Abstract
The main goal of the current study is to biosynthesize titanium dioxide nanoparticles (TiO2-NPs) using green approach to biocontrol of different fungal strains isolated from historical Description of Egypt book. Nineteen fungal strains were isolated from deteriorated parts and identified by the traditional and molecular methods as Aspergillus flavus (8-isolates), Aspergillus versicolor (2-isolates), Aspergillus ustus (4-isolates), Aspergillus chinensis (2-isolates), Penicillium citrinum (2-isolates), and Penicillium chrysogenum (1-isolate). These fungal strains showed high cellulase, amylase, pectinase, and gelatinase activities which have a significant role in biodeterioration. The biomass filtrate of probiotic strain, Lactobacillus rhamnosus, was used to fabricate TiO2-NPs which characterized by UV-Vis, FT-IR, XRD, TEM, SEM, EDX, DLS, and zeta potential. The obtained data showed the successful formation of spherical and anatase phase NPs with sizes of 3–7 nm and zeta potential values of – 19.9 and – 36.8 mV. The main components of as-formed nanomaterial were Ti and O with weight percentages of 55.91 and 46.25, respectively. The biocompatibility of synthesized TiO2-NPs was investigated toward two normal cell lines, WI38 and HFB4, which reveal the low toxicity at high concentrations (IC50 > 300 μg mL–1). Therefore, concentrations ≤ 300 μg mL–1 were used to biocontrol of isolated fungi. Data showed the promising activity of various concentrations (300, 200, and 100 μg mL–1) of TiO2-NPs to inhibit the growth of fungal strains with varied inhibition zones and dose-dependent manner. This study exhibited the efficacy of probiotic bacterial strains in the synthesis of TiO2-NPs that can be used to preserve historical books from fungal deterioration.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Archeological and historical manuscripts that are deposited in libraries, museums, and archives are priceless and considered national wealth for peoples worldwide. The papers which were used up to nineteenth century were vegetable origin and composed pure cellulose [1]. The cellulosic fibers are consisting of D-glucose monomers linked together by glycosidic linkages to form polymer [2]. Several factors including physicals (such as temperature, light, and relative humidity), chemicals (such as particulates and pollutants), and biologicals (such as microorganisms and insects) are responsible for the degradation of cellulosic fibers and emergence of wide variety of deteriorated aspects such as weakness, erosion, and stains [3, 4].
Fungi is considered one of the main sources for biodeterioration of library contents through deep penetration of cellulosic fibers by fungal hyphae, leading to paper deterioration by the action of hydrolytic enzymes, acid corrosion, and mechanical attack [5, 6].
Different environmental conditions enhanced the fungal growth [7, 8]. For instance, Pinzari et al. [9] reported that the temperature and relative humidity of the materials (paper, glues, textiles, or any other organic materials in the book or manuscript) were the main factors that promote fungal spore germination and growth. The susceptibility of paper to colonize by living organisms is considered high due to their compositions (cellulose and other additives) and hygroscopicity, which is considered rich carbon source for heterotrophic organisms [3]. The growth and development of various fungal strains in museums are dependent on different factors including the indoor climate, the number of available nutrients—from the atmosphere and from the materials themselves, and also the cleaning intervals in the museum [7]. In addition to the factors mentioned earlier, improper conservation practices of historical manuscripts, such as wet cleaning in uncontrolled environments and inadequate drying, can contribute to the growth of fungi. These practices create a favorable environment for fungal growth and can lead to further deterioration of the manuscripts.
Some researchers isolated and identified fungi from historical paper manuscripts and leather bindings. Pasquariello and Maggi [10] mention that the paper is susceptible to fungal deterioration by genera of Trichoderma, Chaetomium, Paecilomyces, Cladosporium, Alternaria, and Fusarium. Moreover, the genera of fungi including Alternaria, Aspergillus, Chaetomium, Mucor, Myrothecium, Penicillium, Rhizopus, Stachybotrys, Trichoderma, Trichothecium, and Ulocladium are the main causes of erosion, spots, pigmentation, and change in the mechanical characteristics of paper [5, 11]. Pinheiro et al. [12] surveyed some studies that deal with the isolation and identification of fungi from libraries and archives of some places worldwide. The authors reported that approximately 207 fungal genera and 580 species were identified. Moreover, the fungal strains of Chaetomium sp. and Fusarium sp. that cause contaminants in the air of archives were related to paper biodeterioration. Also, the authors informed that the most dominant fungal strains were Penicillium, Aspergillus, and Alternaria species, which affected historical papers. Nitiu et al. [13] mentioned that foxing stains are considered one of the most important aspects of the deterioration of historical paper by fungi. Several dark fungi (such as Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, and Alternaria alternata) cause staining of the paper documents through their metabolic activity upon both cellulose and non-cellulosic additives, such as binders, filling, adhesives, and sizing agents [13].
Some studies have looked at the most common enzymes and metabolites produced by various fungal strains. El-Gendi et al. [14] mentioned that the enzymes produced by fungal strains are efficient, compatible, and proper products for various uses in industry, biodegradation, medicine, and agricultural applications. Mohammed et al. [15] identified five fungal isolates of Aspergillus spp. that could produce cellulose degradation enzymes at different efficiencies. The authors reported that the Aspergillus versicolor and Aspergillus terreus were very effective, while Aspergillus fumigatus and Aspergillus flavus were moderately effective. Kosel and Ropret [16] confirmed that fungi can produce pigmentation or degrade organic materials with extracellular enzymes and secreted organic acids. In some cases, they can penetrate the polymeric material and cause weakness. They also mentioned that organic acids, such as oxalic, fumaric, citric, itaconic, succinic, lactic, or acetic acid, are responsible for the degradation of the objects. Due to the importance of the historical book used in the current study, it is urgent to construct a safe, eco-friendly, and cost-effective approach to reduce the biodeterioration caused by different fungal strains.
Recently, researchers have turned to construct safe, eco-friendly, rapid, and cost-effective treatment approaches to save the cultural heritage from microbial damage. By the huge development of nanotechnology science and their effectiveness toward diverse kinds of microbes, the researchers used nanoparticles (NPs), especially those synthesized by green approaches in treatment processes [17]. Nanoparticles are synthesized by chemical, physical, and biological methods. Due to excessive cost, hazardous by-products, extreme condition requirement, and harmful effects on the environment of chemical and physical methods, green synthesis are preferred [18, 19]. The biological synthesis are achieved through harnessing metabolites of different biological entities (bacteria, fungi, algae, and plants) to reducing metal and metal oxides to form NPs followed by capping of the final product [20]. Various NPs such as silver-NPs [21], selenium-NPs [22], and iron oxide-NPs [23] have been synthesized by different biological entities. Among metal oxides, titanium dioxide nanoparticles (TiO2-NPs) are very important nanomaterial due to it is safe, high stable, cost-effective, and biocompatible for integration into various medical and technological applications [24]. It is widely used as an antimicrobial agent, in wastewater treatment, photocatalysis, cosmetics, electronic devices, anticancer, mosquitocidal, antioxidant, and pharmaceuticals [25, 26]. Recently, TiO2-NPs were synthesized by seed aqueous extract of Myristica fragrans [27] and peel extract of watermelon [28] for uses as antimicrobial, anticancer, antioxidant, and wastewater treatment. Moreover, bacterial mediated biosynthesis of TiO2-NPs was integrated into various applications. For instance, TiO2-NPs synthesized by Bacillus mycoides were used to construct a green solar cells [29]. Also, those synthesized by bacterial strain, Staphylococcus aureus were used as antibacterial agent toward various Gram-positive and Gram-negative bacteria and as antibiofilm agent [30].
Lactobacillus spp. is a common bacterial species for milk curdling, non-pathogenic strains, oxygen tolerant, and has high metabolic fluxes; therefore, it is highly beneficial [31]. Due to the huge Lactobacillus metabolites, it is a useful microbe utilized for green synthesis of high-stable nanomaterials as a green approach. Although TiO2-NPs were synthesized by different bacterial strains, but this the first report for synthesis of TiO2-NPs using cell-free filtrate of Lactobacillus rhamnosus as a green method. Also, this is the first report for using L. rhamnosus-TiO2-NPs for controlling the growth of fungal strains associated with deteriorated historical book.
Therefore, the main hypothesis of the current study is investigating the efficacy of TiO2-NPs fabricated by the green method to control the highly deteriorated fungal strains isolated from the historical book. To achieve this hypothesis, the historical book namely Description of Egypt was selected to assess the biodeterioration aspects using various methods such as photo-documentation, environmental scanning electron microscope (ESEM), attenuated total reflection/Fourier transform infrared spectroscopy (ATR/FTIR), and color change. Next, several fungal strains associated with the deteriorated parts of the book were isolated and identified using traditional methods. Further a molecular technique that involved amplification and sequencing of the ITS regions was used. Additionally, we investigated the activity of these isolated fungal strains in terms of secreting different hydrolytic enzymes. Finally, TiO2-NPs were synthesized by harnessing metabolites of Lactobacillus rhamnosus to control highly deteriorated fungal strains.
2 Materials and methods
2.1 Materials
The substrate of enzymes (carboxymethyl cellulose, starch, gelatin, and pectin) was obtained from Sigma-Aldrich, Egypt with purity 95–98%. Titanium tetraisopropoxide (Ti[OCH(CH3)2]4, 98%, MW: 284.22) as a precursor of TiO2-NPs was purchased from Advent Chembio Pvt Ltd., India. The two normal cell lines used for investigating the biocompatibility were purchased from the Holding Company for Biological Products and Vaccines (VACSERA), Cairo, Egypt. All media components were analytical grade, and all reactions were achieved using distilled H2O.
2.2 Methods
2.2.1 Historical book and storage conditions
The Description de l’Égypte (English: Description of Egypt) was selected for the current study because of its important value. This volume was published in 1809 AD on the orders of Emperor Napoleon and contains 23 books and select book 11 for the current study due to the highly deterioration. The current historical book was deposited in The Misr Institute in the Hassan Kashif Palace on the outskirts of Cairo, Egypt, until 1932 AD and transferred after that to The Arabic Language Academy, Cairo, Egypt, once established by the order of King Fouad I.
The storage conditions around the selected book were unfavorable and encouraged microbial attacks. The relative humidity (RH) increased by more than 65%, especially in the winter season, and this is due to that The Arabic Language academy building is located directly on the Nile River, as well as open windows, air vents, or poorly sealed windows and doors during daily work. Poor ventilation in the basement of the building (the place where the book is preserved) was noticed. There is a fluctuation in temperature throughout the year, and in all cases, the temperature exceeds 22 °C, especially in the summer season. The lighting conditions in the area are inadequate, lacking proper control of the electric lighting being used. This can result in excessive lighting that may harm the manuscripts. Furthermore, the accumulation of dirt and dust is evident, primarily due to the absence of filters to absorb these particles and prevent air pollutants from settling on the books. Additionally, the display and storage of the books within the building do not meet international standards for preserving manuscripts and historical books. Improper handling and storage practices can pose risks to the long-term preservation of these valuable materials.
2.2.2 Measurement of deterioration aspects
Deterioration aspect documentation
The damage observed in the historical book was described as shown by the naked eye. Moreover, the portable USB digital microscope (model PZ01-Shenzhen Super Eyes Co. Ltd., China) was used to examine the damage and foxing spots on the surface of the studied samples.
Environmental scanning electron microscope (ESEM)
The surface morphologies of Whatman paper no. 1 (as a control) and historical paper were investigated to identify the fibers used, fungal growth among the fibers, and to observe any changes in the fiber’s morphology. The investigation was performed under ESEM, FEL quanta 3D 200i, and was operated under the conditions of a vacuum for 20 kV and an LF detector.
Attenuated total reflection/Fourier transform infrared spectroscopy (ATR/FTIR)
The molecular structure of Whatman no. 1 (control) and historical paper samples was elucidated in the range 4000 to 400 cm–1 by attenuated total reflection–Fourier transform infrared spectroscopy (ATR-FTIR), Bruker Vertex 70 FTIR spectrometer at The Research Centre, Ministry of Tourism and Antiquities, Cairo, Egypt.
Color change
Color changes of the control historical sample compared to the control (Whatman no. 1) were measured using the CIE*Lab system, commonly used to compare the colors of two samples. The L scale measures lightness and varies from 0 (black) to 100 (perfect white), whereas the a*-scale measures red-green; + a means redder and − a means more green. The b*-scale measures yellow-blue, + b meaning more yellow and − b bluer. Differences in color between two specimens are determined using the Greek letter delta (ΔL, Δa, Δb). The total color difference (ΔE) is calculated according to the following equation:
The measurement was made using a portable spectrophotometer by HunterLab-Reston, VA, USA. The measurement was carried out according to El-Gamal et al. [32].
2.2.3 Fungal isolation and identification
The process of isolating various fungal strains from the historical book involved using sterilized cotton swabs. These swabs were pressed against the deteriorated areas of the paper and then directly transferred into sterilized tubes which were transported to the laboratory for further analysis and investigation. Under aseptic conditions, each swab was added separately to the test tube containing 10 mL of sterilized saline solution supplemented with an antibacterial agent (chloramphenicol, 500 mg L–1) for 24 h at 25 ± 2 °C to enhance or reactivate the fungal spore. After that, 100 μL of the previous saline solution was spread over the Czapeck yeast extract (CYA) agar media (ready prepared, Merck) and potato dextrose agar media (ready prepared, Oxoid) using a sterilized glass spatula before being incubated at 25 ± 2 °C. The inoculated plates were observed daily to observe the fungal growth which picked up and reinoculated over new agar plate for future purification [33, 34].
The obtained fungal isolates were subjected to identification using microscopic and cultural characteristics based on standard keys for Aspergillus spp. [35] and Penicillium spp. [36]. The ITS sequence analysis was used for the molecular identification of fungal strains. Six fungal strains were selected based on traditional identification (one from each strain) to confirm their identification. The extraction of fungal DNA was carried out using Gene Jet Plant genomic DNA purification kit (Thermo) protocol. The PCR amplification of the ITS region was achieved using fungal DNA as a template and primers of ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR mixture of 0.5 μM of each primer (50 μL) contained Maxima Hot Start PCR Master Mix (Thermo) and 1 μL of extracted fungal DNA. The protocol of the PCR method was achieved according to Dorit et al. [37]. The obtained sequences were compared with ITS sequences that were deposited in the GenBank using the NCBI-BLAST program. The neighbor-joining method (MEGA v6.1, www.megasoftware.net) was used to construct the phylogenetic tree with confidence-tested bootstrap analysis with 1000 repeats.
2.2.4 Enzyme activity
The activity of various fungal strains isolated from deteriorated historical papers to secrete different enzymes including cellulase, amylase, gelatinase, and pectinase was investigated using the agar plate method. The fresh disk (0.6 mm in diameter) of each purified fungal strain was inoculated in mineral salt agar (MSA) media (composed of (g L−1) KCl, 5; NaNO3, 6; MgSO4.7H2O, 0.5; KH2PO4, 1.5; ZnSO4, 0.01; FeSO4, 0.01; agar, 15, dis. H2O, 1 L) supplemented with 1% of specific substrate (carboxymethyl cellulose for cellulase enzyme, starch for amylase, gelatin for gelatinase, and pectin for pectinase). The antibacterial agent (chloramphenicol) was added to MSA media to suppress bacterial growth. The inoculated plates were incubated at 25 ± 2 °C for 96 h before calculating the results by subtracting the diameter of fungal growth from the diameter of all inhibition zone [38].
The efficacy of fungal strains in producing extracellular enzymes was detected after flooding the plate with iodine solution to investigate the cellulase, amylase, and pectinase activity, whereas acidic mercuric chloride was used for the detection of gelatinase activity [39].
2.2.5 Biosynthesis of TiO2-NPs
Organism used
Biosynthesis of TiO2-NPs was achieved using biomass filtrate of Lactobacillus rhamnosus ATCC-7469. This strain was purchased from the Microbiological Resources Centre (MIRCEN), Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
Preparation of cell-free filtrate and biosynthesis of TiO2-NPs
A single colony of L. rhamnosus was picked up and inoculated into MRS broth media (ready prepared, Merck, Germany) before being incubated at 35 ± 2 °C for 24 h. After that, the inoculated media were centrifuged at 1000 rpm for 10 min to collect the cells which were rinsed twice with sterilized dis. H2O before resuspending approximately 10 g of cells into 100 mL of dis. H2O. The previous mixture was incubated at 35 ± 2 °C for 24 h. At the end of the incubation period, the mixture was subjected to centrifugation to collect the supernatant (cell-free filtrate) which was used as a biocatalyst for the biosynthesis of TiO2-NPs through mixing with Ti[OCH(CH3)2]4 under stirring condition (for 1 h) to get a final concentration of 5 mM [40]. The pH of the mixture was adjusted at 8 using 1 N NaOH which was added drop by drop. The color change from pale yellow to white precipitate indicates the successful formation of TiO2-NPs. After 24 h of incubation, the white precipitate was collected, washed thrice with high pure H2O (Milli-Q), and subjected to oven dry at 200 °C for 3 h.
Characterization
The first monitor for the formation of TiO2-NPs is the color change of cell-free filtrate after mixing with metal precursor from pale yellow to white. After that, the absorbance of the former white color was detected by measuring their absorbance at the wavelength of 200–800 nm using UV-visible spectroscopy (JENWAY 6305, Staffordshire, UK). In this method, the quartz cuvette was filled with 2 mL of the synthesized solution followed by measuring their absorbance at regular-interval wavelength to detect the maximum surface plasmon resonance (SPR) [41].
The functional groups in bacterial cell-free filtrate compared to those in TiO2-NPs were analyzed using Fourier transform infrared (FT-IR, Cary-660 model). In this method, 10 mg of synthesized TiO2-NPs or 5 mL of cell-free filtrate was mixed with KBr, mixed well, and subjected to pressed under pressure to form a disk before being scanning at wavenumbers in the ranges of 400–4000 cm–1 [42].
The nature structure (crystallinity or amorphous) of synthesized TiO2-NPs was investigated by X-ray diffraction (XRD, PANalytical-X’Pert-Pro-MRD). The source of X-ray radiation was CuKα (λ = 1.54 Å) at a current and voltage of 30 mA and 40 kV, respectively. The XRD scanning was carried out at 2θ values of 5–80°. The average crystallite size of biosynthesized TiO2-NPs was measured using XRD analysis by Debye–Scherrer’s equation as follows [43]:
where 0.94 is a Scherrer constant, 1.54 is the wavelength of the X-ray, β is the full width of the diffraction peak at a half maximum, and θ is the diffraction angle.
The sizes, shape, surface morphology, and chemical compositions of biosynthesized TiO2-NPs were detected by transmission electron microscope (TEM, JEOL, Ltd-1010, Tokyo, Japan), scanning electron microscope (SEM, JEOL, JSM-6360LA, Tokyo, Japan), and energy dispersive X-ray (EDX). The as-formed powder was suspended in high pure water (Milli-Q) under sonification followed by drooping a few drops on the surface of the TEM-carbon grid. The loaded grid remains dry before being subjected to analysis [42]. The sample was subjected to SEM (JEOL, JSM-6360LA, Tokyo, Japan) analysis through loading on the SEM holder and coated with gold under vacuum and captured the image at 20 kV. The elementary qualitative and quantitative mapping was detected by EDX connected with the SEM apparatus.
The detection of hydrodynamic sizes and size distribution in colloidal solution was investigated by DLS. The biosynthesized TiO2-NPs were suspended in high-pure H2O to prevent the formation of shadow and extra peaks during particle scattering. The surface charge of synthesized particles was detected by zeta potential analysis using Malvern Zeta-sizer (Nano-ZS, Malvern, UK).
2.2.6 Biocompatibility of TiO2-NPs
The biocompatibility of bacterial-based TiO2-NPs was investigated toward two normal cell lines WI38 (human fibroblast lung tissue) and HFB4 (human normal melanocytes) to detect the safe dose that can be applied to control the fungal strains isolated from historical paper. The biocompatibility test was examined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method. Each type of cell was inoculated separately in a 96-well culture plate with cell intensity of 1 × 105 cells/100 μL/well followed by incubation for 24 h at 37 °C in a 5% CO2 incubator. After incubation time, the monolayer sheet was formed and mixed with 100 μL of maintenance media (RPIM) with 2% serum. The growing cells were treated with double-fold TiO2-NP concentration (1000–31.25 μg mL–1) before being incubated for 48 h. The control was represented by three wells without treatment. At the end of the incubation period, the remaining growth media in each well were discarded and filled with 50 μL of MTT solution (5 mg/mL phosphate buffer saline solution), shaken well for 5 min, and incubated at 37 °C for 4 h. After that, the MTT solution was removed and filled the well with 100 μL of DMSO (10%) for dissolving the formazan crystal that formed as a result of MTT assay metabolism. The DMSO was removed from wells after 30 min and measurement of the absorbance of the formed color was at 570 nm using ELIZA reader [44]. The percentages (%) of cell viability due to TiO2-NPs treatment were measured using the following equation:
2.2.7 Antifungal activity
The activity of biosynthesized TiO2-NPs to inhibit the growth of six fungal strains (selected based on one organism from each species) designated as ED1, ED3, ED4, ED8, ED11, and ED14 was investigated by the well-diffusion method. The different concentrations (lower than the IC50 value from the biocompatibility test) represented as 300, 200, and 100 μg mL–1 were used. In this method, 50 μL from spore suspension (adjusted the OD at 1) was spread thoroughly over the surface of Czapeck yeast extract agar media before being made three wells (0.6 mm in diameter) and filled with 100 μL of each concentration. The loaded plates were kept in the refrigerator for 1 h before being incubated at 27 ± 2 °C for 48 h. The solvent system (DMSO) was used as a control. The results were recorded as a diameter of the inhibition zone (mm) appeared around each well [45]. The experiment was carried out in triplicate.
2.2.8 Statistical analysis
Data collected in the current study are represented by the means of three independent replicates followed by analyzing using ANOVA analysis by a statistical package SPSS v17. The mean difference comparison between the treatments was analyzed by the Tukey HSD test at p < 0.05.
3 Results and discussion
The novelty of the current study is represented by the selection of historical book Description de l’Égypte under study due to its original copy, written by order of Emperor Napoleon while he was in Egypt and is called an imperial copy, and no study was achieved on this copy. There are only two original copies of the selected book in Egypt, one is preserved in the Library of the Academy of the Arabic Language, Cairo, Egypt (which selected for the current study), and the other is deposited in the Alexandria Library, Alexandria, Egypt. Also, the novelty of the current study was represented by biocontrol of different fungal strains obtained from deteriorated parts using green synthesized TiO2-NPs which fabricated by safe probiotic bacterial strain.
3.1 Investigate the deterioration aspects
3.1.1 Deterioration documentation
Upon visual observation, the historical book exhibited a considerable amount of colored spots, manifesting as reddish-brown foxing, scattered across both its internal and external pages (Fig. 1). These spots could be related to bad storage conditions such as RH, temperature, poor ventilation, iron storage cabinet, and growth of some colored molds such as Epicoccum spp. and Monoascus spp. [7]. Moreover, the dust, heavy metal residue, oxidation process, lights, and accumulation of organic and inorganic contaminants can be the reason for the appearance of these foxing spots [46]. Interestingly, the fungal and bacterial attacks to historical paper can be responsible for these foxing spots. Michaelsen and coauthors reported that the colonization of historical papers by fungal and bacterial strains such as Aspergillus versicolor, A. nidulans, A. terreus, A. flavus, Penicillium pinophilum, P. chrysogenum, P. citrinum, Cladosporium spp. Botryotinia fuckeliana, Rhizopus oryzae, Aureobasidium pullulans, Bacillus spp., Kocuria spp., Acinetobacter spp., Stenotrophomonas sp., and Clostridium colinum is responsible for forming foxing spots [47, 48].
3.1.2 Environmental scanning electron microscope (ESEM)
In the current study, the Whatman filter paper (grade no. 1) served as a control during investigation analyses due to it is made from cotton, free of impurities, has a neutral pH, and has not had any foreign additives. As shown, the control (Whatman paper no.1) fiber is mainly cotton fibers (Fig. 2A). These cotton fibers appear as collapsed or twisted ribbon and twisted tube, which namely convolutions. The obtained data are compatible with Dochia et al. [49], who reported that the surface of cotton fibers appears uneven due to the presence of convolutions. Also, these convolutions improve friction between inter-fiber and increase the strength of fine cotton yarns to be spun [50]. The analysis conducted in Fig. 2B reveals that the primary constituent of the historical paper is cotton fibers, resembling a Whatman paper (control). Furthermore, signs of deterioration, including fiber rupture, weakness, and erosion, were observed in certain fibers. Additionally, the presence of fungal and bacterial growth amid the fibers was evident. In a parallel study, SEM analysis was employed to examine the deterioration characteristics of archaeological papers, which exhibited fiber degradation and the presence of microbial strains [11].
3.1.3 Attenuated total reflection/Fourier transform infrared spectroscopy (ATR/FTIR)
Figure 3A shows the ART-FTIR for the historical paper compared to control (Whatman paper). The band at wavenumber of 3332 cm−1 refers to the O–H stretching vibration for the hydroxyl groups [51]. The historical sample and control have the band at the same wavenumber, but the intensity of this band in historical paper sample was higher compared to the control. Bands observed at 2897 cm−1 and 2899 cm−1 in FTIR chart of historical papers and control, respectively, signify to the stretching vibration of C–H. Moreover, these bands are also referring to the ν(CH2) and ν(CH3) stretching vibrations, which refer to the ethyl and methyl groups [52]. The band at 1644 cm−1 in the control chart was referring to the H–O–H deformation vibration which shifts to 1635 cm−1 with high intensity in the historical sample. Bending vibrations of C–O–H and CH2 were observed at the band in the ranges of 1540 cm−1 and 1248 cm−1 (bands at wavenumbers of 1508, 1427, 1360, and 1248 cm−1 were represented the control, whereas bands at the wavenumbers of 1542, 1418, and 1318 cm−1 were for historical sample). The intensities of these bands in historical samples were high compared to the intensity in control. The C–O stretching, C–C–O, and C–O–C bending vibrations were detected in the ranges of 1204 and 1092 cm−1. These bands were classified as 1204, 1159, and 1108 cm−1, and 1161 and 1092 cm−1 for Whatman and historical paper samples, respectively [53]. As shown the intensities of these bands in historical samples were higher than their intensities in control.
Other bands signifying to the cellulose structure was also detected. For instance, the plane bending vibrations bands for H–C–H and O–C–H were detected at wavenumber of 1427 cm−1 for the control and shifted to wavenumber of 1418 cm−1 in the historical sample. Also, the C–H deformation vibration was observed at bands of 1360 cm−1 and 1372 cm−1 for the Whatman and historical paper samples, respectively [52].
It was noticed from the data mentioned above that the bands of the historical paper sample shifted to lower wavenumbers and gave higher intensity compared to the Whatman paper sample. This indicated that the historical paper sample was exposed to deterioration, which may be due to physical, chemical, or biological factors.
3.1.4 Change of color
It was clear from the data represented in Fig. 3B that the L* value (lightness) of the Whatman paper was 95.97. The L* value of the historical paper was decreased compared to the Whatman paper. The reduction in the L* value of the historical sample was 24%. This may be due to the accumulation of dust on the surface of the historical paper, the continuous increasing of the incorrect relative humidity which can adhere the dust to the surface, and uncontrolled pollution in the area of the Arabic Language Academy (storage site) [38]. The results of the a* value of the historical sample tended to be in red color, while the control sample tends to be green color. The red color (b* value) of the historical sample increased by 95% compared to the control sample. Both the Whatman and historical samples displayed a yellowish hue, as indicated by their respective b* values. Notably, the b* value of the historical sample exhibited an 89% increase compared to that of the Whatman paper. Furthermore, the total color difference between the historical sample and the Whatman paper was measured at 29. These findings indicate that the historical paper appears darker in color compared to the Whatman paper. The observed variations in color values and total color difference can be attributed to the influence of environmental conditions, encompassing physical, chemical, and biological factors, present within the book.
3.2 Fungal isolation and identification
The different fungal strains that colonized the deteriorated parts of historical papers were isolated by the dependent culture method. Herein, nineteen fungal isolates designated as ED1–ED19 were isolated from deteriorated sites using two culture media, PDA and CYA media, as mentioned in the “Material and method” section. Similarly, two culture media, namely, PDA and Sabourud dextrose agar (SDA), were used to isolate the fungal strains associated with the stone of Cyrus the Great Tomb [54]. The purified fungal strains were subjected to primary identification based on morphological characteristics and microscopic examination (Fig. 4). Data from traditional identification revealed that all obtained fungal isolates were belonging to the division of Ascomycota. The majority of fungal strains were belonging to Aspergillus spp. with percentages of 84.2% (from the total fungal strains) followed by Penicillium spp. with percentages of 15.8% (Table 1). Among Aspergillus spp., eight fungal strains designated as ED1, ED2, ED5, ED6, ED7, ED9, ED16, and ED17 were identified as A. flavus (42.11%), whereas two isolates ED3 and ED18 were identified as A. versicolor (10.53%), four fungal isolates coded ED8, ED12, ED13, and ED15 identified as A. ustus (21.05%), and two isolates (ED11 and ED19) identified as A. chinensis (10.53%). On the other hand, two Penicillium species were obtained from deteriorated samples and identified as P. citrinum for isolate ED4 and ED10, and P. chrysogenium for isolate ED14. Interestingly, P. citrinum was the most common Penicillium spp. isolated from the historical book with percentages of 10.53% followed by P. chrysogenium with percentages of 5.26% (Fig. 5A).
Traditional identification of isolated fungal strains obtained from deteriorated historical book. A A. flavus, B A. versicolor, C P. citrinum, D A. ustus, E A. chinensis, and F P. chrysogenum. (1) is reverse colony grown on CYA media, (2) is the observed colony, and (3) is the microscopic image using bright field microscopy (X = 800)
A Pie chart of the percentage of detected fungal species; B the phylogenetic tree of six selected fungal strains obtained from deteriorated parts of the historical book based on ITS identification. The obtained sequences were compared with NCBI reference sequences. The tree was constructed by the neighbor-joining method (MEGA 6) with a bootstrap value (1000 replicates)
In a similar study, approximately 199 fungal isolates were obtained from old manuscript deteriorated samples and isolated on a PDA medium. The most common fungal strains were belonging to Aspergillus spp. with a percentage of 45.57% followed by Fusarium with a percentage of 33.1%, Penicillium spp. with a percentage of 8.6%, Alternaria spp. (6.5%), Trichoderma spp. (3.0%), Stymphylium spp. (1.5%), and Nigrospora spp. (1.5%) [55]. On the other hand, thirty-one (31) fungal strains were isolated from two old manuscripts and identified by traditional method into six genera as follows: Aspergillus spp. (14 isolates with percentages of 45.2%), Cladosporium spp. (8 isolates with percentages of 25.8%), Penicillium spp. (5 isolates with percentages of 16%), Curvularia sp. (one isolate with a percentage of 3.2%), Ulocladium sp. (one isolate with a percentage of 3.2%), and yeast-like fungi (2 isolates with a percentages of 7%) [33]. Recently, thirteen fungal strains were obtained from deteriorated parts of a historical manuscript dated back to seventeenth century and identified by cultural and microscopic examination to Aspergillus niger (3 isolates, 23%), A. fumigatus (2 isolates, 15.4%), A. quadrilineatus (3 isolates, 23%), Penicillium citrinum (2 isolates, 15.4%), and P chrysogenum (3 isolates, 23%) [11]. Moreover, twenty (20) fungal isolates belonging to Aspergillus spp. and Penicillium spp. only based on traditional identification colonized the deteriorated historical manuscript dated back to the nineteenth century [56].
The ITS sequence analysis (molecular identification) was used to confirm the identification of traditional method. Therefore, one fungal strain was selected from each species to confirm their identification. Herein, six fungal strains coded as ED1, ED3, ED4, ED8, ED11, and ED14 were undergoing ITS gene sequence analysis (Table 1, Fig. 5B). The molecular identification reveals that the selected strains were similar to A. flavus, A. ustus, A. Chinensis, P. citrinum, and P. chrysogenium with closest accession numbers of KT358861, EU042148, NR131284, NR137441, NR121224, and NR077145 and similarity percentages of 99.47, 99.20, 99.09, 98.98, 98.94, and 98.90%, respectively. Therefore, the six selected fungal strains were identified as A. flavus ED1, A. versicolor ED3, P. citrinum ED4, A. ustus ED8, A. Chinensis ED11, and P. chrysogenium ED14. As shown the molecular identification was matched with traditional methods.
The deterioration percentages of papers are influenced by various environmental factors, including temperature, relative humidity, as well as the presence of fungal strains and paper components [7]. Factors such as the availability of different carbon sources (such as cellulose, xylan, and starch), additives (like gelatin and minerals) incorporated during paper manufacturing, and storage conditions play a role in promoting the growth of different fungal strains, ultimately determining the extent of damage [38]. It is important to note that fungal strains have the potential to secrete various metabolites, including allergenic substances and mycotoxins, which can have detrimental effects on the health of readers, visitors, and workers [57].
3.3 Enzymatic activities
Fungi have the efficacy of producing diverse groups of enzymes extracellularly; fungal proteases, amylases, lipases, xylanase, pectinase, and cellulases represent the most present enzymes [14]. It should be noted that the adhesive types used in the paper industry are water-based adhesives, both synthetic and biopolymer based (such as starch, cellulose, protein, and itaconic acid) with some other additives and synthetic compounds to improve mechanical and chemical properties [57]. Microbes have the capability to secrete extracellular hydrolytic enzymes, including cellulases, lipases, chitinases, amylases, proteases, and pectinases. These enzymes can induce alterations, weakening, and degradation of the structures of biopolymers and additives present in the materials. Fungi are considered the most microbial species that have the capacity to produce these extracellular enzymes [58]. The polymers can be hydrolyzed and converted from complex, water-insoluble materials to low-molecular-weight soluble compounds, ultimate to enhance the biodeterioration processes [59].
In the current study, the potential of various fungal strains that inhabit the historical paper to produce different enzymes including amylase, cellulase, pectinase, and gelatinase was investigated. Analysis of variance reveals that the nineteen fungal strains have the efficacy to secrete these hydrolytic enzymes with varying degrees (Fig. 6).
Data analyses revealed that the fungal strain Aspergillus versicolor ED3 exhibited the highest amylase activity, with an inhibition zone measuring 21.3 ± 3.1 mm. Following this, fungal strains A. flavus ED1, A. flavus ED2, and A. ustus ED13 displayed inhibition zones of 17.3 ± 3.1 mm, 12.3 ± 4.04 mm, and 10.0 ± 2.0 mm, respectively (Fig. 6A). The fungal strains A. flavus ED9, A. ustus ED12, Penicillium chrysogenum ED14, and A. flavus ED17 did not exhibit any amylase activity.
Analysis of variance showed that the highest pectinase activity was recorded for fungal strains P. citrinum ED10 and A. flavus ED2 with inhibition zones of 18.7 ± 1.5 and 18.3 ± 3.2 mm, respectively, followed by fungal strains A. flavus ED1 and A. versicolor ED3 with inhibition zones of 15.3 ± 3.2 and 14.7 ± 4.2 mm, respectively (Fig. 6B). Unfortunately, the fungal strains A. ustus ED12 and A. ustus ED15 do not exhibit any pectinase activity. Interestingly, the pectinase activity between fungal strains (ED1–ED3) and (ED4, ED5, and ED7) was not significant with inhibition zones ranging between (14.7 ± 4.2–18.3 ± 3.2 mm) and (9.0 ± 2.6–10.0 ± 2.6 mm) respectively.
Moreover, the most fungal strains that have the activities to secrete cellulase enzymes with the highest inhibition zones of 22.7 ± 3.2 mm and 21.7 ± 1.5 were ED3 and ED4 respectively, followed by fungal strains ED8 and ED13 with inhibition zones of 21.7 ± 2.02 and 21.3 ± 2.1 mm, respectively (Fig. 6C). Unfortunately, the fungal strains ED1, ED6, ED12, and ED17 do not exhibit any cellulase activity. Analysis of variance revealed that the cellulase activity between fungal strains ED2, ED3, ED4, ED8, ED11, ED13, and ED15 was similar (not significant) with inhibition zones ranging between 21.0 ± 4.6 and 19.7 ± 0.6 mm. The cellulase activity observed for fungal strains ED5, ED7, ED9, ED10, and ED14 was found to be statistically insignificant, with inhibition zones ranging from 7.0 ± 4.4 to 11.0 ± 1.7 mm. The results showed that the highest gelatinase activity was recorded for fungal strains A. ustus ED8 followed by A. versicolor ED3 and A. flavus DE5 with inhibition zones of 24.3 ± 1.2, 18.0 ± 2.6, and 14.7 ± 1.5 mm, respectively (Fig. 6D). Unfortunately, the fungal strains ED.1, ED.7, ED.10, ED.11, ED.13, and ED.15 do not exhibit any gelatinase activity.
Fungi possess the ability to produce a diverse array of enzymes capable of breaking down polysaccharides into individual monosaccharide units. Cellulose, being a prominent polysaccharide component of paper, is susceptible to degradation through fungal activity, leading to the breakdown of cellulose into monosaccharides [3]. Pinheiro and Sequeira [5] reported that the colonization of paper components by fungi leads to penetration of the fibers causing physical changes along with the accumulation of products of fungal metabolism in the fibers. Under suitable conditions for fungal growth, hyphae germinate, developed, and mature to produce different secondary metabolites that lead to enhancement of biodeterioration and loss of mechanical properties of the paper, in addition to aesthetic changes and deformations through the pigments they secrete.
In a similar study, the types of fungal strains responsible for the biodegradation of a nineteenth century art group were studied and their cellular enzymatic activity was determined [60]. The authors reported that the nineteen fungal isolates belonging to the genera Arthrinium, Aspergillus, Chaetomium, Cladosporium, Colletotrichum, Penicillium, and Trichoderma were identified and showed a significant role in the production of enzymes that degrade lignin and cellulose leads to microbial degradation of ancient documents. Moreover, gelatinase and pectinase have the ability to degrade various proteins and carbohydrates such as fibroin, collagen, and keratin, which are used in the manufacture of parchment, leather, silk, and wool [38].
The obtained results showed that the activity of fungal strains associated with the historical book in their efficacy to the secretion of a wide range of enzymes amylase, pectinase, cellulase, and gelatinase with different degrees is important for understanding the role of these strains in the biodeterioration process. In the current study, the RH of the storage site was 65% and temperature was 22 °C, which encourage the fungal growth and hence enhance the production of various metabolites such as acids and enzymes which mainly responsible for the deterioration of historical papers [61]. This finding was compatible with those reported that the fungal strains of A. terreus, A. niger, A. ustus, A. versicolor, P. chrysogenum, P. citrinum, P. commune, and Cladosporium sp. associated with historical paper from seventeenth century have the efficacy to produce acidic metabolites which decrease the pH value up to 4 [11].
3.4 Biosynthesis of TiO2-NPs using Lactobacillus rhamnosus
3.4.1 Characterizations
UV-Vis spectroscopy
Recently, the biosynthesis of TiO2-NPs using a probiotic bacterial strain L. rhamnosus has received great attention due to its sustainable and eco-friendly approach [31]. L. rhamnosus act as a biofactory for the synthesis of TiO2-NPs by the action of their metabolites without the need of high-energy processes and toxic substances, leading to reduction of the hazardous environmental impacts. These probiotic bacteria have diverse metabolites for reducing and stabilizing properties, enhancing the production of controlled size, shape, and crystalline structure, which facilitate their incorporation into various biotechnological and biomedical fields [62]. Moreover, the synthesis of TiO2-NPs using probiotic bacterial strains exhibits increased biocompatibility and decreases the toxicity compared to traditional synthesized methods. Overall, L. rhamnosus-mediated green synthesis of TiO2-NPs offers a biocompatible, efficient, and sustainable alternative to conventional synthesis approaches, opening the windows for the synthesis of new advanced NPs with diverse applications. The first sign for biosynthesis of TiO2-NPs by harnessing probiotic metabolites is color change which measured their intensity by UV-Vis spectroscopy.
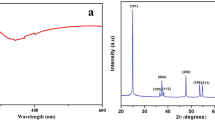
The UV-Vis spectroscopy depends on the UV- or visible-light retention which leads to forming a specific spectrum. The electrons on the surface of nanomaterials at the point of UV light absorption go to excitation. This leads to bouncing of electrons from inert to energized state [63]. In the current study, the absorbance of white color intensity that formed as a result of the reduction of metal precursor to NPs was measured in the range of 200–800 nm to detect the SPR. As shown, the maximum peak was observed at 360 nm which matched with the maximum SPR for TiO2-NPs (Fig. 7A). Data recorded by Kirthi et al. [41] reported that the maximum SPR peak for TiO2-NPs fabricated by cell-free filtrate of Bacillus subtilis was located at 266 nm. On the other hand, TiO2-NPs synthesized by B. mycoids showed a broad absorption peak at a wavelength of 380 nm [29]. Overall, the SPR absorption peak that characterizes the successful formation of TiO2-NPs has been observed in the range of 300–400 nm as reported previously [64, 65].
Fourier transform infrared spectroscopy (FT-IR)
The functional groups in the cell-free filtrate (CFF) and their role in the reduction, capping, and stabilizing of as-formed NPs were investigated using FT-IR. As shown, the bacterial biomass filtrate contains four intense peaks at wavenumbers of 3420, 2066, 1630, and 520 cm–1. After fabrication of TiO2-NPs, the intense peaks in CFF were shifted to 3580, 3360, 2360, 1628, 1425, 1075, 877, 620, and 550 cm–1 (Fig. 7B). The N–H stretching of aliphatic primary amines was detected at a wavenumber of 3420 cm–1 [66], which shifted after formation of TiO2-NPs to 3580 cm–1. On the other hand, the CH bending of aromatic compounds or N=C=S stretching of isothiocyanate from biomass filtrate was detected at peak of 2066 cm–1 [67]. A peak at wavenumber of 1630 cm–1 is corresponding to the asymmetric nitro-compound stretching, bending N–H of amines, or the C═O of polysaccharide moiety secreted by bacterial strain [42]. This peak was shifted after TiO2-NPs synthesize to 1628 cm–1. The peak at 520 cm–1 in bacterial biomass filtrate corresponds to the C–I of halo compounds. The medium peak at 3360 cm–1 is signifying to the stretching N–H of secondary amines, whereas the adsorption of CO2 on the surface of NPs was observed at wavenumber of 2360 cm–1 [53]. The peak at 1425 cm–1 could be attributed to the CH2 vibration bending of lipids and proteins [41]. The strong peak at 1075 cm–1 can be related to the stretching C–O of primary alcohol or stretching S═O sulfoxide [53]. The successful formation of TiO2-NPs was confirmed by the presence of peaks in the range of 500–900 cm–1 that correspond to O–Ti–O [42, 68]. The presence of diverse functional groups associated with various metabolites, including proteins, carbohydrates, polysaccharides, amino acids, and primary and secondary amines, plays a pivotal role in the reduction of metal precursors, leading to the formation of nanoparticles (NPs). Subsequently, these functional groups also contribute to the capping and stabilization of the newly formed compounds, preventing their aggregation.
X-ray diffraction (XRD)
The XRD pattern for biosynthesized TiO2-NPs is shown in Fig. 8 to explain the crystal or amorphous phase. The various Bragg’s reflection peaks were observed at 2θ° of 25.4°, 37.8°, 47.9°, 53.6°, 54.6°, 62.6°, 69.9°, and 74.9° which corresponded to planes of (101), (004), (200), (105), (211), (204), (220), and (216), respectively. The obtained diffraction peaks validated the anatase of biosynthesized TiO2-NPs according to JCPD standard (No. 2-21-1272). This finding was compatible with literature about the green synthesis of TiO2-NPs that have anatase form [24, 68]. No additional peaks in XRD analysis indicate the high purity of synthesized nanomaterial (validated by EDX). Khan and Fulekar reported that the presence of the main peak in the XRD pattern in the ranges of 2θ values of 25.25–25.58° corresponded to the crystallographic plane of (101), indicating the successful formation of anatase crystalline [42]. The crystallite size of synthesized TiO2-NPs was measured using XRD analysis by Debye–Scherrer’s equation based on the main plane (101) which was 7.5 nm. Compatible with the obtained result, the average crystallite size of TiO2-NPs synthesized by aqueous extract of Acacia nilotica based on XRD analysis was 9 nm [68], whereas those fabricated by juice extract of Citrus limon have crystallite size of 15 nm [63].
Morphological characteristics
The activity of nanomaterials is dependent on varied factors such as size, shape, surface area, agglomeration, chemical compositions, and surface charge [69]. Therefore, it is important to investigate these parameters. In the current study, the biomass filtrate of probiotic L. rhamnosus has the potential to fabricate TiO2-NP spherical shapes and sizes in the ranges of 3–10 nm (Fig. 9A) with an average of 5.72 ± 1.93 nm (Fig. 9B). The activity of NPs was inversely proportional with sizes; the activity increased by decreasing the size. This finding could be attributed to the increasing surface-to-volume ratio with decreased sizes; this enhances the penetration of NPs to bacterial cell walls rapidly and improves of antimicrobial activities of biosynthesized TiO2-NPs [70]. The sizes and shapes of synthesized NPs can be varied according to the reducing and capping agents which varied between various biological entities. For instance, the sizes of TiO2-NPs synthesized by cell-free filtrate of Lactobacillus sp. were in the range of 15–35 nm, whereas those fabricated by Sacharomyces cerevisae were 8–20 nm [31]. Moreover, those synthesized by B. subtilis and B. amyloliquefaciens have sizes in the ranges of 66–77 nm and 11.2–97.3 nm, respectively [41, 42]. The selected area electron diffraction (SAED) pattern for bacterial-based TiO2-NPs was shown in Fig. 9C. The SAED pattern reveals the presence of a circular diffraction ring, signifying the successful formation of the anatase form, which is consistent with the X-ray diffraction (XRD) pattern obtained.
Morphological and elemental analysis of biosynthesized TiO2-NPs. A TEM analysis showing a spherical shape, B size distribution according to TEM image, C SAED pattern confirming the anatase phase, D SEM analysis showing a smooth and spherical shape, and E the EDX analysis revealing the elementary mapping
The surface morphology of green synthesized TiO2-NPs was detected by SEM which reveals the smooth surface with a spherical shape and an average particle size of 15 nm. However, some large particles were noticed which were possibly attributed to some aggregation during sample preparation (Fig. 9D). Anbumani et al. [25] reported the presence of some large size or aggregation in SEM analysis due to either the capping agent or irregular distribution during sample preparation. The elemental compositions of as-formed NPs were detected by EDX analysis. As shown, the absorption peaks at the bending energies of 0.4 and 4.5 keV are signifying to the Ti ions, whereas the peak at the bending energy of 0.5 keV is corresponding to O ions which indicates the successful formation of TiO2 (Fig. 9E). The EDX chart reveals that the main component of the sample was Ti with weight and atomic percentages of 55.91% and 46.25% followed by O with percentages of 24.21% and 25.32%, respectively. Compatible with the obtained results, the EDX chart of TiO2-NPs synthesized by Bacillus subtilis showed the presence of absorption peaks of Ti and O ions at bending energies of 0.4 and 4.5 keV and 0.5, respectively, with weight percentages of 67.32% and 32.68% and atomic percentages of and 51.75% and 48.25% [71]. The EDX showed the presence of an absorption peak of C at a bending energy of 0.25 keV which possibly could be the emission of bacterial metabolites that are utilized during NP synthesis and serve as capping agents [24].
Dynamic light scattering (DLS) and zeta potential analysis
DLS analysis was utilized to examine the size distribution of TiO2-NPs in a colloidal solution as well as to determine the residual size of the nanoparticles. Figure 10A illustrates the size distribution graph obtained from DLS, indicating that the average particle size of TiO2-NPs derived from L. rhamnosus was measured at 28.2 nm. The sizes determined through DLS measurements appear to be larger compared to those obtained through TEM, SEM, and XRD analyses. This phenomenon can be attributed to the presence of different capping agents that adhere to the surfaces of NPs, which are derived from the bacterial extract. These capping agents can affect the size measurements obtained through DLS. It is important to note that DLS analyzes the hydrodynamic residue or the hydrated state of the NPs, whereas TEM and SEM measurements are conducted on dry particles. This distinction in measurement conditions can contribute to the observed differences in NP size between the techniques. Moreover, the DLS is affected by the non-homogenous distribution of NPs in colloidal solution which has a negative response on measurement [72]. In a similar result, the size-based TEM, SEM, and XRD analyses of TiO2-NPs fabricated by leave aqueous extract of Syzygium cumini were 11 nm, 18 nm, and 10 nm, respectively, which were considered smaller than the sized obtained by DLS (22 nm) [73]. Similarly, the average particle size of NPs synthesized by extract of Benincasa hispida obtained by DLS was 70 nm which is bigger compared to the size obtained by TEM which was 22.2 nm [74].
The movement of NPs in colloidal solution in the presence of electric charge is defined as electrokinetic or zeta potential, which is considered a useful tool for measurement of the stability of as-formed NPs [75]. In the current study, the zeta potential chart has two peaks at values of – 19.9 mV and – 36.8 mV (Fig. 10B). The presence of only a negative charge in the current study causes the repulsion of as-formed particles from each other and prevents aggregation, which indicates the high stability of synthesized TiO2-NPs. In contrast, if positive and negative charges are present, they lead to attraction between different particles and hence agglomerated or aggregated [76]. The zeta potential value of TiO2-NPs synthesized by aqueous extract of Syzygium cumini was – 18.7 mV [73], whereas those synthesized by extract of Withania somnifera roots and propolis extract was – 24 mV and – 32.4 mV, respectively [77, 78].
3.4.2 Biocompatibility
The biocompatibility of bacterial synthesized TiO2-NPs was evaluated against two mammalian normal cell lines designated as WI38 and HFB4. The purpose of this experiment is to detect a safe dose of NPs to be used for biocontrol of various fungal strains isolated from deteriorated historical book to recommend this dose to be applied in paper treatment. The MTT assay method was used to investigate biocompatibility because it is an accurate, sensitive, and colorimetric method for estimating the proliferation and viability of cells after treatment with various concentrations of active compounds [79]. Herein, the two normal cell lines were treated with double-fold concentrations (1000–32.25 μg mL–1) of TiO2-NPs that exhibited viability in a dose-dependent manner. For instance, the lowest cell viability was attained at a concentration of 1000 μg mL–1 with percentages of 2.4 ± 0.2% and 8.1 ± 1.1% for WI38 and HFB4, respectively. These cell viability percentages were increased by decreasing the NP concentrations to 67.4 ± 1.4% and 80.0 ± 0.9% for WI38 and HFB4, respectively (Fig. 11). Similarly, it was observed that TiO2-NPs did not exhibit any cytotoxic effects on osteoblast-MG63 normal cell lines when used at concentrations below 100 μg mL–1. This lack of cytotoxicity was observed after 24 and 48 h of incubation [80]. In a similar study, the TiO2-NPs showed high activity against tow cancer cell lines, HCT116, and HT29 after treatment with concentrations in the range of 50–400 μg mL–1. Whereas, the viability of normal cell lines, HUVECs (human umbilical vein endothelial cells), does not exhibit any changes at a concentration in the range of 50–100 μg mL–1 and decreased after treatment with concentrations in the range of 200–400 μg mL–1 [81]. This means the biocompatibility of TiO2-NPs against normal cell lines was attained at low concentrations and changes with negative impacts at high concentrations. In the current study, the IC50 (concentration of NPs inhibits 50% of cell viability) values were 341.9 ± 12.8 and 368.9 ± 5.6 μg mL–1 for WI38 and HFB4, respectively. Incompatible with the obtained results, the IC50 of TiO2-NPs against normal cells, HUVECs was 158.7 μg mL–1 [81]. This indicates the synthesized NPs in the current study were safer toward normal cell line at high concentrations (≤ 300 μg mL–1). Therefore, we recommend using TiO2-NPs with a concentration below 300 μg mL–1 to biocontrol of fungal strains and to be added in the future to historical paper for treatment and preservations.
3.4.3 Antifungal activity
The utilization of metal and metal oxide nanoparticles for controlling fungal contamination holds great promise due to their potent antifungal properties against various strains. In this study, six fungal strains, namely, A. flavus ED1, A. versicolor ED3, P. citrinum ED4, A. chinensis ED8, A. ustus ED11, and P. chrysogenum ED14 (one representative strain for each), were selected to assess the inhibitory effect of TiO2-NPs synthesized through a green approach. The growth inhibition of these fungal strains was evaluated using the agar well diffusion method. Data analysis showed that the activity of synthesized NPs was concentration dependent. This finding is compatible with various studies which reported the activity of NPs against various microbes in was dose-dependent manner [25, 78]. All tested concentrations (300, 200, and 100 μg mL–1) showed inhibition zone toward all tested fungal strains with varying degrees. The presence of inhibition zones in inoculated plates indicates the antifungal activity of bacterial-based TiO2-NPs. The maximum inhibition zone against A. flavus ED1 (17.7 ± 0.6 mm), A. versicolor ED3 (20.3 ± 1.5 mm), P. citrinum ED4 (18.7 ± 0.6 mm), A. chinensis ED8 (20.3 ± 0.5), A. ustus ED11 (18.3 ± 0.6 mm), and P. chrysogenum ED14 (17.7 ± 1.2 mm) was recorded for a concentration of 300 μg mL–1. Similarly, the TiO2-NPs fabricated by an aqueous extract of Parmotrema austrosinense showed antifungal activity against Rhizoctonia solani, Fusarium oxysporum, and Sclerotium rolfsii as a diameter of inhibition zone [82]. The diameter of inhibition zones was decreased by lowering the NP concentrations. For instance, at 200 and 100 μg mL–1, the diameters of inhibition zones were (14.7 ± 0.6, 16.3 ± 0.6, 13.3 ± 0.5, 15.3 ± 0.6, 14.7 ± 0.6, and 13.7 ± 0.5 mm) and (12.3 ± 0.6, 13.3 ± 0.5, 11.7 ± 0.6, 12.3 ± 0.6, 12.7 ± 0.6, and 12.0 ± 1.0 mm) for fungal strains ED1, ED3, ED4, ED8, ED11, and ED14, respectively (Fig. 12). In a similar study, the inhibition zones formed due to treatment of A. flavus, A. niger, Rhizopus oryzae, and Sclerotium rolfsii with 40 μg mL–1 TiO2-NPs were 27 ± 0.4, 36 ± 0.5, 34 ± 0.3, and 42 ± 0.3 mm, respectively. These zones of inhibitions decreased gradually with concentrations of 21 ± 0.5, 24 ± 0.3, 18 ± 0.4, and 24 ± 0.3 mm after treatment with 20 μg mL–1 for the same previous sequence of fungi [25]. Recently, because of the unique activities of NPs, it is used to control bacterial and fungal growth that causes deterioration in an archeological and historical manuscript. For instance, silver nanoparticles (Ag-NPs) and zinc oxide nanoparticles (ZnO-NPs) were employed to combat the growth of P. chrysogenum and B. subtilis. These microorganisms were isolated from a historical manuscript dating back to the seventeenth century and exhibited the highest cellulase enzyme activity. The Ag-NPs and ZnO-NPs were utilized as a means to control the proliferation of these strains [83]. Moreover, Ag-NPs and ZnO-NPs were used to inhibit the growth of A. niger (highest cellulase enzyme activity) isolated from deteriorated historical paper [84].
The inhibitory action of NPs against different fungal strains is dependent on several factors such as shapes, sizes, agglomeration, surface area to volume, surface chemistry, and distribution [85]. The antifungal activity of NPs with small sizes is high compared to the large sizes, and this finding could be due to increased surface area with smaller sizes [86]. Therefore, the antifungal activity of bacterial-mediated synthesis of TiO2-NPs could be related to its smaller sizes, which easily penetrate the cells and enhance the toxicity.
The inhibitory effects of NPs could be related to the dissolution and liberation of toxic ions which in the current study is Ti+4 once enter the cells; hence, it interacts with sulfur-containing amino acids such as cysteine and methionine, leading to blocking their functions. In addition, the release of toxic ions can cause dysfunction of cytoplasmic membrane proteins [87]. Moreover, NPs can alter the selective permeability function of the cytoplasmic membrane by reacting with carrier proteins leading to destroying the transporting system [88]. The germination of conidia can be inhibited after reacting with NPs and hence suppress the fungal growth. Another inhibitory mechanism of eukaryotic cells involves the interaction of NPs with the mitochondrial membrane. This interaction subsequently leads to the upregulation of certain oxidative stress genes, including Shgst1, GPx, catalase, and SOD2. These genes are responsible for the increased production of ROS, contributing to the inhibition of cell growth [86]. Oxidative stress is the main reason for the damage of nucleic acids, proteins, enzymes, and other macromolecules in the eukaryotic cells [89].
3.5 Comparison study
To best of our knowledge, this is the first report achieved on the Description de l’Égypte book in the preservation of deterioration aspects. Only one study was achieved on unoriginal copy deposited in Misr library, Mansoura City, Egypt [84]. In this study, nine fungal isolates were obtained from deteriorated parts and undergone the traditional identification, followed by selection one isolate based on the highest cellulase activity for molecular identification. The activity of zinc oxide nanoparticles and silver nanoparticles to inhibit the growth of this fungal strain was investigated. Whereas, in the current study nineteen fungal isolates were obtained from the collected deteriorated samples and identified using traditional methods and select six isolates to confirm their identification by molecular analysis. The activities of all fungal isolates to produce different hydrolytic enzymes (cellulase, amylase, gelatinase, and pectinase) were investigated to examine their activity in biodeterioration of main historical paper and leather components. Finally, different concentrations of TiO2-NPs fabricated by green method were used to control the growth of most potent fungal strains (six strains).
4 Conclusion
The most common culturable fungal strains associated with deteriorated book, namely, Description of Egypt, was isolated and subjected to traditional and molecular identification. The damage signs in selected book were assessed using visual documentation, ESEM, ATR/FTIR, and color change. The identification tools showed that the obtained fungal strains belong to Aspergillus flavus (8-isolates), A. versicolor (2-isolates), A. ustus (4-isolates), A. chinensis (2-isolates), Penicillium citrinum (2-isolates), and P. chrysogenum (1-isolate). The roles of these fungal strains in biodeterioration were assessed by investigating their potential to secrete hydrolytic enzymes including cellulase, amylase, gelatinase, and pectinase. The majority of fungal strains have the efficacy to secrete all recommended hydrolytic enzymes. TiO2-NPs were synthesized using cell-free filtrate of probiotic bacterial strain Lactobacillus rhamnosus to inhibit the growth of deteriorated fungal strains. The synthesized TiO2-NPs were characterized by UV-Vis, FT-IR, XRD, TEM, SAED, SEM, EDX, DLS, and zeta potential. The safe concentrations of TiO2-NPs, which were selected based on biocompatibility toward two normal cell lines, showed promising activity to control the growth of high-deteriorated fungal strains. Therefore, it can be concluded that the TiO2-NPs have the efficacy to inhibit the growth of various fungal strains associated with deteriorated historical papers; hence, our hypothesis was achieved. However, the drawback or shortage of this work is a lack of experimental studies to show the potential of TiO2-NPs to inhibit fungal growth when inoculated on filter paper loaded with NPs.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The sequence in the current study was deposited in NCBI (GenBank) at: https://www.ncbi.nlm.nih.gov/nuccore/OP782309; https://www.ncbi.nlm.nih.gov/nuccore/OP782310; https://www.ncbi.nlm.nih.gov/nuccore/OP782311; https://www.ncbi.nlm.nih.gov/nuccore/OP782312; https://www.ncbi.nlm.nih.gov/nuccore/OP782313; https://www.ncbi.nlm.nih.gov/nuccore/OP804338.
References
Gallo F, Pasquariello G, Valenti P (2003) Libraries and archives. In: Mandrioli P, Caneva G, Sabbioni C (eds) Cultural heritage and aerobiology: methods and measurement techniques for biodeterioration monitoring. Springer, Netherlands, Dordrecht, pp 175–193. https://doi.org/10.1007/978-94-017-0185-3_7
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44(22):3358–3393. https://doi.org/10.1002/anie.200460587
Andlar M, Rezić T, Marđetko N, Kracher D, Ludwig R, Šantek B (2018) Lignocellulose degradation: an overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng Life Sci 18(11):768–778. https://doi.org/10.1002/elsc.201800039
Abdel-Maksoud G, Abed al-Sameh Al-Shazly EE, El-Amin A-R (2011) Damage caused by insects during the mummification process: an experimental study. Archaeol Anthropol Sci 3:291–308
Pinheiro AC, Sequeira SO (2021) Mycological studies in Cultural Heritage settings. In: Zaragoza O, Casadevall A (eds) Encyclopedia of Mycology. Elsevier, pp 27–39
Oetari A, Susetyo-Salim T, Sjamsuridzal W, Suherman EA, Monica M, Wongso R, Fitri R, Nurlaili DG, Ayu DC, Teja TP (2016) Occurrence of fungi on deteriorated old dluwang manuscripts from Indonesia. Int Biodeterior Biodegradation 114:94–103. https://doi.org/10.1016/j.ibiod.2016.05.025
Sterflinger K, Piñar G (2013) Microbial deterioration of cultural heritage and works of art — tilting at windmills? Appl Microbiol Biotechnol 97(22):9637–9646. https://doi.org/10.1007/s00253-013-5283-1
Branysova T, Kracmarova M, Durovic M, Demnerova K, Stiborova H (2021) Factors influencing the fungal diversity on audio-visual materials. Microorganisms 9(12). https://doi.org/10.3390/microorganisms9122497
Pinzari F, Fanelli C, Canhoto O, Magan N (2004) Electronic nose for the early detection of moulds in libraries and archives. Indoor Built Environ 13(5):387–395
Pasquariello G, Maggi O (2003) Museums. In: Mandrioli P, Caneva G, Sabbioni C (eds) Cultural heritage and aerobiology: methods and measurement techniques for biodeterioration monitoring. Springer, Netherlands, Dordrecht, pp 195–206. https://doi.org/10.1007/978-94-017-0185-3_8
Fouda A, Abdel-Nasser M, Khalil AMA, Hassan SE-D, Abdel-Maksoud G (2022) Investigate the role of fungal communities associated with a historical manuscript from the 17th century in biodegradation. npj Mater Degrad 6(1):88. https://doi.org/10.1038/s41529-022-00296-4
Pinheiro AC, Sequeira SO, Macedo MF (2019) Fungi in archives, libraries, and museums: a review on paper conservation and human health. Crit Rev Microbiol 45(5-6):686–700. https://doi.org/10.1080/1040841x.2019.1690420
Nitiu DS, Mallo AC, Saparrat MCN (2020) Fungal melanins that deteriorate paper cultural heritage: an overview. Mycologia 112(5):859–870. https://doi.org/10.1080/00275514.2020.1788846
El-Gendi H, Saleh AK, Badierah R, Redwan EM, El-Maradny YA, El-Fakharany EM (2021) A comprehensive insight into fungal enzymes: structure, classification, and their role in mankind’s challenges. J Fungi 8(1). https://doi.org/10.3390/jof8010023
Mohammed BT, Dakhil MH, Lmutairy T (2018) Manuscripts preserved at the Al-Hussein Holy Shrine: Isolation and diagnosis of fungi causing potential damage. Indian J Ecol 45(1):214–221
Kosel J, Ropret P (2021) Overview of fungal isolates on heritage collections of photographic materials and their biological potency. J Cult Herit 48:277–291. https://doi.org/10.1016/j.culher.2021.01.004
David ME, Ion RM, Grigorescu RM, Iancu L, Andrei ER (2020) Nanomaterials used in conservation and restoration of cultural heritage: an up-to-date overview. Materials 13(9). https://doi.org/10.3390/ma13092064
Hashem AH, Saied E, Amin BH, Alotibi FO, Al-Askar AA, Arishi AA, Elkady FM, Elbahnasawy MA (2022) Antifungal activity of biosynthesized silver nanoparticles (AgNPs) against Aspergilli causing aspergillosis: ultrastructure study. J Funct Biomater 13(4). https://doi.org/10.3390/jfb13040242
Nassar A-RA, Eid AM, Atta HM, El Naghy WS, Fouda A (2023) Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci Rep 13(1):9054. https://doi.org/10.1038/s41598-023-35360-9
Sagadevan S, Lett JA, Fatimah I, Lokanathan Y, Léonard E, Oh WC, Hossain MAM, Johan MR (2021) Current trends in the green syntheses of tin oxide nanoparticles and their biomedical applications. Mater Res Exp 8(8):082001. https://doi.org/10.1088/2053-1591/ac187e
Saied E, Hashem AH, Ali OM, Selim S, Almuhayawi MS, Elbahnasawy MA (2022) Photocatalytic and antimicrobial activities of biosynthesized silver nanoparticles using Cytobacillus firmus. Life 12(9). https://doi.org/10.3390/life12091331
Hashem AH, Abdelaziz AM, Askar AA, Fouda HM, Khalil AMA, Abd-Elsalam KA, Khaleil MM (2021) Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in faba bean plants. J Fungi 7(3). https://doi.org/10.3390/jof7030195
Suppiah DD, Julkapli NM, Sagadevan S, Johan MR (2023) Eco-friendly green synthesis approach and evaluation of environmental and biological applications of iron oxide nanoparticles. Inorg Chem Commun 152:110700. https://doi.org/10.1016/j.inoche.2023.110700
Ngoepe NM, Mathipa MM, Hintsho-Mbita NC (2020) Biosynthesis of titanium dioxide nanoparticles for the photodegradation of dyes and removal of bacteria. Optik 224:165728. https://doi.org/10.1016/j.ijleo.2020.165728
Anbumani D, Kv D, Manoharan J, Babujanarthanam R, Bashir AKH, Muthusamy K, Alfarhan A, Kanimozhi K (2022) Green synthesis and antimicrobial efficacy of titanium dioxide nanoparticles using Luffa acutangula leaf extract. J King Saud Univ Sci 34(3):101896. https://doi.org/10.1016/j.jksus.2022.101896
Sagadevan S, Imteyaz S, Murugan B, Lett JA, Sridewi N, Weldegebrieal GK, Fatimah I, Oh W-C (2022) A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Process Synth 11(1):44–63. https://doi.org/10.1515/gps-2022-0005
Sagadevan S, Anita Lett J, Vennila S, Varun Prasath P, Saravanan Kaliaraj G, Fatimah I, Léonard E, Mohammad F, Al-Lohedan HA, Alshahateet SF, Lee CT (2021) Photocatalytic activity and antibacterial efficacy of titanium dioxide nanoparticles mediated by Myristica fragrans seed extract. Chem Phys Lett 771:138527. https://doi.org/10.1016/j.cplett.2021.138527
Ali OM, Hasanin MS, Suleiman WB, Helal EE-H, Hashem AH (2022) Green biosynthesis of titanium dioxide quantum dots using watermelon peel waste: antimicrobial, antioxidant, and anticancer activities. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02772-y
Órdenes-Aenishanslins NA, Saona LA, Durán-Toro VM, Monrás JP, Bravo DM, Pérez-Donoso JM (2014) Use of titanium dioxide nanoparticles biosynthesized by Bacillus mycoides in quantum dot sensitized solar cells. Microb Cell Factories 13(1):90. https://doi.org/10.1186/s12934-014-0090-7
Landage KS, Arbade G, Khanna P, Bhongale C (2020) Biological approach to synthesize TiO2 nanoparticles using Staphylococcus aureus for antibacterial and anti-biofilm applications. J Microbiol Exp 8(1):36–43
Jha AK, Prasad K, Kulkarni AR (2009) Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf B: Biointerfaces 71(2):226–229. https://doi.org/10.1016/j.colsurfb.2009.02.007
El-Gamal R, Nikolaivits E, Zervakis GI, Abdel-Maksoud G, Topakas E, Christakopoulos P (2016) The use of chitosan in protecting wooden artifacts from damage by mold fungi. Electron J Biotechnol 24:70–78. https://doi.org/10.1016/j.ejbt.2016.10.006
Lintang W, Susetyo-Salim T, Oetari A, Sjamsuridzal W (2021) Isolation and characterization of fungi from deteriorated old manuscripts from Banyumas, collection of Library of Universitas Indonesia. IOP Conf Ser.: Earth Environ Sci 948(1):012031. https://doi.org/10.1088/1755-1315/948/1/012031
Mai B, Liu N, Liu X, Teri G, Liu P, Wang J, Li Y, Cao J (2022) Mould prevention of archive packaging based microenvironment intervention and regulation. J Cult Herit 57:16–25. https://doi.org/10.1016/j.culher.2022.07.005
Diba K, Kordbacheh P, Mirhendi S, Rezaie S, Mahmoudi M (2007) Identification of Aspergillus species using morphological characteristics. Pak J Med Sci 23(6):867
Frisvad JC, Samson RA (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol 49(1):1–174
Dorit RL, Ohara O, Hwang CB, Kim JB, Blackshaw S (2001) Direct DNA sequencing of PCR products. Current protocols in molecular biology Chapter 15:Unit 15.12. https://doi.org/10.1002/0471142727.mb1502s56
Abdel-Maksoud G, Abdel-Nasser M, Sultan MH, Eid AM, Alotaibi SH, Hassan SE, Fouda A (2022) Fungal biodeterioration of a historical manuscript dating back to the 14th century: an insight into various fungal strains and their enzymatic activities. Life 12(11). https://doi.org/10.3390/life12111821
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using gram's iodine. Curr Microbiol 57(5):503–507. https://doi.org/10.1007/s00284-008-9276-8
Aravind M, Amalanathan M, Mary MSM (2021) Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl Sci 3(4):409. https://doi.org/10.1007/s42452-021-04281-5
Kirthi AV, Rahuman AA, Rajakumar G, Marimuthu S, Santhoshkumar T, Jayaseelan C, Elango G, Zahir AA, Kamaraj C, Bagavan A (2011) Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mater Lett 65(17):2745–2747. https://doi.org/10.1016/j.matlet.2011.05.077
Khan R, Fulekar MH (2016) Biosynthesis of titanium dioxide nanoparticles using Bacillus amyloliquefaciens culture and enhancement of its photocatalytic activity for the degradation of a sulfonated textile dye Reactive Red 31. J Colloid Interface Sci 475:184–191. https://doi.org/10.1016/j.jcis.2016.05.001
Londoño-Restrepo SM, Jeronimo-Cruz R, Millán-Malo BM, Rivera-Muñoz EM, Rodriguez-García ME (2019) Effect of the nano crystal size on the X-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones. Sci Rep 9(1):5915. https://doi.org/10.1038/s41598-019-42269-9
Hamza MF, Fouda A, Wei Y, El Aassy IE, Alotaibi SH, Guibal E, Mashaal NM (2022) Functionalized biobased composite for metal decontamination – insight on uranium and application to water samples collected from wells in mining areas (Sinai, Egypt). Chem Eng J 431:133967. https://doi.org/10.1016/j.cej.2021.133967
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Piñar G, Piombino-Mascali D, Maixner F, Zink A, Sterflinger K (2013) Microbial survey of the mummies from the Capuchin Catacombs of Palermo, Italy: biodeterioration risk and contamination of the indoor air. FEMS Microbiol Ecol 86(2):341–356. https://doi.org/10.1111/1574-6941.12165
Michaelsen A, Piñar G, Montanari M, Pinzari F (2009) Biodeterioration and restoration of a 16th-century book using a combination of conventional and molecular techniques: a case study. Int Biodeterior Biodegrad 63(2):161–168. https://doi.org/10.1016/j.ibiod.2008.08.007
Michaelsen A, Piñar G, Pinzari F (2010) Molecular and microscopical investigation of the microflora inhabiting a deteriorated Italian manuscript dated from the thirteenth century. Microb Ecol 60(1):69–80. https://doi.org/10.1007/s00248-010-9667-9
Dochia M, Sirghie C, Kozłowski RM, Roskwitalski Z (2012) 2 - Cotton fibres. In: Kozłowski RM (ed) Handbook of natural fibres, vol 1. Woodhead Publishing, pp 11–23. https://doi.org/10.1533/9780857095503.1.9
Costa MN, Veigas B, Jacob JM, Santos DS, Gomes J, Baptista PV, Martins R, Inácio J, Fortunato E (2014) A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: lab-on-paper. Nanotechnology 25(9):094006. https://doi.org/10.1088/0957-4484/25/9/094006
Özgörüş NK, Ünlü CH, Grupče O, Bakan F, Sezen M (2017) Analysis of deterioration phenomena in a Koran by nineteenth century ottoman Calligrapher Mehmed Şevki. Restaurator Int J Preserv Lib Arch Mater 38(4):331–354
Kruer-Zerhusen N, Cantero-Tubilla B, Wilson DB (2018) Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 25(1):37–48. https://doi.org/10.1007/s10570-017-1542-0
Coates J (2006) Interpretation of infrared spectra, a practical approach. In: Encyclopedia of analytical chemistry. https://doi.org/10.1002/9780470027318.a5606
Mohammadi P, Maghboli-Balasjin N (2014) Isolation and molecular identification of deteriorating fungi from Cyrus the Great tomb stones. Iran J Microbiol 6(5):361–370
Sahab A, Dissoki B, Sahaba S, Badie S, Hanafy O, Monir A (2014) Studies on fungal isolates involved in biodeterioration of ancient manuscripts of the general Egyptian book organization (GEBO). Egypt J Archeol Restor Stud 4(1):47–54
Oetari A, Natalius A, Komalasari D, Susetyo-Salim T (2018) Sjamsuridzal W Fungal deterioration of old manuscripts of European paper origin. In: AIP Conference Proceedings, vol 1. AIP Publishing LLC, p 020156
Gadhave RVI, Gadhave CR (2022) Adhesives for the paper packaging industry: an overview. Open J Polym Chem 12(2):55–79
Sabatini L, Sisti M, Campana R (2018) Evaluation of fungal community involved in the bioderioration process of wooden artworks and canvases in Montefeltro area (Marche, Italy). Microbiol Res 207:203–210. https://doi.org/10.1016/j.micres.2017.12.003
Željko S, Miloš S, Nikola U, Aleksandar K, Jelena V, Milica Ljaljević G (2021) Fungal deterioration of cultural heritage objects. In: Kassio Ferreira M, de Rodrigo Nogueira S, Kamila Cabral M (eds) Biodegradation technology of organic and inorganic pollutants. IntechOpen, Rijeka, p 15. https://doi.org/10.5772/intechopen.98620
Coronado-Ruiz C, Avendaño R, Escudero-Leyva E, Conejo-Barboza G, Chaverri P, Chavarría M (2018) Two new cellulolytic fungal species isolated from a 19th-century art collection. Sci Rep 8(1):7492. https://doi.org/10.1038/s41598-018-24934-7
Cao D, Lou Y, Jiang X, Zhang D, Liu J (2022) Fungal diversity in barley under different storage conditions. Front Microbiol 13:895975. https://doi.org/10.3389/fmicb.2022.895975
Chakravarty A, Ahmad I, Singh P, Ud Din Sheikh M, Aalam G, Sagadevan S, Ikram S (2022) Green synthesis of silver nanoparticles using fruits extracts of Syzygium cumini and their bioactivity. Chem Phys Lett 795:139493. https://doi.org/10.1016/j.cplett.2022.139493
Singh Jassal P, Kaur D, Prasad R, Singh J (2022) Green synthesis of titanium dioxide nanoparticles: development and applications. J Agric Food Res 10:100361. https://doi.org/10.1016/j.jafr.2022.100361
Taran M, Rad M, Alavi M (2018) Biosynthesis of TiO(2) and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. BioImpacts: BI 8(2):81–89. https://doi.org/10.15171/bi.2018.10
Liu Y, Sun D, Askari S, Patel J, Macias-Montero M, Mitra S, Zhang R, Lin WF, Mariotti D, Maguire P (2015) Enhanced dispersion of TiO2 nanoparticles in a TiO2/PEDOT:PSS hybrid nanocomposite via plasma-liquid interactions. Sci Rep 5:15765. https://doi.org/10.1038/srep15765
Hamza MF, Alotaibi SH, Wei Y, Mashaal NM (2022) High-performance hydrogel based on modified chitosan for removal of heavy metal ions in borehole: a case study from the Bahariya Oasis, Egypt. Catalysts 12(7). https://doi.org/10.3390/catal12070721
Hamza MF, Wei Y, Guibal E (2020) Quaternization of algal/PEI beads (a new sorbent): Characterization and application to scandium sorption from aqueous solutions. Chem Eng J 383:123210. https://doi.org/10.1016/j.cej.2019.123210
Rao TN, Riyazuddin BP, Ahmad N, Khan RA, Hassan I, Shahzad SA, Husain FM (2019) Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi J Biol Sci 26(7):1385–1391. https://doi.org/10.1016/j.sjbs.2019.09.005
Salem SS, Fouda A (2021) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199(1):344–370. https://doi.org/10.1007/s12011-020-02138-3
Sundrarajan M, Bama K, Bhavani M, Jegatheeswaran S, Ambika S, Sangili A, Nithya P, Sumathi R (2017) Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J Photochem Photobiol B, Biol 171:117–124. https://doi.org/10.1016/j.jphotobiol.2017.05.003
Mansoor A, Khan MT, Mehmood M, Khurshid Z, Ali MI, Jamal A (2022) Synthesis and characterization of titanium oxide nanoparticles with a novel biogenic process for dental application. Nanomaterials 12(7). https://doi.org/10.3390/nano12071078
Tomaszewska E, Soliwoda K, Kadziola K, Tkacz-Szczesna B, Celichowski G, Cichomski M, Szmaja W, Grobelny J (2013) Detection limits of DLS and UV-vis spectroscopy in characterization of polydisperse nanoparticles colloids. J Nanomater 2013:313081. https://doi.org/10.1155/2013/313081
Sethy NK, Arif Z, Mishra PK, Kumar P (2020) Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process Synth 9(1):171–181. https://doi.org/10.1515/gps-2020-0018
Al Saqr A, Khafagy ES, Alalaiwe A, Aldawsari MF, Alshahrani SM, Anwer MK, Khan S, Lila ASA, Arab HH, Hegazy WAH (2021) Synthesis of gold nanoparticles by using green machinery: characterization and in vitro toxicity. Nanomaterials 11(3). https://doi.org/10.3390/nano11030808
Kaszuba M, Corbett J, Watson FM, Jones A (2010) High-concentration zeta potential measurements using light-scattering techniques. Philos Trans Series A, Math, Phys Eng Sci 368(1927):4439–4451. https://doi.org/10.1098/rsta.2010.0175
Dhanjal S, Cameotra SS (2010) Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb Cell Fact 9:52. https://doi.org/10.1186/1475-2859-9-52
Al-Shabib NA, Husain FM, Qais FA, Ahmad N, Khan A, Alyousef AA, Arshad M, Noor S, Khan JM, Alam P, Albalawi TH, Shahzad SA (2020) Phyto-mediated synthesis of porous titanium dioxide nanoparticles from Withania somnifera root extract: broad-spectrum attenuation of biofilm and cytotoxic properties against HepG2 cell lines. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.01680
Hamed MT, Bakr BA, Shahin YH, Elwakil BH, Abu-Serie MM, Aljohani FS, Bekhit AA (2021) Novel synthesis of titanium oxide nanoparticles: biological activity and acute toxicity study. Bioinorg Chem Appl 2021:8171786. https://doi.org/10.1155/2021/8171786
Ghasemi M, Turnbull T, Sebastian S, Kempson I (2021) The MTT assay: utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci 22(23). https://doi.org/10.3390/ijms222312827
Chellappa M, Anjaneyulu U, Manivasagam G, Vijayalakshmi U (2015) Preparation and evaluation of the cytotoxic nature of TiO2 nanoparticles by direct contact method. Int J Nanomed 10(Suppl 1 (Suppl 1)):31–41. https://doi.org/10.2147/ijn.s79978
Rahmani Kukia N, Rasmi Y, Abbasi A, Koshoridze N, Shirpoor A, Burjanadze G, Saboory E (2018) Bio-effects of TiO2 nanoparticles on human colorectal cancer and umbilical vein endothelial cell lines. Asian Pac J Cancer Prev: APJCP 19(10):2821–2829. https://doi.org/10.22034/apjcp.2018.19.10.2821
Ali Alasmary F, Rajaram SK, Rajabathar J, Innasimuthu GM, Sankar K, Stephen Muthaiah S, AlKahtani AMA, Salem Almalki A, Mohammed Alhajri H (2022) Titanium dioxide nanoparticles fabrication from Parmotrema austrosinense (Zahlbr.) Hale extracts and its antimicrobial efficacy against plant pathogens. Inorg Chem Commun 145:110007. https://doi.org/10.1016/j.inoche.2022.110007
Fouda A, Abdel-Maksoud G, Abdel-Rahman MA, Salem SS, Hassan SE-D, El-Sadany MA-H (2019) Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int Biodeterior Biodegrad 142:160–169. https://doi.org/10.1016/j.ibiod.2019.05.012
Fouda A, Abdel-Maksoud G, Abdel-Rahman MA, Eid AM, Barghoth MG, El-Sadany MA-H (2019) Monitoring the effect of biosynthesized nanoparticles against biodeterioration of cellulose-based materials by Aspergillus niger. Cellulose 26(11):6583–6597. https://doi.org/10.1007/s10570-019-02574-y
Huston M, DeBella M, DiBella M, Gupta A (2021) Green synthesis of nanomaterials. Nanomaterials 11(8). https://doi.org/10.3390/nano11082130
Rai M, Ingle AP, Paralikar P, Anasane N, Gade R, Ingle P (2018) Effective management of soft rot of ginger caused by Pythium spp. and Fusarium spp.: emerging role of nanotechnology. Appl Microbiol Biotechnol 102(16):6827–6839. https://doi.org/10.1007/s00253-018-9145-8
Cruz-Luna AR, Cruz-Martínez H, Vásquez-López A, Medina DI (2021) Metal nanoparticles as novel antifungal agents for sustainable agriculture: current advances and future directions. J Fungi 7(12). https://doi.org/10.3390/jof7121033
Hamza MF, Hamad DM, Hamad NA, Abdel-Rahman AAH, Fouda A, Wei Y, Guibal E, El-Etrawy A-AS (2022) Functionalization of magnetic chitosan microparticles for high-performance removal of chromate from aqueous solutions and tannery effluent. Chem Eng J 428:131775. https://doi.org/10.1016/j.cej.2021.131775
Huerta-García E, Pérez-Arizti JA, Márquez-Ramírez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, López-Marure R (2014) Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med 73:84–94. https://doi.org/10.1016/j.freeradbiomed.2014.04.026
Acknowledgements
The authors extend their appreciation to the Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt; Conservation Department, Faculty of Archaeology, Cairo University, Giza, Egypt; Department of Manuscripts Conservation, Al-Azhar Al-Sharif Library, Cairo, Egypt; and Food Toxicology and Contaminants Department, National Research Centre, Cairo, Egypt for the great support and cooperation in the achievement and publication of this research work. Also, the authors extend their thanks to the staff of the Library of the Arabic Language Academy for their cooperation and support during the study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Gomaa Abdel-Maksoud and Amr Fouda: conceptualization, methodology, validation, formal analysis, data curation, supervision, writing—original draft, and writing—review and editing. Mahmoud Abdel-Nasser, Saad El-Din Hassan, Ahmed M. Eid, and Aya Abdel-Nasser: methodology, validation, formal analysis, software, data curation, resources, and writing—original draft. All authors approved the final version of the manuscript to be published.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Maksoud, G., Abdel-Nasser, M., Hassan, S.ED. et al. Biosynthesis of titanium dioxide nanoparticles using probiotic bacterial strain, Lactobacillus rhamnosus, and evaluate of their biocompatibility and antifungal activity. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04587-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04587-x