Abstract
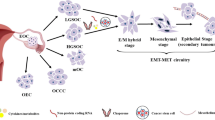
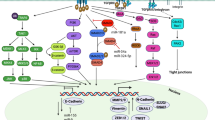
The mesodermally derived normal ovarian surface epithelium (OSE) displays both epithelial and mesenchymal characteristics and exhibits remarkable phenotypic plasticity during post-ovulatory repair. The majority of epithelial ovarian carcinomas (EOC) are derived from the OSE and represent the most lethal of all gynecological malignancies, as most patients (∼70%) present at diagnosis with disseminated intra-abdominal metastasis. The predominant pattern of EOC metastasis involves pelvic dissemination rather than lymphatic or hematologic spread, distinguishing EOC from other solid tumors. Acquisition of the metastatic phenotype involves a complex series of interrelated cellular events leading to dissociation (shedding) and dispersal of malignant cells. A key event in this process is disruption of cell–cell contacts via modulation of intercellular junctional components. In contrast to most carcinomas that downregulate E-cadherin expression during tumor progression, a unique feature of primary well-differentiated ovarian cancers is a gain of epithelial features, characterized by an increase in expression of E-cadherin. Subsequent reacquisition of mesenchymal features is observed in more advanced tumors with concomitant loss of E-cadherin expression and/or function during progression to metastasis. The functional consequences of this remarkable phenotypic plasticity are not fully understood, but may play a role in modulation of cell survival in suspension (ascites), chemoresistance, and intraperitoneal anchoring of metastatic lesions.






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics. Clin 57(1):43–66 2007 Jan-Feb
Wheeler JE (1993) Pathology of malignant ovarian epithelial tumors and miscellaneous and rare ovarian and paraovarian neoplasms. In: Rubin SC, Sutton GP (eds) Ovarian Cance, McGraw Hill, pp 87–130
Cannistra SA (2004) Cancer of the ovary. N Engl J Med 351:2519–2529
DuBeau L (1999) The cell or origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gyn Oncol 72:437–442
Shedden KA, Kshisagar MP, Schwartz DR, Wu R, Yu H, Misek DE, Hanash S, Katabuchi H, Ellenson LH, Fearon ER, Cho KC (2005) Histologic type, organ or origin and wnt pathway status: effect on gene expression in ovarian and uterine carcinomas. Clin Can Res 11:2123–2131
Shih IM, Kurman RJ (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Path 164:1511–1518
Schwartz DR, Kardia SLR, Shedden KA, Kuick R, Michailidis G, Taylor JMG, Misek DE, Wu R, Zhai Y, Darrah DM, Reed H, Ellenson LH, Giordano TJ, Rearon ER, Hanash SM, Cho KR (2002) Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Can Res 62:4722–4729
Scully RE, Young RH, Clement PB (1998) Tumors of the ovary, maldeveloped gonads, fallopian tube and broad ligament. Atlas of tumor pathology, third series, Fascicle 23
Ghosh S, Wu Y, Stack MS (2002) Ovarian cancer proteinases. Cancer Treat Res 107:331–351
Skubitz APN (2002) Adhesion molecules. Cancer Treat Res 107:305–329
Hoskins WJ (1995) Prospective on ovarian cancer: why prevent? J Cell Biochem suppl 23:189–199
Naora H, Montell DJ (2005) Ovarian cancer metastasis: integrating insights from disparate model organisms. Nature Rev Cancer 5:355–366
Wong AST, Auersperg N (2002) Normal Ovarian Surface Epithelium. Cancer Treat Res 107:161–183
Czernobilsky B, Moll R, Levy M, Franke WW (1985) Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J of Cell Biology 37:175–190
Auersperg N, Ota T, Mitchell GW (2002) Early events in ovarian epithelial carcinogenesis: progress and problems in experimental approaches. Int J Gynecol Cancer 12(6):691–703
Sundfeldt K, Piontkewitz Y, Ivarsson K, Nilsson O, Hellberg P, Brannstrom M, Janson PO, Enerback S, Hedin L (1997) E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int J Can 74:275–280
Okamura H, Katabuchi H, Nitta M, Ohtake H (2006) Structural changes and cell properties of human ovarian surface epithelium in ovarian pathophysiology. Microsc Res Tech 69:469–481
Peralta-Soler A, Knudsen KA, Tecson-Miguel A, McBrearty FX, Han AC, Salazar H (1997) Expression of E-cadherin and N-cadherin in surface epithelial stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Human Path 28:734–739
Wong AST, Maines-Bandiera SL, Rosen B, Wheelock MJ, Johnson KR, Leung PCK, Roskelley CD, Auersperg N (1999) Constitutive and conditional cadherin expression in cultured human ovarian surface epithelium: influence of family history of ovarian cancer. Int J Can 81:180–188
Patel IS, Madan P, Getsios S, Bertrand MA, MacCalman CD (2003) Cadherin switching in ovarian cancer progression. Int J Can 106:172–177
Darai E, Scoazec JY, Walker-Combrouze F, Mlika-Cabanne N, Feldmann G, Madelenat P, Potet F (1997) Expression of cadherins in benign, borderline and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Path 28:922–928
Alper O, De Santis ML, Stromberg K, Hacker NF, Cho-Chung YS, Salomon DS (2000) Anti-sense suppression of epidermal growth factor receptor expression alters cellular proliferation, cell-adhesion and tumorigenicity in ovarian cancer cells. Int J Cancer 88(4):566–574
Alper O, Bergmann-Leitner ES, Bennett TA, Hacker NF, Stromberg K, Stetler-Stevenson WG (2001) Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J Natl Cancer Inst 93:1375–1384
Auersperg N, Maines-Bandiera Sl, Dyck HG, Kruk PA (1994) Characterization of cultured human ovarian surface epithelial cells: phenotypic plasticity and premalignant changes. Lab Invest 71:510–518
Naora H (2007) The heterogeneity of epithelial ovarian cancers: reconciling old and new paradigms. Expert Rev Mol Med 9:1–12
Hay ED (2005) EMT concept and examples from the vertebrate embryo In: Savagner P (ed) Rise and fall of epithelial phenotype: concepts of epithelial–mesenchymal transition. Springer, Berlin, pp 111–134
Peinado H, Portillo F, Cano A (2004) Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol 48:365–375
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558
Van Marck VL, Bracke ME (2005) Epithelial–mesenchymal transitions in human cancer. In: Savagner P (ed) Rise and fall of epithelial phenotype: concepts of epithelial–mesenchymal transition. Springer, Berlin, pp 111–134
Guarino M, Rubino B, Ballabio G (2007) The role of epithelial–mesenchymal transition in cancer pathology. Pathology 39:305–318
Tse JC, Kalluri R (2007) Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem 101(4):816–829
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T (2005) Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer 5:744–749
Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66:8319–8326
Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N (2006) Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol 290(6):C1532–C1542
Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P (2006) Snail and slug play distinct roles during breast carcinoma. Clin Cancer Res 12:5395–5402
Arnoux V, Come C, Kusewitt D, Hudson L, Savagner P (2005) Cutaneous Wound Reepithelializaton: A partial and reversible EMT. In: Savagner P (ed) Rise and fall of epithelial phenotype: concepts of epithelial–mesenchymal transition. Springer, Berlin, pp 111–134
Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F, Palacios J, Cano A (2006) Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for snail, slug, and e47 factors in epithelial–mesenchymal transition. Cancer Res 66:9543–9556
Fodde R, Brabletz T (2007) Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19:150–158
Chaffer CL, Thompson EW, Williams ED (2007) Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 185:7–19
McLachlan RW, Yap AS (2007) Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med 85:545–554
Derycke LDM, Bracke ME (2004) N-cadherin in the spotlight of cell–cell adhesion, differentiation, embryogenesis, invasion and signaling. Int J Dev Biol 48:463–476
Conacci-Sorrell M, Zhurnisnsky J, Ben-Ze’ev A (2002) The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest 109:987–991
Arthur WT, Noren NK, Burridge K (2002) Regulation of Rho family GTPases by cell–cell and cell–matrix adhesion. Biol Res 35:239–246
Pece S, Chiariello M, Murga C, Gutkind JS (1999) Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell–cell junctions. J Biol Chem 274:19347–19351
Munshi HG, Ghosh S, Mukhopadhyay S, Wu YI, Sen R, Green KJ, Stack MS (2002) Proteinase suppression by E-cadherin-mediated cell–cell attachment in premalignant oral keratinocytes. J Biol Chem 277:38159–38167
Pece S, Gutkind JS (2000) Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell–cell contact formation. J Biol Chem 275:41227–41233
Wheelock MJ, Johnson KR (2003) Cadherin-mediated cellular signaling. Curr Opin Cell Biol 15:509–14
Li G, Satyamoorthy K, Herlyn M (2001) N-cadherin-mediated inter-cellular interactions promote survival and migration of melanoma cells. Cancer Res 61:3819–3825
Tran NL, Adams DG, Vaillancourt RR, Heimark RL (2002) Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem 277:32905–32914
Wheelock MJ, Johnson KR (2003) Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 19:207–235
Darai E, Bringuier AF, Walker-Combrouze F, Feldmann G, Madelenat P, Scoaze JY (1998) Soluble adhesion molecules in serum and cyst fluid from patients with cystic tumors of the ovary. Hu Reprod 13:2831–2835
Silvertsen S, Berner A, Michael CW, Bedrossian C, Davidson B (2006) Cadherin expression in ovarian carcinoma and malignant mesothelioma cell effusions. Acta Cytol 50:603–607
Roveri Marques F, Fonsechi-Carvasan GA, Andrade LAL, Luiz FB (2004) Immunohistochemical patterns for αand β catenin, E and N cadherin expression in ovarian epithelial tumors. Gyn Onc 94:16–24
Marques RF, Fonsechi-Carvasan GA, De Angelo Andrade LA, Bottcher-Luiz F (2004) Immunohistochemical patterns for alpha- and beta-catenin, E- and N-cadherin expression in ovarian epithelial tumors. Gynecol Oncol 94:16–24
Sarrio D, Moreno-Bueno G, Sanchez-Estevez C, Banon-Rodriguez I, Hernandez-Cortes G, Hardisson D, Palacios J (2006) Expression of cadherins and catenins correlates with distinct histologic types of ovarian carcinomas. Hum Pathol 37:1042–1049
Faleiro-Rodrigues C, Macedo-Pinto I, Pereira D, Lopes CS (2004) Prognostic value of E cadherin immunoexpression in patients with primary ovarian carcinomas. Ann Oncol 15(10):1535–1542
Maines-Bandiera SL, Auersperg N (1997) Increased E-cadherin expression in ovarian surface epithelium: an early step in metaplasia and dysplasia? Int J Gynecol Path 16:250–255
Imai T, Horiuchi A, Shiozawa T, Osada R, Kikuchi N, Ohira S, Oka K, Konishi I (2004) Elevated expression of E-cadherin and alpha-, beta-, and gamma-catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum Pathol 35(12):1469–1467
Davies BR, Worsley SD, Ponder BA (1998) Expression of E cadherin, α-catenin and β-catenin in normal ovarian surface epithelium and epithelial ovarian cancers. Histopath 32:69–80
Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR (2004) E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J 23:1739–1748
ho EY, Choi Y, Chae SW, Sohn JH, Ahn GH (2006) Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int 56:62–70
Voutilainen KA, Anttila MA, Sillanpaa SM, Ropponen KM, Saarikoski SV, Juhola MT, Kosma VM (2006) Prognostic significance of E-cadherin-catenin complex in epithelial ovarian cancer. J Clin Pathol 59:460–467
Veatch AL, Carson LF, Ramakrishnan S (1994) Differential expression of the cell-cell adhesion molecule E cadherin in ascites and solid human ovarian tumor cells. Int J Can 58:393–399
Bryant DM, Stow JL (2004) The ins and outs of E-cadherin trafficking. Trends Cell Biol 14:427–434
D’Souza-Schorey C (2005) Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol 15:19–26
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428
Wu R, Zhai Y, Fearon ER, Cho KR (2001) Diverse mechanisms of beta-catenin deregulation in ovarian endometroid carcinomas. Cancer Res 61(22):8247–8255
Oliva E, Sarrio D, Brachtel EF, Sanchez-Estevez C, Soslow RA, Moreno-Bueno G, Palacios J (2006) High frequency of beta-catenin mutations in borderline endometrioid tumours of the ovary. J Pathol 208:708–713
Rathi A, Virmani AK, Schorge JO, Elias KJ, Maruyama R, Minna JD, Mok SC, Girard L, Fishman DA, Gazdar AF (2002) Methylation profiles of sporadic ovarian tumors and nonmalignant ovaries from high-risk women. Clin Cancer Res 8:3324–3331
Makarla PB, Saboorian MH, Ashfaq R, Toyooka KO, Toyooka S, Minna JD, Gazdar AF, Schorge JO (2005) Promoter hypermethylation profile of ovarian epithelial neoplasms. Clin Cancer Res 11:5365–5369
Yuecheng Y, Hongmei L, Xiaoyan X (2006) Clinical evaluation of E-cadherin expression and its regulation mechanism in epithelial ovarian cancer. Clin Exp Metastasis 23:65–74
Kurrey NK, Amit K, Bapat SA (2005) Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol 97:155–165
Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B (2005) Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103:1631–1643
Elloul S, Silins I, Trope CG, Benshushan A, Davidson B, Reich R (2006) Expression of E-cadherin transcriptional regulators in ovarian carcinoma. Virchows Arch 449:520–528
Terauchi M, Kajiyama H, Yamashita M, Kato M, Tsukamoto H, Umezu T, Hosono S, Yamamoto E, Shibata K, Ino K, Nawa A, Nagasaka T, Kikkawa F (2007) Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. May 9; [Epub ahead of print]
Hosono S, Kajiyama H, Terauchi M, Shibata K, Ino K, Nawa A, Kikkawa F (2007) Expression of twist increases the risk for recurrence and for poor survival in epithelial ovarian carcinoma patients. Br J Cancer 96:314–320
Sundfeldt K, Ivarsson K, Rask K et al (2001) Higher levels of soluble E-cadherin in cyst fluid from malignant ovarian tumors than in benign cysts. Anticancer Res 21:65–70
Wheelock MJ, Buck CA, Bechtol KM, Damsky CH (1983) Soluble 80-kd fragment of cell-CAM120/80 disrupts cell–cell adhesion. J Cell Biochem 34:187–202
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ (1997) Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861–1872
Noe V, Fingleton B, Jacobs K et al (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114:111–118
Maretzky T, Reiss K, Ludwig A et al (2005) ADAM10 mediated E-cadherin shedding and regulates epithelial cell–cell adhesion, migration and b-catenin translocation. Proc Natl Acad Sci USA 102:9182–9187
Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS (2007) Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent e-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res 67:2030–2039
Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED (2006) Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 66:11271–11278
Pon YL, Auersperg N, Wong AS (2005) Gonadotropins regulate N-cadherin-mediated human ovarian surface epithelial cell survival at both post-translational and transcriptional levels through a cyclic AMP/protein kinase A pathway. J Biol Chem 280:15438–15448
Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Skubitz AP (2004) Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gyn Oncol 93:170–181
Burleson KM, Boente MP, Pambuccian SE, Skubitz AP (2006) Disaggregation and invasion of ovarian carcinoma ascites spheroids. J Transl Med 4:6
Wu C, Cipollone J, Maines-Bandiera S, Tan C, Karsan A, Auersperg N, Roskelley CD (2007) The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation. Jul 2; [Epub ahead of print]
Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, Lundin E, Ottander U, Rytinki M, Liu K (2005) Formation of E-cadherin mediated cell–cell adhesion activates AKT and MAPK via PI3kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol 19:2564–2578
Shen X, Kramer RH (2004) Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol 165:1315–1329
Lin Rz, Chou LF, Chien CC, Chang HY (2006) Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res 324:411–422
Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH (2007) E-cadherin cell–cell adhesion in weing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res 67:3094–3105
Shield K, Riley C, Quinn MA, Rice GE, Ackland ML, Ahmed N (2007) alpha2beta1 integrin affects metastatic potential of ovarian carcinoma spheroids by supporting disaggregation and proteolysis. J Carcinog 6:11
Sutherland RM (1988) Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240:177–184
Green SK, Francia G, Isidoro C, Kerbel RS (2004) Anti-adhesive antibodies targeting E-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer Ther 3:149–159
Desoize B, Jardillier J (2000) Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol 36:193–207
Bates RC, Edwards NS, Yates JD (2000) Spheroids and cell survival. Crit Rev Oncol Hematol 36:61–74
Minchinton AI, Tannock IF (2006) Drug penetration in solid tumors. Nat Rev Cancer 6:583–92
Frankel A, Rosen K, Filmus J, Kerbel RS (2001) Induction of anoikis and suppression of human ovarian tumor growth in vivo by down-regulation of Bcl-X(L). Cancer Res 61:4837–4841
Ning Y, Buranda T, Hudson LG (2006) Activated epidermal growth factor receptor induces integrin alpha2 internalization via caveolae/raft-dependent endocytic pathway. J Biol Chem 282:6380–6387
Zeineldin R, Rosenberg M, Ortega D, Buhr C, Chavez MG, Stack MS, Kusewitt DF, Hudson LG (2006) Mesenchymal transformation in epithelial ovarian tumor cells expressing epidermal growth factor receptor variant III. Mol Carcinog 45:851–860
Ning Y, Zeineldin R, Liu Y, Rosenberg M, Stack MS, Ludson LG (2005) down-regulation of integrin alpha2 surface expression by mutant epidermal growth factor receptor (EGFRvIII) induces aberrant cell spreading and focal adhesion formation. Cancer Res 65:9280–9286
Do TV, Symowicz JC, Berman DM, Liotta LA, Petricoin EF, Stack MS, Fishman DA (2007) Lysophosphatidic acid down-regulates stress fibers and up-regulates pro-matrix metalloproteinase-2 activation in ovarian cancer cells. Mol Cancer Res 5:121–131
Cowden-Dahl KD, Zeineldin R, Hudson LG (2007) PEA3 is necessary for optimal epidermal growth factor receptor-stimulated matrix metalloproteinase expression and invasion of ovarian cancer cells. Mol Cancer Res 5:413–421
Fishman DA, Liu Y, Ellerbroek SM, Stack MS (2001) Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res 61:3194–3199
Ellerbroek SM, Hudson LG, Stack MS (1998) Proteinase requirements of epidermal growth factor-induced ovarian cancer cell invasion. Int J Cancer 78:331–337
Ellerbroek SM, Halbleib JM, Benavidez M, Warmka JK, Wattenberg EV, Stack MS, Hudson LG (2001) Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res 61:1855–1861
Fishman DA, Bafetti LM, Banionis S, Kearns AS, Chilukuri K, Stack MS (1997) Production of extracellular matrix-degrading proteinases by primary cultures of human epithelial ovarian carcinoma cells. Cancer 80:1457–1463
Young TN, Rodriguez GC, Rindhart AR, Bast RC Jr, Pizzo SV, Stack MS (1996) Characterization of gelatinases linked to extracellular matrix invasion in ovarian adenocarcinoma: purification of matrix metalloproteinase 2. Gynecol Oncol 62:89–99
Choi JH, Choi KC, Auersperg N, Leung PC (2004) Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J Clin Endocrinol metab 89:5508–16
Acknowledgments
This work was supported by National Cancer Institute Research Grants RO1CA090492 (LGH), CA109545 (MSS), and CA86984 (MSS). The authors wish to gratefully acknowledge the Robert H. Lurie Comprehensive Cancer Center Pathology Core Facility for ovarian tumor tissues and Dr. Brian P. Adley (Lutheran General Hospital, IL) for scoring of the immunohistochemical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hudson, L.G., Zeineldin, R. & Stack, M.S. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis 25, 643–655 (2008). https://doi.org/10.1007/s10585-008-9171-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-008-9171-5