 12 datasets were combined and global pooled survival analyses were performed. Survival curves are based on Kaplan–Meier estimates and the log-rank p value is shown for difference in survival. Cox regression analysis was used to calculate hazard ratios. The R package survival was used to calculate and plot the Kaplan–Meier survival curves.
Abbreviations
- EMT:
-
Epithelial to mesenchymal transition
- RPMI:
-
Roswell Park Memorial Institute
- HR:
-
Hazard ratio
- OS:
-
Overall survival
- PBS:
-
Phosphate buffered saline
References
Low EL, Waller J, Menon U, et al. Ovarian cancer symptom awareness and anticipated time to help-seeking for symptoms among UK women. J FamPlannReprod Health Care. 2013;39:163–71.
Office for National Statistics. Cancer survival in England—adults diagnosed. 2022.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet Lond Engl. 2019;393:1240–53. https://doi.org/10.1016/S0140-6736(18)32552-2.
Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–82.
Stordal B, Hamon M, McEneaney V, et al. Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PLoS One. 2012;7:e40717.
Kajiyama H, Shibata K, Terauchi M, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–83.
Ahmed N, Abubaker K, Findlay J, Quinn M. Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer. Curr Cancer Drug Targets. 2010;10:268–78.
Klymenko Y, Kim O, Stack MS. Complex determinants of epithelial: mesenchymal phenotypic plasticity in ovarian cancer. Cancers. 2017;9:104. https://doi.org/10.3390/cancers9080104.
Rosano L, Cianfrocca R, Spinella F, et al. Acquisition of chemoresistance and EMT Phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res. 2011;17:2350–60.
Yue P, Zhang X, Paladino D, et al. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene. 2012;31:2309–22.
Zhang J, Guan W, Xu X, et al. A novel homeostatic loop of sorcin drives paclitaxel-resistance and malignant progression via Smad4/ZEB1/miR-142-5p in human ovarian cancer. Oncogene. 2021;40:4906–18. https://doi.org/10.1038/s41388-021-01891-6.
Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581–8.
Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25:643–55.
Chen D, Wang J, Zhang Y, et al. Effect of down-regulated transcriptional repressor ZEB1 on the epithelial-mesenchymal transition of ovarian cancer cells. Int J Gynecol Cancer. 2013;23:1357–66.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. https://doi.org/10.1038/nmeth.2089.
Ma J, Maliepaard M, Kolker HJ, et al. Abrogated energy-dependent uptake of cisplatin in a cisplatin-resistant subline of the human ovarian cancer cell line IGROV-1. Cancer Chemother Pharmacol. 1998;41:186–92.
Himmelstein KJ, Patton TF, Belt RJ, et al. Clinical kinetics of intact cisplatin and some related species. ClinPharmTher. 1981;29:658–64.
Bielack SS, Erttmann R, Looft G, et al. Platinum disposition after intraarterial and intravenous infusion of cisplatin for osteosarcoma. Cooperative Osteosarcoma Study Group COSS. Cancer Chemother Pharmacol. 1989;24:376–80. https://doi.org/10.1007/BF00257446.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28.
Cui Y, Qin L, Tian D, et al. ZEB1 promotes chemoresistance to cisplatin in ovarian cancer cells by suppressing SLC3A2. Chemotherapy. 2018;63:262–71. https://doi.org/10.1159/000493864.
Wang M, Zhang R, Zhang S, et al. MicroRNA-574-3p regulates epithelial mesenchymal transition and cisplatin resistance via targeting ZEB1 in human gastric carcinoma cells. Gene. 2019;700:110–9. https://doi.org/10.1016/j.gene.2019.03.043.
Deng Z, Wang H, Guo G, et al. Next-generation sequencing analysis of mRNA profile in cisplatin-resistant gastric cancer cell line SGC7901. Med Sci Monit Int Med J Exp Clin Res. 2019;25:2386–96. https://doi.org/10.12659/MSM.915866.
Chen Y, Zuo J, Liu Y, et al. Inhibitory effects of miRNA-200c on chemotherapy-resistance and cell proliferation of gastric cancer SGC7901/DDP cells. Chin J Cancer. 2010;29:1006–11. https://doi.org/10.5732/cjc.010.10236.
Chang L, Guo F, Wang Y, et al. MicroRNA-200c regulates the sensitivity of chemotherapy of gastric cancer SGC7901/DDP cells by directly targeting RhoE. Pathol Oncol Res POR. 2014;20:93–8. https://doi.org/10.1007/s12253-013-9664-7.
Yan X-D, Li M, Yuan Y, et al. Biological comparison of ovarian cancer resistant cell lines to cisplatin and taxol by two different administrations. Oncol Rep. 2007;17:1163–9.
Sakata J, Utsumi F, Suzuki S, et al. Inhibition of ZEB1 leads to inversion of metastatic characteristics and restoration of paclitaxel sensitivity of chronic chemoresistant ovarian carcinoma cells. Oncotarget. 2017;8:99482–94. https://doi.org/10.18632/oncotarget.20107.
Gemmill RM, Roche J, Potiron VA, et al. ZEB1-responsive genes in non-small cell lung cancer. Cancer Lett. 2011;300:66–78.
Benard J, Da Silva J, De Blois MC, et al. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985;45:4970–9.
Kurman RJ, Shih I-M. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186:733–47. https://doi.org/10.1016/j.ajpath.2015.11.011.
Soslow RA, Han G, Park KJ, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25:625–36. https://doi.org/10.1038/modpathol.2011.183.
Domcke S, Sinha R, Levine DA, et al. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. https://doi.org/10.1038/ncomms3126.
Spentzos D, Levine DA, Kolia S, et al. Unique gene expression profile based on pathologic response in epithelial ovarian cancer. J Clin Oncol. 2005;23:7911–8.
Bonome T, Levine DA, Shih J, et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008;68:5478–86.
Crijns APG, Fehrmann RSN, de Jong S, et al. Survival-related profile, pathways, and transcription factors in ovarian cancer. PLoS Med. 2009;6:e1000024.
Denkert C, Budczies J, Darb-Esfahani S, et al. A prognostic gene expression index in ovarian cancer—validation across different independent data sets. J Pathol. 2009;218:273–80.
Mok SC, Bonome T, Vathipadiekal V, et al. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: microfibril-associated glycoprotein 2. Cancer Cell. 2009;16:521–32.
Yoshihara K, Tajima A, Yahata T, et al. Gene expression profile for predicting survival in advanced-stage serous ovarian cancer across two independent datasets. PLoS One. 2010;5:e9615.
Yoshihara K, Tsunoda T, Shigemizu D, et al. High-risk ovarian cancer based on 126-gene expression signature is uniquely characterized by downregulation of antigen presentation pathway. Clin Cancer Res. 2012;18:1374–85.
Konstantinopoulos PA, Cannistra SA, Fountzilas H, et al. Integrated analysis of multiple microarray datasets identifies a reproducible survival predictor in ovarian cancer. PLoS One. 2011;6:e18202.
Ferriss JS, Kim Y, Duska L, et al. Multi-gene expression predictors of single drug responses to adjuvant chemotherapy in ovarian carcinoma: predicting platinum resistance. PLoS One. 2012;7:e30550.
The Cancer Genome Atlas. 2013.
Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–208.
Konstantinopoulos PA, Spentzos D, Karlan BY, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28:3555–61.
Li X, Huang R, Li RH, et al. Expression of zinc finger E-box-binding homeobox factor 1 in epithelial ovarian cancer: a clinicopathological analysis of 238 patients. Mol Clin Oncol. 2016;4:18–22. https://doi.org/10.3892/mco.2015.662.
Markman M, Kennedy A, Webster K, et al. Evidence that a “treatment-free interval of less than 6 months” does not equate with clinically defined platinum resistance in ovarian cancer or primary peritoneal carcinoma. J Cancer Res Clin Oncol. 1998;124:326–8.
Poveda A, Marth C. Platinum or nonplatinum in recurrent ovarian cancer: that is the question. Future Oncol Lond Engl. 2017;13:11–6. https://doi.org/10.2217/fon-2017-0317.
Ma J, Maliepaard M, Nooter K, et al. Synergistic cytotoxicity of cisplatin and topotecan or SN-38 in a panel of eight solid-tumor cell lines in vitro. Cancer Chemother Pharmacol. 1998;41:307–16.
Young L, Sung J, Stacey G, Masters JR. Detection of Mycoplasma in cell cultures. Nat Protoc. 2010;5:929–34.
Affymetrix. Quality assessment of exon and gene arrays. Affymetrix genechip gene and exon array white paper collection, 2018.
Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–7.
Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93.
Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64.
Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92.
Jeffery IB, Higgins DG, Culhane AC. Comparison and evaluation of methods for generating differentially expressed gene lists from microarray data. BMC Bioinform. 2006;7:359.
Smith CA. annaffy: Annotation tools for Affymetrix biological metadata. R package version 1.30.0. 2010.
Albini A. Tumor and endothelial cell invasion of basement membranes. The matrigel chemoinvasion assay as a tool for dissecting molecular mechanisms. Pathol Oncol Res. 1998;4:230–41.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. NatProtocols. 2008;3:1101–8.
Madden SF, Clarke C, Stordal B, et al. OvMark: a user-friendly system for the identification of prognostic biomarkers in publically available ovarian cancer gene expression datasets. Mol Cancer. 2014;13:241.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Acknowledgements
This research was funded by a Marie Curie International Incoming Fellowship and a Career Integration Grant from the European Union FP7 programme, and an Irish Cancer Society Postdoctoral Fellowship (BS). This research was also funded by Middlesex University.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments—BS, JK, CS, SM. Performed the experiments—SR, BS, JK, CS. Analysed the data—SR, BS, JK, CS, SM, GB. Contributed reagents/materials/analysis tools—BS, JK, CS, SM, GB. Wrote the paper—BS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rae, S., Spillane, C., Blackshields, G. et al. The EMT-activator ZEB1 is unrelated to platinum drug resistance in ovarian cancer but is predictive of survival. Human Cell 35, 1547–1559 (2022). https://doi.org/10.1007/s13577-022-00744-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00744-y